Abstract
Atopic asthma is poorly controlled by current therapies. Newer therapies and novel antihistamines are, therefore, required to treat patients whose atopic asthma is not controlled. For the first time, C-027 is shown to antagonize histamine, IgE-mediated and serotonin-induced contraction in human airways and vessels. Human precision-cut lung slices (PCLS, 250 µm thick), containing an airway or blood vessel, were pretreated with either C-027 (2 hours) or with vehicle alone and were contracted with histamine or serotonin. Known antihistamine was used as a comparator in antihistamine studies. Also, human airways were contracted via IgE passive sensitization in the presence or absence of C-027 or fexofenadine. Affinity of C-027 toward human G-protein coupled receptors was also determined, as well as the drug's biodistribution in murine model. C-027 was shown to have the highest affinity toward human histamine and serotonin receptors. Subsequently, C-027 was shown to antagonize histamine- and serotonin-induced airway and vascular smooth muscle contraction, respectively, and histamine-released bronchocontraction mediated by IgE passive sensitization in human small airways. C-027 also inhibited histamine-mediated single-cell calcium ion release. Low levels of C-027 were found in murine brain tissue. Collectively, these data suggest that C-027 markedly inhibits IgE-induced bronchoconstriction and antagonizes histamine and serotonin-contraction with little biodistribution in the brain. The compound may offer a future therapy for allergen-induced airway hyperresponsiveness in patients with asthma.
Keywords: Airway smooth muscle, allergy, antagonist, anti-histamine, anti-serotonic, asthma, IgE mediated, novel compound, PCLS, reverse agonist
INTRODUCTION
Patients with a history of diseases characterized by atopy and increased immunoglobulin E (IgE) are at greater risk for developing airway hyperresponsiveness (AHR) and asthma.1–3 Allergic asthma occurs when repeated exposure to allergens increases IgE levels that result in cross-linking of FcεRI receptors on mast cells and basophils, causing the release of preformed mediators such as leukotrienes, prostaglandins, serotonin, and histamine. These mediators evoke bronchoconstriction, increase vasopermeability, and sustain airway inflammation. Current popular therapies for asthma include β2-adrenergic receptor (β2-AR) agonists (albuterol, formoterol, and salmeterol) and anticholinergics (ipratropium bromide and tiotropium bromide). Although these aforementioned treatments alleviate the symptoms of allergic asthma by maintaining airflow, antihistamines (fexofenadine, levocetirizine, and loratadine) can advantageously decrease airway reactivity to methacholine challenges4 and may also offer other anti-inflammatory properties such as decreasing secretions of IL-6 and IL-8.5
Although β2-AR agonists have proven to benefit the majority of patients with asthma, studies show that the use of these therapies alone can induce tolerance and thereby reduce the efficacy of the drug while increasing asthma morbidity.6,7 The use of a steroid in combination with a β2-AR agonist significantly decreases the effect of β2-AR desensitization.8,9 However, a small population of asthma and chronic obstructive pulmonary disease patients are steroid insensitive.10 Some clinicians advocate the use of anticholinergics rather than β2-AR agonists; however, such approaches remain controversial. Anticholinergic agents may offer benefit to patients with predominant large airway disease rather than lower airway obstruction.11 Typically, anticholinergics are used in emergency treatment rooms in combination with β2-AR agonists after a severe asthma exacerbation.12
Antihistamines (histamine receptor inverse agonists) typically have been used to treat patients with mast cell–dependent atopic diseases.13–15 They are used to treat allergic rhinitis by abruptly blocking the procontractile actions of histamine, therefore, promoting bronchodilation and preventing allergen-induced increases in airway resistance.4,16,17 Furthermore, antihistamines have also been shown to decrease infiltrating neutrophils and eosinophils in patients with allergic rhinitis.17 Historically, antihistamines typically induce drowsiness and were not considered to be optimal therapies for asthma, with clinical studies showing modest effects concluding antihistamines to be unsuitable as a single therapy against asthma.18,19 New evidence suggests third-generation antihistamines have fewer central nervous system effects and may offer therapeutic value in asthma, especially in combination therapy with antileukotrienes.16 The administration of antihistamines in subsets of atopic children has also been shown to delay or prevent the development of asthma.15 Controversy over the suitability of antihistamines to manage the symptoms of airway resistance is also determined by the frequency of administration. Numerous studies have shown that continuous treatment with antihistamines are more effective than an on-demand/symptom reliever administration.20,21 The differences in the clinical studies may offer somewhat contradictory results and hence create debate on their suitability. Collectively, these data suggest that antihistamines may have beneficial effects in the management of asthma by controlling symptoms of allergic rhinitis when administered continuously rather than on demand.
In the present study, a novel long-acting compound, C-027 (2-amino-N-(2-{4-[3-(2-trifluoromethyl-phenothiazin-10-yl)-propyl]-piperazin-1-yl}-ethyl)-acetamide trihydrochloride) is shown to act as an antihistamine and antiserotonergic agent. C-027 showed high affinity toward histamine and serotonin receptors and antagonized histamine-induced bronchoconstriction in human small airways, similar to that of fexofenadine using precision-cut lung slice (PCLS), with no effect on carbachol (CCh)-induced contractions. Furthermore, C-027 inhibited histamine-induced calcium release from single-cell human airway smooth muscle cells. C-027 also antagonized serotonin-induced vasoconstriction. Taken together, C-027 is a promising novel compound and it may offer unique therapeutic advantages in additional studies in its treatment for asthma and allergic airways disease.
METHODS
Reagents
CCh, isoproteronol, histamine, low melting point agarose (IX-A), dexamethasone, myeloma human IgE (Calbiochem, San Diego, CA) and goat anti-human IgE (Sigma, St. Louis, MO), Ham's F-12 medium (supplemented with 2.0 mM of glutamine, 100 U/mL of penicillin, 100 µg/mL of streptomycin, 1.0 mg/mL of primocin [Amaxa, Walkersville, MD], and 1.0 M of HEPES, pH 7.6). All reagents were obtained from Sigma unless stated otherwise.
C-027 Receptor Binding Assays
Radioligand binding assays were performed against histamine H1–4, β1–3-adrenergic, M1–5 muscarinic, and 5-HT2A–C, 5-HT4, 5-HT6, and 5-HT7 serotonin receptors and corresponding known agonists. Briefly, C-027, or an unlabeled positive control antagonist compound, were added to a receptor preparation plus radiolabeled agonist, either at a single antagonist concentration for screening to generate a single point percent inhibition, or at a graded series of antagonist concentrations to generate a sigmoidal inhibition curve from which a Ki was derived (Caliper Life Sciences, Hopkinton, MA). Radioligands and reference antagonists used for individual receptors were as follows: H1, 3H-pyrilamine 1.2 nM, pyrilamine; H2, 3H-aminopotentidine 0.1 nM, tiotidine; H3, 3H-R(−)-α-methylhistamine 3 nM, R(−)-α-methylhistamine; H4, 3H-histamine 8.2 nM, histamine; M1–53H-N-methylscopalamine 0.8 nM, 4-diphenylacetoxy · N-piperidine (4-DAMP); 5-HT2A3H-ketanserin 2 nM, ketanserin; 5-HT2B, H-lysergic acid diethylamide (3H-LSD) 1 nM, 5-HT; 5-HT2C3H-mesulergine 1 nM, mianserin; 5-HT4,3H-GR113808 0.2 nM, 5-HT; 5-HT5A,6,3H-LSD 1.5 nM, methiothepin mesylate; and 5-HT7, 3H-LSD 2.5 nM, 5-CT.
Pharmacokinetics and Biodistribution of C-027 in Mice
C-027 was dosed intravenously (i.v.), via oral gavage (o.g.), or intraperitoneally (i.p.) in overnight-fasted male CD-1 mice (20–40 g), 4 per time point. C-027 was dissolved (weight-free base/volume) in PBS and dosed within 30 minutes. Plasma was collected into sodium heparin on ice, centrifuged at 13,000 rpm, at 4°C (5 minutes); plasma was stored at −80°C. Using a Tomtec Quadra 96-Model 320 liquid handling system, plasma samples (55 µL) were added to microtiter wells preloaded with 150 µL of acetonitrile plus 0.1% formic acid containing warfarin (100 ng/mL) as internal standard in 96-well Siroco Protein Precipitation plates (Waters Corp., Milford, MA). After mixing via aspiration, contents of the wells were vacuum filtered into clean polypropylene 96-well plates and 50-µL aliquots mixed with an equal volume of distilled water. Liquid chromatography mass spectromatic (LC/MS) analysis was performed using a PE Sciex API3000 instrument equipped with Perkin Elmer series 200 micropumps and a LEAP autosampler. Samples (20 µL) were injected onto a Phenomenex Synergi PolarRP (Torence, CA) column (50 × 2 mm internal diameter (id), 4 µm) and eluted with a mobile phase of 40 mM of ammonium formate, pH 3.5, with a water/acetonitrile gradient at a flow rate of 300 µL/min. An eight-point calibration curve was prepared at concentrations ranging from 0.5 to 100 ng/mL. Each batch had at least 60% of standards within an accuracy of +20% of nominal, except at the lower limit of quantitation where +25% was considered acceptable. Mean plasma concentration time data were analyzed using noncompartmental methods (area moment analysis). The peak concentration (Cmax) and time of Cmax (Tmax) were taken directly from the observed data. The area under the curve (AUC), apparent volume of distribution (Vss), and bioavailability (F) were estimated with the linear trapezoidal rule using WinNonlin software, Version 4.2 (Pharsight, Mountain View, CA). Except for calculation of descriptive statistics, statistical analysis was not performed.
In the biodistribution studies, animals (n = 4/dose group) were dosed as in the pharmacokinetics experiments. Lung tissue was weighed, placed in 20:80 methanol/water to make 4 mL/g of tissue, homogenized in a Virsonic 100 ultrasonic homogenizer (VirTis, Gardner, NY) on ice, and then stored at −80°C until analyzed. Homogenates were extracted with acetonitrile, vacuum filtered, and analyzed by LC/MS using a PE Sciex API3000 as described previously for pharmacokinetic samples.
Human PCLS Preparation and Airway Function
PCLS from healthy whole lungs (received from National Disease Research Interchange) were prepared as previously described.8,22 After their preparation, the lung slices were placed in a 12-well plate in 1.0 mL of Ham's F-12 medium and were held in place using a platinum weight with nylon attachments and viewed under a microscope (magnification: ×40). A baseline image was taken (0% contraction) followed by the addition of the lowest concentration of CCh to begin the concentration response (10−8–10−4 M) or histamine (10−10–10−4 M). Images were collected 10 minutes after each dose. Previous in-house experiments have determined that these time points are sufficient to allow maximal effect at each concentration. Airway lumen area was measured using a macro written within Image Pro-Plus (Version 6.0) software (Media Cybernetics, Bethesda, MD) and given in units of micrometers squared. A log EC50 and Emax value for each airway was derived from a concentration–response curve. At least two airways from each donor were used per condition; n values indicate number of donors. Data were expressed as mean + SEM. Statistical difference was shown by using a nonpaired t-test. Vasoconstriction was assessed in the same manner as bronchoconstriction, except that the lumen of a pulmonary vessel located on a PCLS was measured to increasing concentrations of 5-HT.
Single Cell Ca2+ Imaging
Human ASM cells cultured on coverslips were washed twice with PBS and serum starved in 0.1% bovine serum albumin media for an additional 24 hours. On the day of the experiments, the cells were pretreated with C-027 or diluent alone for 2 hours. Cells were washed and maintained in a saline solution containing 130 mM of NaCl, 4 mM of KCl, 2 mM of CaCl2, 2 mM of MgCl2, HEPES, and 15 mM of glucose (pH 7.4). The cells were loaded with 1 µM pf fura-2 AM (Invitrogen, Carlsbad, CA) for ~45 minutes at room temperature and washed. The imaging experiments were performed at room temperature and the cells were continually perfused with the saline solution at ~1 mL/min. The dye-loaded ASM cells were excited at 340- and 380-nm wavelength and emissions were collected at ≥400-nm wavelength (Photon Technology International, Birmingham, NJ) using a CCD camera mounted on a Nikon Diaphot inverted microscope (Nikon Instruments, Inc., Melville, NY). The background fluorescence was estimated in the presence of 10 mM of MnCl2 and 5 µM of ionomycin in the nominal absence of Ca2+. The imaging results were processed using a custom routine implemented in IGOR Pro (WaveMetrics, Lake Oswego, OR) to estimate the ratio of the background-subtracted emission at 340 and 380 nm. To record differential effects of C-027 to varied contractile agonists, responses were recorded in response to stimulation with histamine (100 µM for 10 minutes and wash for 35 minutes) and subsequent treatment with bradykinin (2 µM for 10 minutes). In each group, responses from 15 to 17 cells were analyzed.
RESULTS
C-027 Receptor Binding Assay
C-027 bound avidly to the histamine H1-receptor (Ki = 3.6 nM) but did not bind significantly to β1-, β2-, or β3-ARs, or to the M1, M2, M3, M4, or M5 muscarinic receptors (Fig. 1). C-027 also bound to several serotonin receptors including 5-HT7 and 5-HT2B (Ki in the range 4–6 nM) and 5-HT2A, 5HT2C, 5-HT4, and 5-HT6 (Ki in the range of 60–1000 nM).
Figure 1.
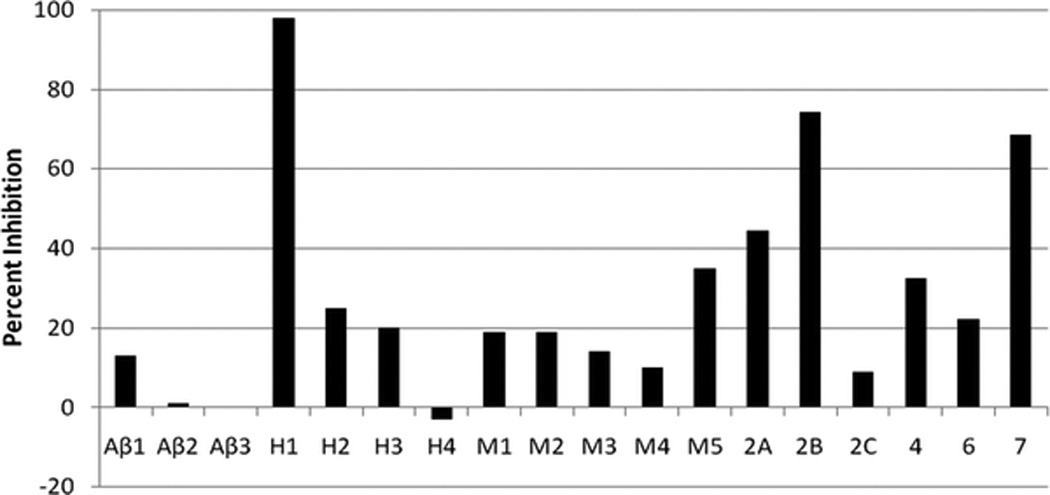
C-027 preferentially binds to H1 and selected serotonin receptors. Radiolabeled known agonist plus unlabeled C-027 at a concentration of 10−7 M or an unlabeled positive control compound at appropriate concentration (data not shown) were combined in a screening assay to generate a single point percent inhibition. In a separate experiment (not shown) C-027 was added at a graded series of antagonist concentrations to generate a sigmoidal inhibition curve from which a Ki could be derived. Receptors designations: Aβ, β-adrenergic; H, histamine; M, muscarinic; serotonin receptors are shown only as subtypes 2A–7.
Bioavailability and Pharmacokinetics of C-027
C-027 was administered to CD-1 mice either via i.v., i.p., or o.g. routes at various concentrations. After an i.v. dose of 5 mg/kg the highest plasma concentration (Cmax) of 1424 ng/mL occurred at the earliest time point measured (5 minutes). After i.p administration at 1 and 10 mg/kg, Cmax occurred at 30 minutes at concentrations of 12.9 and 333 ng/mL, respectively. After 10 mg/kg of C-027 administered o.g., Cmax of 22.7 ng/mL occurred at 2 hours (Table 1). When administered orally at 10, 1.0, and 0.1 mg/kg, C-027 concentrations in the lung after 24 hours were measured to be 9625, 326, and 52 ng/mL, respectively, indicating that delivery to lung tissue is approximately dose proportional over a wide range of oral dosing. The highest drug concentration was found in the small intestine. The tissue content of C-027 in the brain was ~1% of that in most other organs, indicating that C-027 is restricted from crossing the blood-brain barrier (Fig. 2).
Table 1.
C-027 Pharmacokinetics in mice
| Dose (mg/kg) | Route | Cmax (ng/mL) | Tmax (hr) | AUC0-last (hr * ng per mL) | Vss (L/kg) | F* (%) |
|---|---|---|---|---|---|---|
| 5 | i.v. | 1424 | 0.083 | 302 | 37.1# | ND |
| 1 | i.p. | 12.9 | 0.5 | 21.5 | ND | ND |
| 10 | i.p | 333 | 0.5 | 363 | ND | ND |
| 10 | o.g. | 22.7 | 2 | 134 | ND | 22 |
Bioavailability.
Represents ~63 × the total body water volume.
C-027 was administered to CD-1 mice either via i.v., i.p., or o.g. routes at various concentrations. The peak concentration (Cmax) and time of Cmax (Tmax) were token directly from the observed data. The AUC, apparent volume of distribution (Vss), and bioavailability (F) were estimated with the linear trapezoidal rule using WinNonlin software Version 4.2 (Pharsight, Mountain View, CA).
AUC = area under the curve; i.v. = intravenous; i.p. = intraperitoneal; o.g. = oral lavage.
Figure 2.
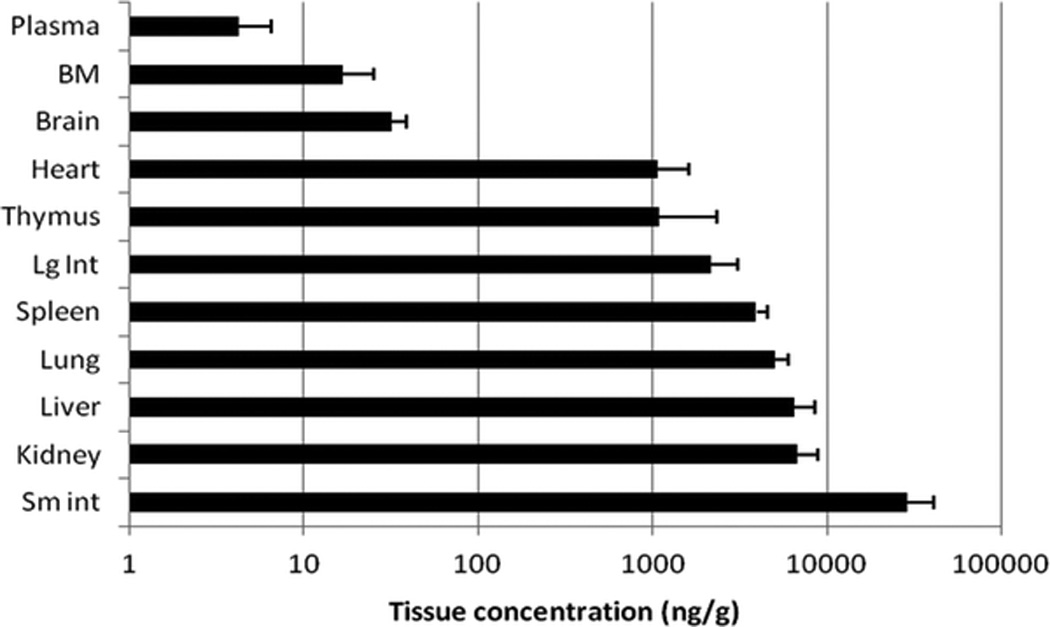
Biodistribution of C-027. The content of C-027 (mean + SD) in organs. CD-1 mice (n = 4) were administered C-027 via oral gavage and tissue concentrations of the drug were measured after 24 hours.
C-027 Inhibits IgE Passive Sensitization-Induced Contraction
The passive sensitization in the early allergic response in human PCLS has been previously shown to be dependent on IgE.22,23 The incubation with human IgE sensitizes the lung tissue to an allergic response, when the secondary antibody, an anti-human IgE, is added at a later stage, the IgE is activated by their cross-linking, and airway contraction occurs. This is presumed to be caused by the release of histamine via the degranulation of mast cells in the human lung tissue. Human IgE from myeloma (4 µg/mL) was incubated with a human slice for 18 hours. After addition of goat anti-human IgE (20 µg/mL) airway lumen area measurements were taken every 30 seconds. As shown previously,22 IgE passive sensitization induced bronchoconstriction to 61.0 + 3.6%. The control slices incubated with human IgG followed by goat anti-human IgE induced a 20.1 + 10.1% bronchoconstriction, probably caused by endogenous nonspecific IgE already located on mast cells in the lung slice. Three concentrations of C-027 were then incubated 2 hours before the addition of the secondary antibody, and a dose-dependent inhibition of contraction was observed. The highest concentration of 10 µM completely abrogated any contraction, 3.0 µM induced a 42.1 + 11.5% inhibition of the maximum passive-sensitized induced bronchoconstriction from 61 to 34%; and the lowest concentration of C-027 (1.0 µM) had little effect (Fig. 3A). Because the mechanism of bronchoconstriction in this model is thought to be caused by the release of histamine, the effects of C-027 were compared with a known antihistamine, fexofenadine. Slices were incubated with fenofexadine at 3 µM for 2 hours before anti-human IgE administration, and the same level of inhibition was seen as an equal concentration of C-027. The steroid dexamethasone (1.0 µM; 2 hours) had little effect (Fig. 3B).
Figure 3.
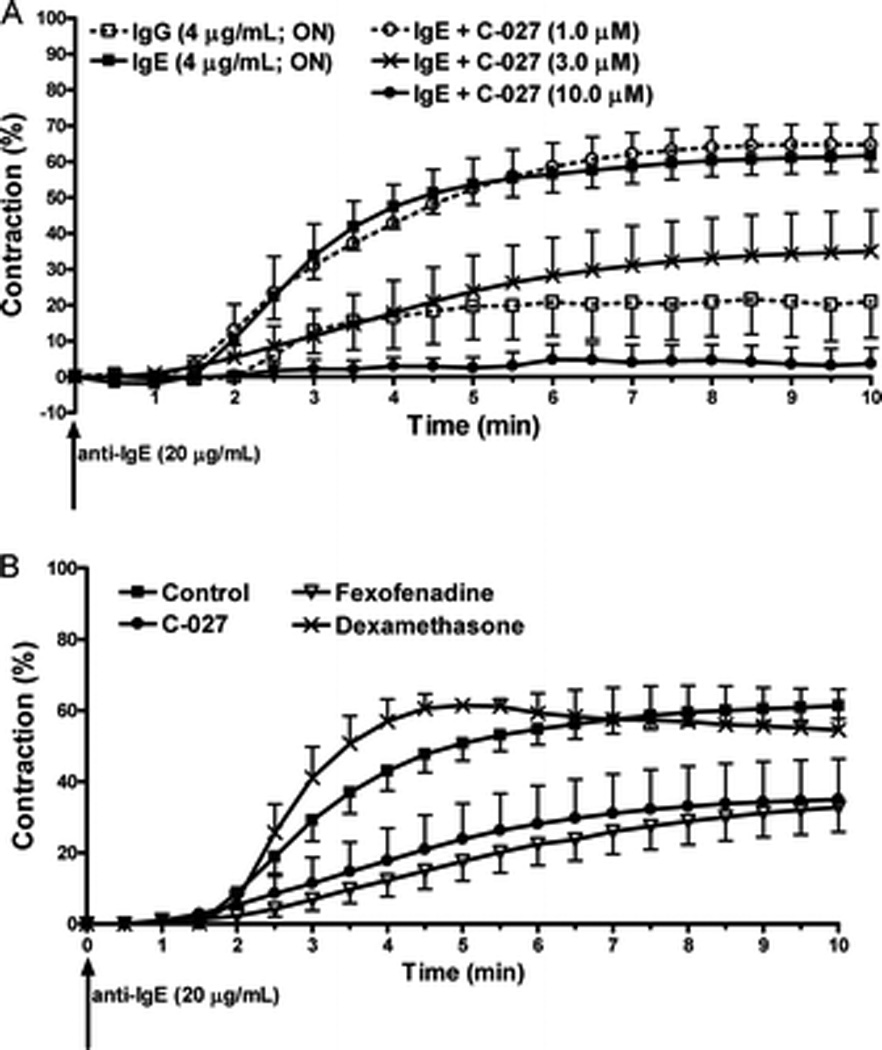
C-027 attenuates IgE-induced human airway contraction similar to a histamine antagonist. Precision-cut lung slices containing a small human airway were incubated with either immunoglobulin G (IgG) or IgE (4 µg/mL; overnight). Human small airway contraction was measured by changes in lumen area after the incubation of anti-IgE (20 µg/mL). Images were taken every 30 seconds. (A) C-027 (1.0, 3.0, or 10.0 µM) were preincubated with slices for 2 hours before the addition of anti-IgE. (B) C-027 (3 µM), fexofenadine (3 µM), or dexamethasone (1.0 µM) were preincubated for 2 hours before the addition of anti-IgE.
C-027 Antagonizes Histamine-Induced Bronchoconstriction
C-027 was incubated with a human lung slice at three different concentrations (10, 3.0, or 1.0 µM) for 2 hours. The drug was removed and a histamine concentration–response curve was performed. C-027 antagonized histamine-induced contraction in a concentration-dependent manner. The lowest concentrations of C-027 significantly decreased airway sensitivity to histamine as shown by an increase in the EC50 values from 0.09 + 0.08 µM (control) to 0.23 + 0.28 µM (3.0 µM) or 0.82 + 0.17 µM (1.0 µM; Fig. 4A). Interestingly, 10 µM of C-027 decreased the maximal effect (data not shown).
Figure 4.
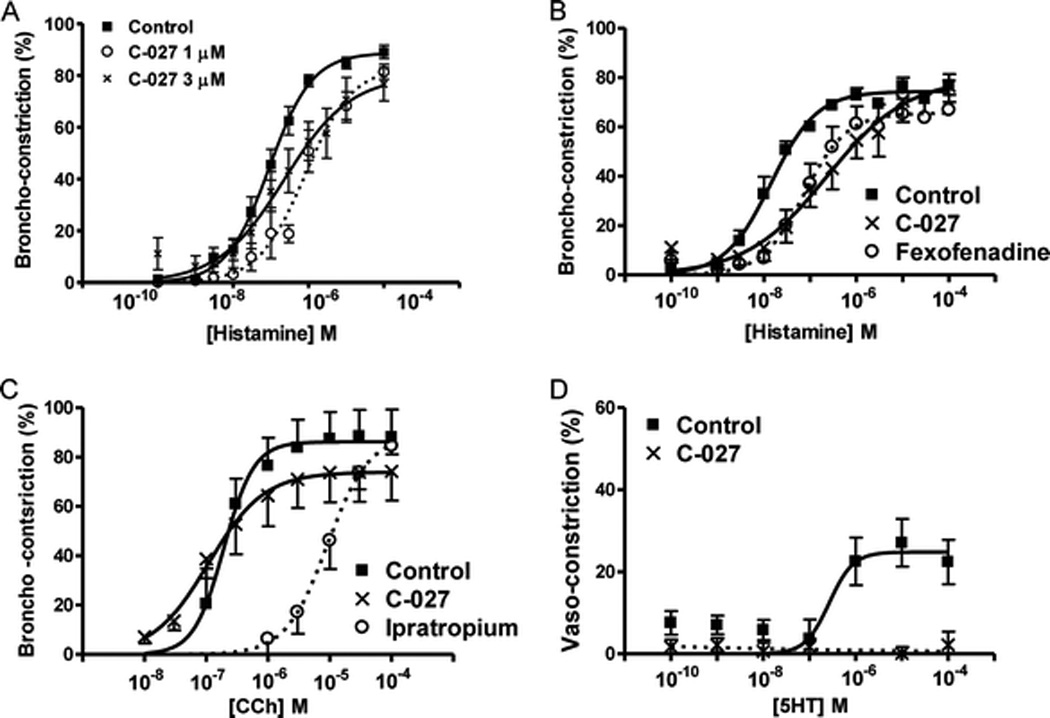
C-027 antagonizes histamine but not muscarinic-induced bronchoconstriction. Precision-cut lung slices containing a small human airway were pretreated with (A) C-027 (1.0 or 3.0 µM) for 2 hours. Airways were contracted with histamine. In separate studies, slices were pretreated with C-027 (3 µM) and (B) fexofenadine (3 µM) or (C) ipratropium (3 µM) and airways were contracted with the antagonist's respective agonist with no drug removal. (C) Pulmonary arterioles were contracted with 5-HT in the presence and absence of C-027 (3 µM). At least three airways or arterioles were used in each group from at least two human donors (therefore six airways total).
C-027 Acts as an Antihistamine and a Serotonin Antagonist but Not as a Muscarinic Antagonist
C-027 (3.0 µM) or fexofenadine (3.0 µM) was incubated with human lung slices containing a small airway for 2 hours and then removed. A histamine concentration–response curve was then generated. C-027 and fexofenadine induced a similar right shift in the histamine concentration–response curve and a significant increase in the EC50 values compared with the control (Fig. 4B). Ipratropium bromide (3.0 µM; 2 hours), induced a right shift in the CCh concentration–response curve as expected for a muscarinic antagonist; however, C-027 had little effect on CCh-induced contraction (Fig. 4C). Pulmonary vessels located on a human PCLS contracted in response to serotonin and an EC50 value of 0.27 µM was achieved with a 25% maximum vasoconstriction. Incubation with C-027 (3.0 µM; 2 hours) completely abrogated the 5HT-induced vasoconstriction (Fig. 4D).
C-027 Inhibits Histamine-Induced Ca2+ Release
Histamine (100 µM) dramatically increased the intracellular Ca2+ concentration ([Ca2+]i) in cultured human airway smooth muscle cells as measured using fura-2 (Fig. 5A, left). However, pretreatment of the cells with C-027 (3 µM; 2 hours) completely abrogated the histamine-induced Ca2+ signal (Fig. 5B, left). Subsequent application of bradykinin (2 µM; after wash for 35 minutes) elicited robust increases in [Ca2+]i in the cells in both groups (Fig. 5, A and B, right), showing specificity of C-027 for histamine-mediated effects.
Figure 5.
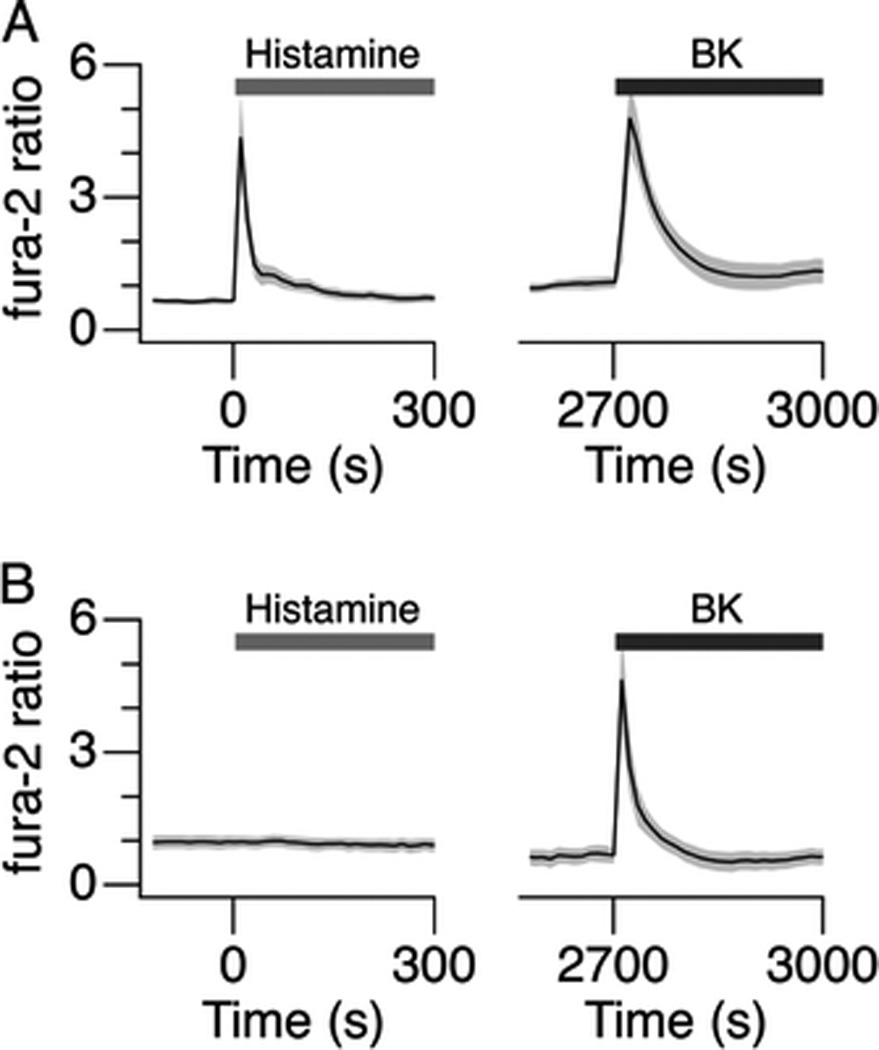
C-027 inhibits histamine-induced calcium ion release. (A and B) Mean fura-2 ratio signals in response to application of histamine (100 µM) and BK (2 µM) in (A) control cells and (B) the cells pretreated with C-027. The shaded gray area represents SD. The cells were washed for 35 minutes between the applications of the agonists.
DISCUSSION
In the current study, we introduce a novel antihistamine and serotonin antagonist, C-027, that abrogates histamine-induced Ca2+ release from human airway smooth muscle cells, blocks IgE-mediated human small airway bronchoconstriction, antagonizes histamine-induced airway closure in human small airways, antagonizes serotonin-induced vascular contraction in human pulmonary vessels, and, most interestingly, shows a low biodistribution in murine brains, indicating minimal potential to induce drowsiness. C-027 is described here as an antihistamine and serotonin antagonist because of its low Ki values, hence, high affinity, toward the respective receptor.
We have previously shown that Aspergillus fumigates–induced increases of IgE in airways are associated with eosinophil-dependent AHR24; others have reported an attenuation of allergen-induced increases in IgE by histamine and serotonin antagonists.25,26 Using an IgE passive sensitization model in human small airways to replicate atopic conditions,22,23 the effects of C-027 on IgE passively sensitized induced bronchoconstriction were examined. C-027 showed a dose-dependent inhibition of passive sensitized-induced bronchoconstriction with an approximate EC50 value of 3.0 µM. A maximum effect concentration (1.0 µM) of dexamethasone, previously shown to induce an effect in human PCLS8 was incubated with slices for an identical time period and failed to inhibit the bronchoconstriction. A prolonged, overnight incubation to induce genomic effects of the steroid also failed to inhibit the airway contraction (data not shown). As a comparator to C-027, the H1-antagonist, fexofenadine, was incubated with separate human lung slices at an equal concentration and time (3.0 µM; 2 hours) and displayed a very similar response to C-027 after the secondary IgE antibody was added. Atopic IgE-mediated bronchoconstriction in humans is thought to be caused by the release of histamine and other bronchoconstrictors. Because human mast cells do not release serotonin, the actions of C-027 in this experiment are predominantly those of an antihistamine. Others have reported that serotonin antagonists have little effect on passively sensitized human airways.23
Antagonism that was not dose dependent to histamine-induced contraction in human small airways was also noted. However, in these experiments, unlike the IgE-mediated bronchoconstriction, C-027 at 1.0 µM did have an effect. The apparent non–dose-dependent inhibition of histamine-induced contraction can not be fully explained at this time. C-027 showed similar properties to the antihistamine, fexofenadine, in that both induced a right shift in the histamine concentration–response curve when incubated for 2 hours. Antihistaminic activity of C-027 also was evident in experiments where the drug selectively blocked intracellular calcium mobilization in human airway smooth muscle cells. A robust response to bradykinin showed that cells were viable and fully capable of calcium mobilization after drug treatment.
Despite various serotonin receptors located on human airway smooth muscle cells, we and others27 have failed to show serotonin-induced bronchoconstriction. To show antiserotonergic properties of C-027, PCLS containing a pulmonary arteriole (approximately the same size as airways used in this study) was treated with increasing concentrations of 5-HT. A decreased maximum arteriole contraction (22%) compared with airway contraction was observed; however, C-027 completely blocked 5-HT–mediated vasoconstriction. The 5-HT2A receptor has been shown to mediate serotonin-induced contraction of human pulmonary arterioles.28 That the observed EC50 value of 0.27 µM for the vascular contractile response is ~10-fold greater than the functional EC50 measured for serotonin acting on a cloned human 5-HT2A receptor (0.031 µM),29 may be caused by destruction of the agonist by monoamine oxidase in sectioned lung tissue. The concentration of C-027 (3 µM) tested exceeds the Ki of the drug for the 5-HT2A receptor by a factor of 5.
Histamine has beneficial actions as a neurotransmitter responsible for maintaining states of awareness in the central nervous system, but some antihistamines cause sedation due to their transport across the blood–brain barrier. Furthermore, first generation anti-histamine drugs also acted as potent anti-cholinergics. The newer second-generation antihistamines lacked some side effects because of their hydrophilic chemical properties. C-027 shows properties similar to the second-generation antihistamines by having little affinity for muscarinic receptors and low biodistribution in the brain of mice showing its restricted ability to cross the blood-brain barrier.
In summary, C-027, a novel antihistamine and serotonergic antagonist inhibits IgG-induced bronchoconstirction in human airways. With additional properties of low biodistribution in the brain, lack of muscarinic activity, and a prolonged half-life, C-027 warrants further studies as a unique therapeutic in asthma and allergic airways disease.
Acknowledgments
Funded by Corridor Pharmaceuticals, Inc.
Footnotes
D.A. Zopf is an employee of Corridor Pharmaceuticals. The remaining authors have no conflicts to declare pertaining to this article
REFERENCES
- 1.Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Simpson BM, Custovic A, Simpson A, et al. NAC Manchester Asthma and Allergy Study (NACMAAS): Risk factors for asthma and allergic disorders in adults. Clin Exp Allergy. 2001;31:391–399. doi: 10.1046/j.1365-2222.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- 3.Sears MR, Burrows B, Flannery EM, et al. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 4.Dizdar EA, Sekerel BE, Keskin O, et al. The effect of regular versus on-demand desloratadine treatment in children with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2007;71:843–849. doi: 10.1016/j.ijporl.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Molet S, Gosset P, Lassalle P, et al. Inhibitory activity of loratadine and descarboxyethoxyloratadine on histamine-induced activation of endothelial cells. Clin Exp Allergy. 1997;27:1167–1174. [PubMed] [Google Scholar]
- 6.Cheung D, Timmers MC, Zwinderman AH, et al. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992;327:1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- 7.Haney S, Hancox RJ. Tolerance to bronchodilation during treatment with long-acting beta-agonists, a randomised controlled trial. Respir Res. 2005;6:107. doi: 10.1186/1465-9921-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper PR, Panettieri RA. Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 10.Yang IA, Fong KM, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;2:CD002991. doi: 10.1002/14651858.CD002991.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigo GJ, Rodrigo C. The role of anticholinergics in acute asthma treatment: An evidence-based evaluation. Chest. 2002;121:1977–1987. doi: 10.1378/chest.121.6.1977. [DOI] [PubMed] [Google Scholar]
- 12.Dotson K, Dallman M, Bowman CM, Titus MO. Ipratropium bromide for acute asthma exacerbations in the emergency setting: A literature review of the evidence. Pediatr Emerg Care. 2009;25:687–692. doi: 10.1097/PEC.0b013e3181b95084. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Trujillo V. Antihistamines treatment for allergic rhinitis: Different routes, different mechanisms? Allergy Asthma Proc. 2009;30:584–588. doi: 10.2500/aap.2009.30.3289. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman P. Intranasal antihistamines for allergic rhinitis: Mechanism of action. Allergy Asthma Proc. 2009;30:345–348. doi: 10.2500/aap.2009.30.3263. [DOI] [PubMed] [Google Scholar]
- 15.Wilson AM. The role of antihistamines in asthma management. Treat Respir Med. 2006;5:149–158. doi: 10.2165/00151829-200605030-00001. [DOI] [PubMed] [Google Scholar]
- 16.Richter K, Gronke L, Janicki S, et al. Effect of azelastine, montelukast, and their combination on allergen-induced bronchoconstriction in asthma. Pulm Pharmacol Ther. 2008;21:61–66. doi: 10.1016/j.pupt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Ciprandi G, Passalacqua G, Mincarini M, et al. Continuous versus on demand treatment with cetirizine for allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79:507–511. doi: 10.1016/S1081-1206(10)63057-8. [DOI] [PubMed] [Google Scholar]
- 18.Spector SL, Nicodemus CF, Corren J, et al. Comparison of the bronchodilatory effects of cetirizine, albuterol, and both together versus placebo in patients with mild-to-moderate asthma. J Allergy Clin Immunol. 1995;96:174–181. doi: 10.1016/s0091-6749(95)70005-6. [DOI] [PubMed] [Google Scholar]
- 19.Bewtra AK, Hopp RJ, Nair NM, Townley RG. Effect of terfenadine on cold air-induced bronchospasm. Ann Allergy. 1989;62:299–301. [PubMed] [Google Scholar]
- 20.Ciprandi G, Ricca V, Passalacqua G, et al. Seasonal rhinitis and azelastine: Long- or short-term treatment? J Allergy Clin Immunol. 1997;99:301–307. doi: 10.1016/s0091-6749(97)70046-0. [DOI] [PubMed] [Google Scholar]
- 21.Canonica GW, Fumagalli F, Guerra L, et al. Levocetirizine in persistent allergic rhinitis: Continuous or on-demand use? A pilot study. Curr Med Res Opin. 2008;24:2829–2839. doi: 10.1185/03007990802395927. [DOI] [PubMed] [Google Scholar]
- 22.Cooper PR, Lamb R, Day ND, et al. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L530–L537. doi: 10.1152/ajplung.00133.2009. [DOI] [PubMed] [Google Scholar]
- 23.Wohlsen A, Martin C, Vollmer E, et al. The early allergic response in small airways of human precision-cut lung slices. Eur Respir J. 2003;21:1024–1032. doi: 10.1183/09031936.03.00027502. [DOI] [PubMed] [Google Scholar]
- 24.Haczku A, Atochina EN, Tomer Y, et al. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25:45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]
- 25.Gelfand EW, Cui ZH, Takeda K, et al. Fexofenadine modulates T-cell function, preventing allergen-induced airway inflammation and hyperresponsiveness. J Allergy Clin Immunol. 2002;110:85–95. doi: 10.1067/mai.2002.124770a. [DOI] [PubMed] [Google Scholar]
- 26.Lima C, Souza VM, Soares AL, et al. Interference of methysergide, a specific 5-hydroxytryptamine receptor antagonist, with airway chronic allergic inflammation and remodelling in a murine model of asthma. Clin Exp Allergy. 2007;37:723–734. doi: 10.1111/j.1365-2222.2007.02700.x. [DOI] [PubMed] [Google Scholar]
- 27.Ressmeyer AR, Larsson AK, Vollmer E, et al. Characterisation of guinea pig precision-cut lung slices: Comparison with human tissues. Eur Respir J. 2006;28:603–611. doi: 10.1183/09031936.06.00004206. [DOI] [PubMed] [Google Scholar]
- 28.Frishman WH, Huberfeld S, Okin S, et al. Serotonin and serotonin antagonism in cardiovascular and non-cardiovascular disease. J Clin Pharmacol. 1995;35:541–572. doi: 10.1002/j.1552-4604.1995.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 29.Porter RH, Benwell KR, Lamb H, et al. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]


