I. Synopsis
Trauma in childhood is a grave psychosocial, medical, and public policy problem that has serious consequences for its victims and for society. Chronic interpersonal violence in children is common worldwide. Developmental traumatology, the systemic investigation of the psychiatric and psychobiological effects of chronic overwhelming stress on the developing child, provides a framework and principles when empirically examining the neurobiological effects of pediatric trauma.
Despite the widespread prevalence of childhood trauma, less is known about trauma's biological effects in children as compared to adults with child trauma histories; and even less is known about how these pediatric mechanisms underlie trauma's short-term and long-term medical and mental health consequences. This article focuses primarily on the peer-reviewed literature on the neurobiological sequelae of childhood trauma in children and adults with histories of childhood trauma. We also review relevant studies of animal models of stress to help us better understand the psychobiological effects of trauma during development. Next, we review the neurobiology of trauma, its clinical applications and the biomarkers that may provide important tools for clinicians and researchers, both as predictors of posttraumatic stress symptoms and as useful tools to monitor treatment response. Finally, we offer suggestions for future researchers.
Keywords: Childhood trauma, developmental traumatology, developmental psychopathology, posttraumatic stress symptoms, stress, biological stress systems, brain development, genes, polymorphisms, epigenetics, cortisol
III. Introduction
Trauma in childhood has serious consequences for its victims and for society. For the purposes of this critical review, childhood trauma is defined according to the Diagnostic and Statistical Manual of Mental Disorders IV and V as exposure to actual or threatened death, serious injury, or sexual violence [1, 2]. This includes experiences of direct trauma exposure, witnessing trauma or learning about trauma that happened to a close friend or relative. In children, motor vehicle accidents, bullying, terrorism, exposure to war, child maltreatment (physical, sexual, and emotional abuse; neglect) and exposure to domestic and community violence are common types of childhood traumas that result in distress, posttraumatic stress disorder (PTSD), and posttraumatic stress symptoms (PTSS). Childhood traumas, particularly those that are interpersonal, intentional, and chronic are associated with greater rates of PTSD [3], PTSS [4, 5], depression [6] and anxiety [7], antisocial behaviors [8] and greater risk for alcohol and substance use disorders [9-12].
The traditional categorical cluster of symptoms that form the diagnosis of PTSD are each associated with differences in biological stress symptoms and brain structure and function; and are thought to individually contribute to delays in or deficits of multisystem developmental achievements in behavioral, cognitive and emotional regulation in traumatized children and lead to PTSS and co-morbidity [13]. Thus, we examine PTSD as a dimensional diagnosis encompassing a range of pathological reactions to severe stress, rather than as a dichotomous variable.
Developmental traumatology, the systemic investigation of the psychiatric and psychobiological effects of chronic overwhelming stress on the developing child, provides the framework used in this critical review of the biological effects of pediatric trauma.[13] This field builds on foundations of developmental psychopathology, developmental neuroscience, and stress and trauma research. The DSM-IV-TR diagnosis of PTSD is made when criterion A, a Type A trauma, is experienced and when three clusters of categorical symptoms are present for more than one month after the traumatic event(s). These three clusters are Criterion B: intrusive reexperiencing of the trauma(s), Criterion C: persistent avoidance of stimuli associated with the trauma(s), and Criterion D: persistent symptoms of increased physiological arousal.[1] These criteria are complex and each Criterion is thought to be associated with dysregulation of at least one major biological stress system as well as several different brain circuits. This makes both the psychotherapeutic and the psychopharmacological treatment of individuals with early trauma complex and challenging.
Criterion symptoms have an experimental basis in classical and operant conditioning theory, where animals learn to generalized behaviors based on previous experiences or “reinforcements”[14] and in animal models of learned helplessness where animals under conditions of uncontrollable shock do not learn escape behaviors, and have exaggerated fear responses as well as social isolation and poor health [15]. For example, Cluster B reexperiencing and intrusive symptoms can best be conceptualized as a classically conditioned response that is mediated by the serotonin system and is similar in some ways to the recurrent intrusive thoughts experienced in obsessive compulsive disorder, where serotonin and norepinephrine transmitter deficits play an important role [16]. An external or internal conditioned stimulus (e.g., the traumatic trigger) activates unwanted and distressing recurrent and intrusive memories of the traumatic experience(s) (e.g., the unconditioned stimulus). However, other Criterion B symptoms, such as nightmares or night terrors, may involve the dysregulation of multiple neurotransmitter systems (serotonin, norepinephrine, dopamine, choline, gamma-amino butyric acid (GABA) [17]). Criterion C symptoms represent both avoidant behaviors and negative alterations in cognitions. In the DSM-V, Criterion C was divided into avoidant behaviors and Criterion D negative alterations in cognitions [2]. Avoidant behaviors can be thought of as ways to control painful and distressing reexperiencing of symptoms. These symptoms are likely associated with the dopamine system and overactivation of the opioid system and associated with anhedonia and numbing of responses [18]. In the DSM-IV the former Criterion D persistent symptoms of increased physiological arousal and reactivity is now Criterion E, and likely involves dysregulation of several biological stress systems [13, 19] as discussed in further detail below.
We will review the known differences in pediatric victims’ stress biology compared to those children who have not experienced trauma. These differences are likely the causes of the greater rates of psychopathology (PTSD, depression, disruptive behaviors, suicidality, substance use disorders) and of the common medical disorders (cardiovascular disease, obesity, chronic pain syndromes, gastrointestinal disorders, immune dysregulation) seen in child victims [20]. Throughout, we will associate the relationship to biological stress systems and common stress symptoms. On many levels, childhood trauma can be regarded as “an environmentally induced complex developmental disorder”[13].
Exposure to a traumatic event or series of chronic traumatic events (e.g., child maltreatment) activates the body's biological stress response systems [21-23]. Stress activation has behavioral and emotional effects that are similar to individual PTSS symptoms [24]. Further, an individual's biological stress response system is made up of different, interacting systems, that work together to direct the body's attention toward protecting the individual against environmental life threats and to shift metabolic resources away from homeostasis and toward a “fight or flight” (and/or freezing) reaction [19, 25]. The stressors associated with the traumatic event are processed by the body's sensory systems through the brain's thalamus, which then activates the amygdala, a central component of the brain's fear detection and anxiety circuits. Cortisol levels become elevated through transmission of fear signals to neurons in the prefrontal cortex, hypothalamus, and hippocampus, and activity increases in the locus coeruleus and sympathetic nervous system. Subsequent changes in, catecholamine levels contribute to changes in heart rate, metabolic rate, blood pressure, and alertness [19]. This process also leads to the activation of other biological stress systems.
In the review of the pertinent literature section, we will review the main biological stress response systems. We will focus on the limbic-hypothalamic-pituitary-adrenal (LHPA) axis, and the locus coeruleus-norepinephrine/sympathetic nervous system (SNS) or catecholamine system. We will also review the serotonin system, oxytocin and the oxytocin system, the immune system, and new data in genetic and epigenetic factors and gene-environment interactions that influence these systems and contribute to an individual's experience of vulnerability and resilience to childhood trauma. For each of these systems, we will provide an explanation of the mechanisms that drive them, followed by an examination of how these systems compare in children and adults who have been exposed to childhood trauma, thereby highlighting how early life adversity can disrupt the body's ability to regulate its response to stress.
Experiencing trauma during development along with dysregulation of biological stress systems can adversely impact childhood brain development [13] and we will discuss brain imaging studies in children who experienced trauma and adults with trauma histories. Recently, the field of neuroscience has become increasingly aware of gender as an important moderator of experience, so throughout, we review peer reviewed publications that highlight gender differences, if available. Little is known about trauma's neurobiological, genetic, and epigenetic effects in children as compared to adults with trauma histories. Since longitudinal psychobiological research in pediatric trauma is a severely understudied area, most of our review will be based on cross-sectional studies. Although we will highlight studies where longitudinal research is available, more longitudinal research in trauma-exposed children is needed to understand the pediatric mechanisms underlying trauma's short-term and long-term adverse effects in adolescence and adulthood. We will review the clinical applications of this knowledge and discuss how stress related biomarkers may provide important tools for clinicians and researchers to objectively examine predictors of PTSS and to monitor treatment response. We then offer suggestions for future directions.
A literature search of Pubmed and PsychInfo articles published to 2013 using keywords and MeSH terms “childhood,” “trauma,” “stress,” and/or “posttraumatic stress,” where crossed individually with “hypothalamic pituitary axis (HPA)”, “corticotrophin releasing hormone” corticotrophin releasing factor,” “immune,” “serotonin,” “dopamine,” “oxytocin,” “brain,”, “brain imaging,” “brain structure,” “brain function,” “cognitive,” “genes,” “polymorphisms,” and “epigenetics,” that were limited to the English language, were reviewed and selected for this critical review. Trauma studies involving physical head trauma or medical illnesses were not included. Our criteria were that the articles be peer-reviewed and methodologically sound, with emphasis placed on the paucity of longitudinal studies in this field. When reviews were needed to describe the foundations of biological stress systems and brain development, meta-analyses or peer-reviewed critical reviews published by known stress researchers were cited.
IV. Review of the pertinent literature: The Neurobiology of Biological Stress Systems Limbic-Hypothalamic-Pituitary-Adrenal (LHPA) Axis
The LHPA axis plays a central role in regulating the body's response to stress and is the most studied biological stress system in animals and humans. Activation of the LHPA axis triggers the hypothalamus to secrete corticotrophin releasing hormone (CRH). This neuropeptide, also called corticotrophin releasing factor (CRF), is a key mediator of the stress response [26]. The term CRH is used when describing its function in the neuroendocrine system and the term CRF is commonly used when describing its function as a neurotransmitter. However the term CRH is the older term and authors are not always consistent in using these rules. CRH stimulates the release of adrenocorticotrophic hormone (ACTH) by binding to CRH receptors in the anterior pituitary. ACTH in turn binds to G protein-coupled receptors in the adrenal cortex, especially in the zona fasciculata of the adrenal glands. ACTH also stimulates the secretion of cortisol, a glucocorticoid hormone that plays an important role throughout the central nervous system (CNS). Cortisol activates glucocorticoid and mineralocorticoid receptors, which are located and expressed throughout the brain. Glucocorticoid receptors act as transcription factors and regulate gene expression for metabolism and immune function, as well as for cognitive and brain development [27]. Increased levels of cortisol suppress the immune system, gluconeogenesis, and inhibit its own secretion via negative feedback to glucocorticoid receptors in the hippocampus [19].
CRF is widely distributed throughout the brain and is involved in the stress response and learning and memory [28]. Cortisol regulates the stress response system both in the hippocampus and medial prefrontal cortex (mPFC), where it works to attenuate the stress response, and in the medial and central nuclei of the amygdala, where it works to promote the stress response via CRF-1 receptors [29]. Through negative feedback, cortisol controls its own secretion, inhibiting the hypothalamus’ release of CRH and the pituitary's release of ACTH, thereby bringing the body back to a state of homeostasis rather than arousal[19]. Cortisol levels have a consistent diurnal pattern where levels are typically close to their peak during morning awakening, rise further in the 20-minute period after awakening and then progressively fall, reaching the nadir in the afternoon in children [30] adolescents [31], and adults [32]. Furthermore, cortisol levels and pituitary volumes increase with age [33].
The LHPA Axis and Childhood Trauma
Exposure to severe stress and trauma in youth can disrupt the regulatory processes of the LHPA axis across the life span in both animals and humans [26, 27, 34-36]. In animals, injections of CRF in early life produces a delayed effect in later life that is associated with reduced cognitive function, reduced number of number of CA3 hippocampal neurons, and decreased branching of hippocampal pyramidal neurons [37, 38]. Although the pediatric trauma literature suggest that the LHPA system is dysregulated in youth exposed to trauma, the cortisol regulation data seem contradictory, where baseline morning and 24-hour cortisol concentrations showed no differences [39, 40] , were higher [41-47] or in a few studies, lower[47-49] compared to youth without trauma. Additionally, no cortisol response differences [39, 50, 51], blunted cortisol responses[52], and increased cortisol concentration responses [53, 54] have been reported in maltreated children and adults with histories of childhood maltreatment under psychological and pharmacological challenges. Other measures of the LHPA axis such as ACTH also show these contradictory findings where both blunted [39, 55] and increased [50, 53, 54, 56] ACTH levels have been reported in maltreated depressed children and adults with histories of childhood maltreatment under psychological and pharmacological challenges. Furthermore, in one meta-analysis, lower morning but higher afternoon/evening cortisol levels, a flatter diurnal rhythm, and greater daily cortisol output were seen in adults retrospectively reporting trauma[47]; while another meta-analysis demonstrated that individuals with adulthood trauma exposure and adults with PTSD showed no differences in cortisol levels [57], indicating that the developing LHPA axis is vulnerable to dysregulation as a result of childhood trauma.
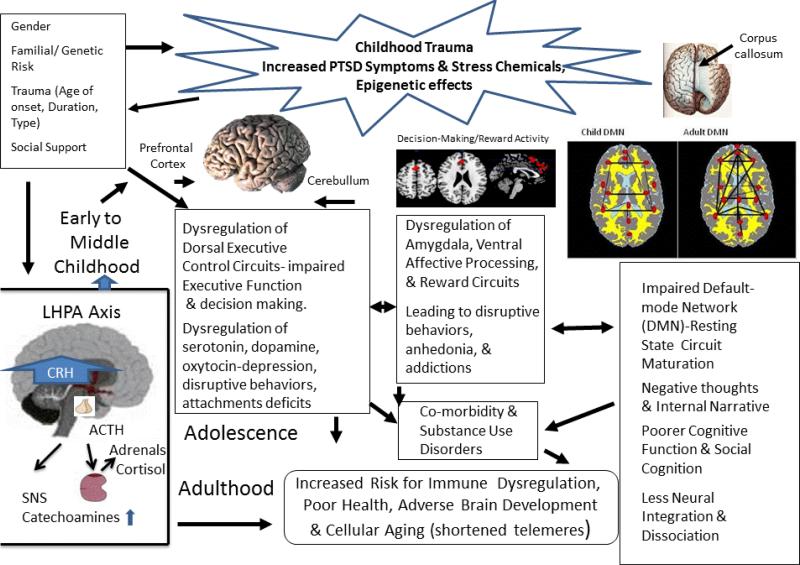
As outlined by De Bellis [13, 39, 42] , the discrepant findings described above may be related to several mediator and moderator mechanisms. A detailed examination of the factors associated with these mechanisms is important in studies endophenotyping an individual's response to the effects of early trauma on the development of biological stress systems. Endophenotyping is a term which is used to describe emotional and behavioral symptoms into stable phenotypes or observable traits with genetic associations. Identifying endophenotypes in traumatized children will be an important new tool for novel approaches to treatments such as personalized medicine [58]. Several of these mediation and moderation mechanisms for the biological effects of trauma on the developing child can be found in a previously published critical review (i.e., Figure 2[13]) and twelve updated mechanisms are detailed below for the LHPA system as this system is the most studied, providing the most data to synthesize into mechanisms (see Figure 1). Other biological stress systems will be reviewed and these mechanisms discussed only if there are published data available.
Figure 1.
Developmental Traumatology Model of the Biological Effects of Trauma
1. Permanent changes occur as a result of childhood trauma onset
Elevated central CRH and CRF occurs with the onset of trauma. While this CRF elevation persists into adulthood; initial elevations of ACTH and cortisol levels become attenuated with chronic exposure to elevated CRH (a.k.a. CRF). High CRH in turn causes adaptive down-regulation of pituitary CRH and neural CRF receptors after trauma onset. These ideas agree with McEwen's [26] theory of allostatic load, which hypothesizes that organisms adapt to re-regulate psychobiological responses to chronic stress to prevent physical harm to the organism. Increasing allostatic load over the lifespan, however, increases vulnerability to stress disorders in response to new stressors. Thus, this down-regulation of CRF receptors may be an adaptive mechanism that regulates pituitary hypertrophy (a finding seen in maltreated children with PTSD[59]); while a down-regulation of CRF receptors would make neurons less responsive to CRF induced neuronal damage and seizures [60, 61]. It should be noted that the opposite of chronic stress (i.e. environmental enrichment) delays seizures and neuronal damage in seizure prone animals through a CRF mechanism [62]. In support of this idea, higher levels of cerebrospinal fluid (CSF) CRF are seen in juvenile primates raised under unpredictable and adverse early rearing conditions [63] which longitudinally persists into young adulthood[64]. CSF CRF levels are higher in adults with childhood trauma histories [20]. In combat-related PTSD, elevated levels of central CRH were found [65, 66]; childhood trauma is also a risk factor for a diagnosis of PTSD after combat experiences [67]. Chronically elevated CRF causes generalized arousal, anxiety, aggression, hypervigiliance, and stimulation of the sympathetic nervous system (SNS), all core symptoms of the PTSD hyperarousal cluster [24]. It also causes inhibition of feeding and sexual behavior, core symptoms of major depression, another common outcome of traumatic experiences in childhood [68].
2. Childhood trauma re-regulates biological stress systems
The long-term consequence of early trauma experiences and elevated CRF resets the regulation of the LHPA axis so that ACTH and cortisol secretions are set at lower 24-hour levels during baseline and non-stressful conditions. Adult studies of victims of childhood trauma consistently show lower cortisol levels [47, 69]. In a meta-analysis, one of the most robust findings was that the longer the time since the trauma, the lower the morning cortisol, daily cortical volume, ACTH and postdexamethasone cortisol levels [47]. Since the adult PTSD studies focus on past trauma, the latter hypothesis may best explain the main differences in the data in childhood PTSD studies, where higher baseline cortisol levels were reported in most pediatric studies [41-47]; while lower 24 hour cortisol levels were seen in adults maltreated in youth [69]. This attenuation hypothesis is supported by data from the only longitudinal psychobiological study published to date; where nonstress cortisol levels were assessed at six time points from childhood through young adulthood in sexually abused and non-abused girls. In this study, nonstress cortisol activity was initially significantly higher in sexually abused girls (post abuse disclosure) compared to non-abused girls; but cortisol activity was significantly attenuated starting in adolescence and significantly lower during young adult follow-up compared to non-abused females [70].
3. Priming also called sensitization occurs as a result of childhood trauma
Priming occurs as a reflection of chronic compensatory adaptation of the LHPA axis long after trauma exposure, and it may be more likely to occur after pubertal maturation. Thus, studies which show greater cortisol or ACTH response after childhood trauma exposure may be the result of “priming.” LHPA axis regulation is affected by other hormones that are stress mediated such as arginine vasopressin and the catecholamines, both of which act synergistically with CRH.[19] A ‘primed system’ will ‘hyper’-respond during acute stress or during the occurrence of traumatic reminders because of the interactive neuroendocrine and neurotransmitter effects activated by current life stressors on the dysregulated LHPA axis. Another term used to describe priming is sensitization, defined as enhanced neuroendocrine, autonomic and behavioral responsiveness to stress as well as LHPA axis dysregulation [20, 71]. Thus, when a new emotional stressor or traumatic reminder is experienced, the LHPA axis response will be enhanced (higher ACTH and higher cortisol levels). This has been seen in findings of increased ACTH secretion in depressed, abused youth who were continuing to experience chronic adversity [50] as well as in depressed women with a history of abuse who reported more recent chronic mild stress than abused women without major depressive disorder, who had blunted ACTH responses to CRF [56]. In a study of post institutionalized children that examined the effect of interactions between children and their caregivers, basal cortisol levels increased in response to parental interactions only in children who had previously been exposed to severe neglect from institutional rearing [72]. In addition, a prolonged elevation in cortisol levels occurred only when the previously neglected children were interacting with their caregivers, as interactions with unfamiliar adults led to similar cortisol levels in previously neglected and non-neglected children. These results therefore illustrated that previously neglected children generalized caregiver interactions as traumatic reminders and stressful experiences and thus demonstrated a disruption in the regulatory processes of the LHPA axis in response to these social interactions. Moreover, in adults with a history of child abuse, higher cortisol levels occur after exposure to traumatic reminders compared to neutral memories [73]. These results are the effects of ‘priming’ or sensitization.
4. Trauma timing and duration influence biological stress systems
Timing of trauma such as duration (single episode or chronic), age of trauma onset and stage of development influence cortisol levels post trauma. Cross-sectional studies show that trauma in infant primates [74] and very young or prepubertal children living in orphanages show low morning and daytime cortisol production [75], suggesting that prepubertal children may be more sensitive to negative feedback control mechanisms for cortisol output than older school-age children who show higher cortisol levels [41-47]. Sexually abused prepubertal children with major depression exhibited significantly lower mean baseline ACTH concentrations over the first 4 hours after sleep onset compared with control children[76]. Results of these cross-sectional studies suggest tight down-regulation of ACTH and cortisol due to elevated central CRF in very young children. Pituitary volumes increased with age [59] along with increasing cortisol levels, which are also associated with increasing body fat [77]. Significantly larger pituitary volumes are seen in pubertal and postpubertal maltreated children and adolescents with PTSD compared with non-maltreated controls [59]. Thus, elevated central CRH levels may lead to pituitary hypertrophy in traumatized children, which may be most pronounced during very early childhood and puberty, due to trophic factors. An adaptive response to elevated CRH levels, particularly during the sensitive periods of very early childhood and adolescence to elevated CRH levels, must be down-regulation of CRH receptors, or the resultant high cortisol levels would result in medical illness and brain structure damage. Tight control of cortisol secretion in infancy and attenuation of cortisol secretion after trauma onset and in response to increasing levels of cortisol that occur with increasing age and puberty are in accord with the theory of allostatic load [26], which hypothesizes that organisms adapt to chronic stress to prevent physical harm.
5. Individual differences in response to childhood trauma are associated with different types of biological stress system regulation
Individual differences in behavioral and emotional responses are associated with different types of LHPA axis dysregulation. Most studies show LHPA axis dysregulation consistent with elevated central CRF in youth who experience childhood trauma and depressive and anxiety symptoms [39, 41-47, 50, 78-80] or co-morbid internalizing and internalizing behaviors [42, 79]; while traumatized children with marked disruptive behavioral disorders or anti-social behaviors show lower cortisol levels [81].In addition, in a study of adults with moderate to severe child maltreatment histories and no diagnosable psychopathology, lower concentrations of cortisol and ACTH were seen in response to the Trier Social Stress Test compared to healthy adults without maltreatment histories [82], further suggesting that elevated central CRF is likely seen as a result of maltreatment even in resilient outcomes.
6. Early trauma type and trauma severity influence biological stress systems
Certain trauma types and increased overall trauma severity are more likely to result in LHPA dysregulation. For example, children who suffered from physical and sexual abuse occurring in the first 5 years of life were more likely to experience internalizing symptoms and LHPA axis dysregulation than those who suffered from abuse occurring after age 5, or from neglect, or emotional abuse [40]. Increasing severity of childhood trauma is associated with dysregulation of the LHPA axis. Children who experienced multiple maltreatment or those who experienced severe sexual abuse were more likely to have elevated cortisol levels [44]. Furthermore, in maltreated children with PTSD, 24 hour urinary cortisol concentrations correlated positively with increased trauma duration, and with PTSD intrusive and hyperarousal symptoms [42].
7. Genetic factors influence biological stress system responses to childhood trauma
LHPA-related genetic factors influence the effect of childhood trauma on the LHPA axis and its associated outcomes. Gene × environment interplay is important for the expression of both negative and resilience outcomes following childhood trauma. Gene x environment investigations are a relatively new area of study, so these data should be considered preliminary since this part of the field is still in its infancy.
Polymorphisms are normal variations in genes, which code for important proteins that build the body and its functions. Single nucleotide polymorphisms (SNPs) are the most common type of genetic variation. Specific polymorphisms that are needed to form LHPA axis related structures (CRH and glucocorticoid receptors) appear to moderate the effect of child abuse on the risk for childhood neuroticism and adult depressive symptoms. The brain's CRH type 1 receptors (CRHR1) are located throughout the brain [27] and, when activated, produce symptoms of anxiety and depression [24, 83].
There are few studies of gene × environment interplay in children. In one study, physically abused, emotionally abused, and neglected children who carried two copies of the TAT haplotype of the CRHR1 had significantly higher levels of neuroticism, a prelude to anxiety and depression, than non-maltreated children who had two copies of the TAT haplotype [84]. However, in this study, sexually abused children and children who had experienced 3 or 4 types of abuse, who had two copies of the TAT haplotype appeared to be protected from neuroticism compared to children who experienced other types of maltreatment [84]. In a follow-up study of these children, only maltreated children who carried two copies of the TAT haplotype exhibited a blunted slope of diurnal cortisol change, a sign of increasing allostatic load and dysregulation of the LHPA axis. However, the CRHR1 haplotype groups (zero or one copy vs. two copies) were not related to internalizing symptoms [85].
In contrast, one study of adult carriers of both the TCA (i.e., T-alleles, formed of SNP, rs7209436, and C allele formed by SNP rs4792887) and TAT haplotypes (i.e., A-allele formed of SNP rs110402) as well as the 2 SNPs (rs7209436 and rs242924) located in intron 1 of the CRHR1 gene, were significantly protected from having major depression despite histories of child abuse [86]. This finding was replicated in a relatively large study of women with child maltreatment [87], and in African American men and women [88], but not replicated in a study of European men and women, who experienced childhood maltreatment [87]. On the other hand, adults with moderate to severe child maltreatment histories and the GG polymorphisms of the CRHR1 gene (rs110402) showed a significant interaction with maltreatment for increased cortisol responses to dexamethasone/corticotropin-releasing hormone pharmacological challenge compared to those adults with maltreatment and the A allele [88]. Higher cortisol is a well-replicated finding in adults with major depression [19, 89]. Furthermore, sex effects may be important in gene × environment interplay. In another study of adults with child abuse histories, the protective effect of the CRHR1 polymorphisms (rs110402 A-allele) against developing adult depression and with decreased cortisol response in the dexamethasone/ corticotropin-releasing hormone pharmacological challenge were observed only in men, and not in women [90] , a finding opposite to the one described above [87].
Gene × environment interplay was seen in a prospective study of the FKBP5 gene, a gene that inhibits glucocorticoid-receptor-mediated glucocorticoid activity [91]. In this study, 884 Caucasians with no history of depression were enrolled at age 12 to 14 years, and followed for 10 years [92]. Those who were homozygous for the minor alleles and had traumatic (but not separation (i.e., lost of parent through death or divorce) events (particularly severe child maltreatment) prior to age 24 years, showed an increased incidence of depression on follow-up, suggesting that the minor allele of the FKBP5 polymorphism and childhood trauma interacted to predict adult depression. Three variants in the FKBP5 gene (rs4713916, rs1360780, and rs3800373) were associated with a failure of cortisol responses to return to baseline in healthy adults after psychosocial stress, suggesting a genotype-dependent risk of chronically elevated plasma cortisol levels in the context of acute stress as a possible mechanism for the increased risk of stress related mental disorders, such as depression and PTSD in adults with these alleles [93]. Cross-sectional studies have also found interaction for adults who carry the minor FKBP5 allele and have child maltreatment histories as they have increased rates of depression [94], PTSD [95, 96], and suicide risk [97]. Interestingly, in one cross sectional study, the less common FKBP5 haplotype (H2) was associated with an increased risk of overt aggressive behavior in adult male prisoners who have a history of physical abuse[98]. In an imaging study, healthy young adults, were genotyped for 6 FKBP5 polymorphisms (rs7748266, rs1360780, rs9296158, rs3800373, rs9470080 and rs9394309) previously associated with psychopathology and/or LHPA axis function [99]. Interactions between each SNP and increased levels of emotional neglect, were associated with heightened reactivity to angry and fear faces in the dorsal amygdala which suggests a neurobiological mechanism linking PTSS and depressives symptoms of hyperarousal and hypervigilience to negative affect and to psychopathology [99]. Hence, investigations of gene × environment interplay suggest that risk genes may interact with childhood trauma to produce different adult emotional, behavioral, and neurobiological outcomes.
8. Epigenetic factors influence biological stress system responses to childhood trauma
LHPA-related epigenetic factors influence the effect of childhood trauma on the LHPA axis and its associated negative behavioral and emotional outcomes. Epigenetics is also a relatively new area of study, so the limited data to date will be described here. The epigenome consists of chromatin, the protein-based structure around DNA, and a covalent modification of the DNA itself by the methylation of cytosine rings found at CG dinucleotides [100]. The epigenome determines the accessibility of the DNA to convert genetic information into the messenger RNA necessary for gene function. Early traumatic experiences are associated with hyper- and demethylation of specific regulatory sites in key biological stress system genes including the gene encoding of the glucocorticoid receptor [101] and the neuropeptide arginine vasopressin (AVP) which is co-localized with CRH and released with CRH from the paraventricular nucleus of the hypothalamus during stress [102]. Increased methylation of key biological stress system genes can silence a gene's activity by making the gene inaccessible for transcription, while demethylation may make a gene accessible for transcription. Thus, childhood trauma can have a long-term impact on gene activity without changing an individual's DNA sequence (i.e., genes)[103, 104]. Epigenetic effects may account for the inconsistent main gene and gene x environment results in the studies previously reported.
Animal studies have provided the first evidence of epigenetic effects caused by early trauma. Rats developing in optimal environments show less stress reactivity [105]. Because lactating female Long-Evans rats exhibit individual variation in the frequency of pup licking/grooming, high or low levels of pup licking/grooming are considered a maternal phenotype [106]. As adults, the offspring of high licking/grooming mothers show less plasma ACTH and cortisol responses to acute stress in comparison with animals reared by low licking/grooming mothers [106, 107]. The offspring of high licking/grooming mothers also show significantly increased hippocampal glucocorticoid receptor mRNA and protein expression, enhanced glucocorticoid negative feedback sensitivity, and decreased hypothalamic CRF mRNA levels, which all indicate decreased stress reactivity as a result of optimal quality of care.[105] Furthermore, DNA methylation patterns differ in high licking/grooming versus low licking/grooming offspring. The glucocorticoid receptor promoter sequence in the hippocampus of adult offspring of low licking/grooming mothers is hypermethylated and functionally less sensitive to cortisol feedback. Maternal behaviors also affect other biological systems that are associated with the LHPA axis. Prolonged periods of maternal separation alter the methylation state of the promoter for the arginine vasopressin gene (AVP) in the pup, increasing hypothalamic vasopressin AVP synthesis and LHPA responses to stress, along with memory deficits and learned helplessness behaviors [108].
In a rodent model of infant maltreatment, abuse and neglect during infancy decreases brain-derived neurotropic factor (BDNF) gene expression in the adult prefrontal cortex (PFC) [109]. In addition, these investigators found that chronic treatment with a DNA methylation inhibitor lowered levels of methylation in male and female rats exposed to early maltreatment. They also showed not only that infant trauma was associated with poor mothering in the next generation and that these epigenetic changes in DNA methylation were passed on from one generation to the next generation, even if the offspring of an abusive dam (mother rat) was cross-fostered with a non-maltreating dam, thereby indicating heritability of these epigenetic changes [109].
Increased methylation in a neuron-specific glucocorticoid receptor (NR3C1) promoter was seen in human postmortem hippocampus obtained from suicide victims with a history of childhood abuse compared to suicide victims without child abuse histories and controls who died of non-suicide related causes, demonstrating an association between early trauma and epigenetic alterations [101]. Decreased hippocampal glucocorticoid receptor expression due to epigenetic changes likely increased LHPA activity and enhanced the risk of both depression and suicide in adults who were child abuse victims [101]. These findings link the previously described data from rats to humans and suggest a common effect of quality of parental care on the epigenetic regulation of hippocampal glucocorticoid receptor expression that can lead to health or the LPHA axis dysregulation seen in childhood PTSD and PTSS and adult depression.
In addition, a preliminary investigation showed that experiencing foster care during childhood is associated with changes in methylation of genes related to both the HPA axis and the immune system [110]. This study therefore supports the idea that childhood trauma may be linked to changes in genetic expression and resulting mental and medical health problems.
9. Gender differences influence the effects of childhood trauma on biological stress systems
Gender differences influence the effect of childhood trauma on the HPA axis. Research involving men and women who were exposed to early trauma but who did not have any psychopathological diagnoses has shown stronger associations between trauma and increased CRF levels in men than in women [111]. In children, girls with histories of physical abuse had higher levels of urinary oxytocin, a neuroendocrine peptide that down-regulates cortisol and is associated with complex social behaviors, and lower levels of salivary cortisol following an experimental stressor when compared to non-abused girls; while abused and non-abused boys did not differ in their hormonal responses [112]. Early trauma and gender differences are an area of research that warrants further investigations, as a prospective investigation showed that maltreated males may be more likely to be arrested for violent offenses as adults [113], thus becoming prisoners and less likely to be involved in retrospective research studies. This fact can lead to a selection bias in retrospective studies, and a possibly mistaken idea that females are more vulnerable to early trauma. Early trauma experiences may lead to greater down-regulation of cortisol due to high levels of CRF and other stress markers, in males compared to females, findings that are commonly seen in individuals with antisocial behaviors [81].
10. Social support buffers biological stress system dysregulation and its associated negative behavioral and emotional outcomes
As adults, the offspring of high licking/grooming mothers show decreased stress and cortisol reactivity as a result of optimal quality of care [105]. In preschool children, quality of childcare is associated with a buffering of hypothalamic-pituitary-adrenal axis to stress [114]. In adult studies, social support was associated with a decrease in the cortisol response to the Trier Social Stress Test in men [115]. Furthermore, decreased cortisol response and activity in the dorsal anterior cingulate, a brain region involved in distress separation, were seen in response to an exclusion neuroimaging task when daily social support was part of the research paradigm [116]. Further research is needed on the impact of social support in traumatized children.
11. Individual biological stress systems dysregulation in response to childhood trauma and the genes associated with the function of these systems influence other biological systems during development to contribute to psychopathology
For example, the LHPA modulates the locus coeruleus-norepinephrine/SNS system and the immune system [19]. When the hypothalamus releases CRH in response to stress, the locus coeruleus becomes activated indirectly through the central amygdala [19]. Serotonin modulates LHPA activity [117]. Furthermore, having more than one type of a depression risk allele in two different biological stress systems (i.e., the risk CRHR1 polymorphisms in the LHPA axis and the short allele of serotonin transporter gene promoter polymorphism (5-HTTLPR) in the serotonin system) is associated with current depressive symptoms in adults with less severe levels of child abuse and neglect as measured on the Childhood Trauma Questionnaire [118].
12. Biological stress systems dysregulation in response to childhood trauma adversely influences cellular, cognitive and brain development [42, 119, 120]
Human brain maturation is marked by the acquisition of progressive skills in physical, behavioral, cognitive, and emotional domains. Myelin, a fatty white substance produced by glial cells, is a vital component of the brain. Myelin encases the axons of neurons, forming an insulator, the myelin sheath and is responsible for the color of white matter. Myelination of newly formed neuronal networks increases neural connectivity and parallels these developmental changes. Brain development occurs with an overproduction of neurons in utero, increases in neuron size, synapses, and neural connections during childhood and adolescence, selective elimination of some neurons (apoptosis) with corresponding decreases in some connections and strengthening of others and corresponding increases in myelination to hasten these connections. Synapses, dendrites, cell bodies, and unmyelinated axons, which form the brain's gray matter, decrease during development [121]. Glucocorticoids are important for normal brain maturation including initiation of terminal maturation, remodeling axons and dendrites and affecting cell survival [26, 122]. Both suppressed and elevated glucocorticoid levels can impair brain development and function [26, 122]. During brain maturation, stress and elevated levels of stress hormones and neurotransmitters may lead to adverse brain development through apoptosis [123-125], delays in myelination[126], abnormalities in developmentally appropriate pruning [127, 128], the inhibition of neurogenesis[129-131], or stress induced decreases in brain growth factors[132]. Indeed, maternal deprivation increases the death of infant rat brain cells [133]. Consequently, dysregulation of a maltreated child's major stress systems likely contributes to adverse brain development and leads to psychopathology [13].
Telomeres are the repetitive TTAGGG sequence at the end of linear chromosomes and with each division, telomeres get shorter, and are considered a molecular clock for cellular aging [134]. In a cross sectional study of children who were previously institutionized, telomere length was shorter than children without such histories[135]. In a prospective longitudinal study of children, from ages 5 to 10 years, children who experienced two or more types of violence (measured as bullying, witnessing domestic violence, and physical abuse), have increased telomere erosion, a marker of premature cellular aging, compared to children who did not experience violence [120]. This landmark study suggests that children, who experience trauma, have decreased telomere maintenance, a potential mechanism (“premature aging”) for adverse brain development, mental health problems, and chronic health problems in adults with a childhood history of trauma [13, 136].
The Locus Coeruleus-Norepinephrine/Sympathetic Nervous System (SNS)/Catecholamine System and Childhood Trauma
When the hypothalamus releases CRH in response to a stressor, the locus coeruleus (LC)-norepinephrine/SNS system becomes activated indirectly through the central amygdala. Activation of the locus coeruleus causes an increase in the release of norepinephrine throughout the brain and results in symptoms of PTSD and anxiety [13]. The locus coeruleus is an ancient brain structure that increases activation of the sympathetic nervous system (SNS), a part of the autonomic nervous system that controls the “fight or flight or freeze” response [13]. The catecholamines (epinephrine, dopamine, and norepinephrine) and corresponding increased activity in the SNS work to generally prepare an individual for action by redistributing blood away from the skin, intestines, and kidneys and to the brain, heart and skeletal muscles [137], and by diverting energy through a central dopamine mechanism that inhibits the prefrontal cortex, from a thinking and planning mode to a survival and alertness mode [138, 139].
Sexually abused girls with dysthymia demonstrated greater 24-hour levels of total urinary catecholamines than non-abused girls [140]. Maltreated boys and girls with PTSD showed greater levels of urinary catecholamines at baseline than non-maltreated healthy children and non-maltreated children with generalized anxiety disorder [42]. Urinary catecholamines positively correlated with duration of PTSD trauma and number of PTSD internalizing and child dissociative symptoms [42]. In a study of children who experienced motor vehicle accidents, significantly elevated plasma noradrenaline concentrations were seen prospectively, at both months 1 and 6, after the accident, compared to the non-PTSD and control groups, further suggesting that higher catecholamines occur as a result of trauma and PTSD [141]. In a study of police academy recruits, those with early trauma exposure demonstrated heightened 3-methoxy-4-hydroxy-phenylglycol (MHPG) (the major metabolite of norepinephrine) response after watching critical incident videos [142]. An adult positron emission tomography imaging study demonstrated significantly reduced norepinephrine transporter (NET) availability in the locus coeruleus in PTSD which would lead to chronic stimulation of the locus coeruleus as a result of increased levels of norepinephrine. This finding positively correlated with PTSD hypervigilance symptoms. Furthermore, in a gene x environment analysis, adult carriers of the Val allele of the Catechol-O-methyltransferase (COMT) polymorphism (a gene involved in dopamine degradation) with a history of sexual abuse showed a higher disposition toward anger, symptoms commonly seen in PTSD patients, compared with adults homozygous for the Met allele. The Val allele is associated with increased dopamine neurotransmission in the prefrontal cortex, which is associated with deficits in executive function [138, 139] and increased risk for impulsive anger. On the other hand, adults with early trauma and the Val/Val genotype showed increasing levels of dissociation corresponding to increased exposure to higher levels of childhood trauma [143]. Dissociation is a failure to integrate sense of self with current and past memories and emotions and is a pathological defense to ward off anxiety that is also associated with non-intentional antisocial behaviors. Dissociation is a different construct from depression and anxiety. Thus studies in both children and adults have consistently demonstrated higher locus coeruleus (LC)-norepinephrine/SNS system activity associated with childhood trauma and PTSD, while down-regulation of this system due to trauma may be associated with antisocial behavior and dissociation.
The Serotonin System and Childhood Trauma
Serotonin is a critical element of the stress response system [13]. Serotonergic neurons project diffusely from the central serotonin raphe nuclei in midbrain to important cortical and subcortical brain regions (e.g., prefrontal cortex, amygdala, hippocampus) that play known roles in regulating emotions (e.g., mood), behaviors (e.g., aggression, impulsivity)[144, 145], cognitive function, motor function, appetite, and the regulation of many physiological processes (e.g. cardiovascular, circadian, neuroendocrine respiratory, and sleep functions [144, 146]). Serotonin is an important regulator of morphogenetic activities during early brain development, influencing cell proliferation, migration, and differentiation, thus influencing child brain development [147]. In preclinical animal studies, decreased levels of serotonin activity are associated with increased levels of aggressive behaviors in rodents and primates exposed to early adversity [148]. Mice genetically engineered to lack the serotonin transport gene show increased LHPA axis activation to stress, suggesting that serotonin modulates LHPA activity [117]. Disruptions in serotonin's regulatory functioning are linked with several psychopathological disorders that are commonly seen in children and adults with childhood trauma. For example, decreased levels of serotonin activity have been associated with mental health problems such as depression and anxiety [149] as well as with aggressive behaviors in individuals with personality disorders such as borderline personality disorder[150].
Early trauma dysregulates serotonin in humans. The serotonin transporter protein is involved in the reuptake of serotonin from the synapse, and is critical to serotonin regulation in the brain. The short allele of the serotonin transporter gene promoter polymorphism (5-HTTLPR) interacts with maltreatment in the development of childhood depression. The short allele is associated with reduced transcriptional activity of serotonin, so that there is less central serotonin available in the brain; while the long allele has at least twice the basal level of transcriptional activity of the short variant [151]. Most studies regarding the effects of 5-HTTLPR are in adults maltreated as children. A large epidemiological study demonstrated that there were no main genetic effects, but carriers of the S-allele had a higher risk of developing depressive and suicidal symptoms when exposed to stressful life events and childhood maltreatment [152]. Children who were homozygous for the short allele of 5-HTTPR demonstrated a significantly elevated vulnerability to depression, but only in the presence of maltreatment; but the presence of positive supports reduced this risk [153]. Having two short-short alleles of the 5-HTTLPR gene moderated the association between bully victimization and emotional problems, such that frequently bullied children were at an increased risk as adolescents for depression or anxiety; the short-long and long-long genotypes did not confer an increased risk [154]. However other studies have not shown an increased vulnerability to depression as a function of interactions between the short allele and maltreatment. Instead, they have shown increased risk with the long-long allele [85, 155]. One study of 595 youth suggests that genetic variation has a negligible effect on promoting resilience among maltreated children [156]. In other words, non-maltreated children with the short-short genotype were more likely to have higher resilient functioning; while maltreated children with the short-short genotype were more likely to have lower resilience [156]. This study agrees with controversial meta-analyses that have found that adverse childhood events had a main effect on depressive outcomes regardless of 5-HTTLPR polymorphisms [157, 158]. The discrepant findings in adults may be caused by failure to include trauma versus a stressful life event, trauma age of onset, trauma duration, and trauma type in the gene x environment interaction analyses, as this interaction showing that adults with histories of child maltreatment were found in another meta-analysis to be at increased risk for depression [159]. On the other hand, in a forensic sample of 237 men with elevated levels of child abuse and neglect given the Childhood Trauma Questionnaire, measures of psychopathy were highest among carriers of the 5-HTTPR long allele and carriers of the low activity monoamine oxidase A (MAOA) gene [160]. Thus resilience is a complex issue, as psychopathy as an outcome may be more harmful to society than depressive symptoms.
Genes associated with the serotonin system have been linked to other adverse outcomes in the presence of childhood trauma. The MAOA gene codes for an enzyme that selectively degrades the biogenic amines dopamine, serotonin, and norepinephrine after reuptake from the synaptic cleft, and influences behavioral regulation [161]. Meta-analyses revealed that the association between early family adversity (particularly between neglect or physical abuse), and the short version of the MAOA gene, was significantly associated with a general index of mental health problems, antisocial behavior, attentional problems, and hyperactivity in boys [162]. Adolescent boys with the short MAOA allele who were exposed to maltreatment or poor-quality family relations had more alcohol-related problems than maltreated boys with the longer MAOA allele [163]. Early use of alcohol in youth was predicted by an interaction of the short alleles of 5-HTTLPR and maltreatment [164]. Further, women with a history of sexual abuse and the short MAOA allele were more likely to demonstrate alcoholism and antisocial personality disorder than were women with a history of sexual abuse and the long allele [165].
Furthermore, genetic polymorphisms can have additive genetic effects. Children who were homozygous for the short allele of 5-HTTLPR, and had the val66met variant of the brain-derived neurotropic factor (BDNF) gene, and had been maltreated, were at increased risk of depression.
In summary, the serotonin system and the genes regulating the serotonin system are influenced by early trauma. However, the field has not yet advanced to the point where treatment can be tailored to an individual child. More work needs to be done on gene-gene interactions, possible epigenetic effects, trauma variables, and other factors such as social supports, to achieve this aim.
The Oxytocin System and Childhood Trauma
Oxytocin plays an important role in interpersonal relationships. Involved in the regulation of an individual's sexual response and milk production, this hormone is also responsible for the regulation of a wider range of social interactions, including social memory and cognition, emotion recognition, empathy, and attachment [166]. In addition, the oxytocin system is involved in the regulation of the body's response to stress. Research in rats demonstrated a relationship between oxytocin and the mother's relationship with her offspring, as those mothers who engaged in more licking/grooming behaviors exhibited higher levels of oxytocin receptor binding in the amygdala [167]. Similarly, rat mothers who demonstrated high levels of licking/grooming had increased levels of oxytocin gene expression and a subsequent increase in dopamine reward production. Mothers who demonstrated lower levels of licking/grooming behaviors, had lower levels of oxytocin gene expression, thereby highlighting the important role that oxytocin plays in the attachment bond [168].
Research involving humans has similarly demonstrated that negative life events can disrupt the body's regulation of oxytocin. Decreased levels of oxytocin have been found in women exposed to early maltreatment—a relationship that was shown to be especially strong when the form of maltreatment was emotional abuse [20]. Gender differences have also been found in the relationship between childhood trauma and oxytocin regulation. Focusing on oxytocin response to an experimental stressor in abused girls and abused boys, girls exposed to physical abuse exhibited higher levels of oxytocin, as well as decreased levels of cortisol, in response to the experimental stress; while there was no difference in hormone response to the stress in the abused boys [112]. In the Adverse Childhood Experiences Study, a relationship was found between exposure to early trauma and increased promiscuity[136]. To explain this relationship, the researchers pointed out that disruptions in oxytocin regulation of social attachments during childhood can lead to high oxytocin and thus problems forming fast and less discriminate personal attachments during adulthood [136].
On the other hand, a strong moderating effect of a positive social environment has also been found in adults with a specific allele of the oxytocin receptor gene OXTR who had been exposed to early stress, as increased resilience in adulthood was found only in those individuals who had been surrounded by a positive family environment during childhood [169]. Further research is needed on the impact of oxytocin on emotional and behavioral outcomes in traumatized children.
The Immune System and Childhood Trauma
Activation of the immune system involves the production of cytokines, which promote an inflammatory reaction to infection or pathogens in the body. Although one of the main effects of this reaction is to produce the physical symptoms of sickness (e.g., fever, nausea, and fatigue), activation of cytokines that promote inflammation are implicated in depression, a common outcome of early trauma [170]. A recent systematic review provided evidence that supports the relationship between pro-inflammatory cytokines and increased levels of depression and anxiety in adolescents [171]. Higher levels of plasma antinuclear antibody titers have been found in girls who have been sexually abused, suggesting that exposure to this form of stress may inhibit the body's means of suppressing the B lymphocytes, or the lymphocytes that produce antibodies, thereby leading to the increased levels of the antibody titers found in the abused girls [172]. Increased levels of inflammatory cytokines, as well as decreased levels of anti-inflammatory cytokines, have been associated with adult PTSD and exposure to chronic stress [173, 174]. In women who had experienced early maltreatment, those who had been diagnosed with PTSD had higher activation levels of T cells than those who did not have PTSD More specifically, investigators found a positive correlation between T-cell activation levels and intrusive symptoms of PTSD [175]. Similar results were found in a study of women with PTSD secondary to physical or sexual abuse during childhood, where these women demonstrated increased inflammatory and immune activity[176]. Other investigators demonstrated that greater concentrations of interleukin-6 (IL-6), a proinflammatory cytokine, concentrations were seen during the Trier Social Stress Test in adults with child maltreatment histories compared to adults without such histories [177]. Furthermore in a longitudinal study of a New Zealand birth cohort (n=1037), it was demonstrated that adults with histories of poverty, social isolation or maltreatment had elevated rates of depression and age-related metabolic disease risks in adulthood including higher body mass index, total cholesterol, glycalated hemoglobin, and low levels of high-density lipoprotein, low maximum oxygen consumption, and higher levels of C-reactive protein, a measure of inflammation [178]. Further children exposed to a greater number of these adverse childhood risk factors had greater age-related disease risk in adult life [178]. The increased activation of cytokines and dysregulation of the immune system, along with the other biological stress response systems, such as the HPA axis and the LC/SNS systems, that occurs in response to early adversity, can lead to hypertension, accelerated atherosclerosis, metabolic syndrome, impaired growth, and immune system suppression and poorer medical health in adults with child trauma histories [179].
The Effect of Childhood Trauma on Neuropsychological Functioning and Cognitive Development
Cross-sectional studies examining maltreatment trauma in childhood have shown lower IQs and deficits in language and academic achievement in maltreated children compared to children who have not been exposed to maltreatment [180-185]. The link between early trauma and IQ has been demonstrated through a twin study, where after controlling for the effect of shared heritability, domestic violence was associated with lower IQ (e.g., mean of 8 points) in exposed versus non-exposed children [186]. Exposure to trauma in childhood has also been associated with executive deficits [187-189].
Fewer studies have examined the effect of child maltreatment on a broader, more comprehensive range of neuropsychological functioning. A study comparing previously institutionalized children exposed to long periods of neglect with those exposed to brief periods of institutionalization as well as children raised in their biological families found that the children who experienced prolonged neglect performed more poorly in the domains of visual attention, memory, learning and inhibitory control, but that these previously institutionalized children did not demonstrate deficits when the tasks involved auditory or executive processing [190]. These results therefore highlight that specific domains of cognitive functioning may be more sensitive to early neglect than others. Lower performance in IQ, complex visual attention, visual memory, language, verbal memory and learning, planning, problem solving, speeded naming and reading and mathematics achievement were seen in neglected children with and without PTSD compared to socioeconomically similar controls [191]. Furthermore, PTSD symptom number, and the failure to supervise, witnessing violence, and emotional abuse variables were each associated with lower scores in IQ, academic achievement, and neurocognitive domains [191]. However, neglected children with PTSD due to witnessing interpersonal violence had lower performance levels on the NEPSY Memory for Faces-Delayed than both neglected children who witnessed domestic violence and did not have PTSD and children not exposed to maltreatment or violence indicting that PTSD was associated with impaired consultation of memory [191]. Childhood PTSD subsequent to witnessing interpersonal violence has also been associated with lower levels of performance on the California Verbal Learning Test-Children's Version when compared to those without PTSD, while both PTSD and non-PTSD groups of youth exposed to domestic violence demonstrated deficits in executive functioning, attention, and IQ standardized scores[192].
In a study comparing the performance of abused youth with PTSD, abused youth without PTSD, and non-maltreated youth on comprehensive neuropsychological testing, both groups of maltreated youth performed worse than the control youth, with deficits in IQ, academic achievement, and all neurocognitive domains expect for fine-motor functioning[193]. The maltreated youth with PTSD showed greater deficits in visuospatial abilities than the maltreated youth without PTSD, and sexual abuse was found to be negatively associated with language and memory scores. A negative relationship was found between the number of maltreatment types experienced and academic achievement, demonstrating that cumulative trauma leads to neuropsychological problems that are unrelated to PTSD symptoms[193]. Data from the National Survey of Child and Adolescent Well-Being (NSCAW) study demonstrated that maltreated children who experienced maltreatment during multiple developmental periods had more externalizing and internalizing problems and lower IQ scores than children maltreated in only one developmental period suggesting that trauma duration has negative and cumulative cognitive effects [194].
Longitudinal prospective studies involving adolescents and adults exposed to maltreatment in childhood agree with the cross-sectional studies and have demonstrated lower IQ scores and deficits in reading ability [195-200]. The research on the effects of early trauma on cognitive function indicates that early trauma is associated with adverse cognitive development and that this is likely reflected in adverse brain development.
The Effect of Childhood Trauma on Brain Development
An early, unexpected, trauma, maternal deprivation, increases the death of both neurons and glia cells in cerebral and cerebellar cortexes in infant rats [133]. Increased exposure to cumulative life stress (e.g., exposure to severe marital conflict, severe chronic illness of a close family member or friend) was associated with poorer spatial working memory performance and decreased volumes of white and gray matter in the prefrontal cortex of non-maltreated youth [201]. Pediatric imaging studies demonstrated that both cerebral and cerebellar volumes are smaller in abused and neglected youth compared to non-maltreated youth [202-206]. In one research study, maltreated subjects with PTSD had 7.0 % smaller intracranial and 8.0% smaller cerebral volumes than non-maltreated children [119]. The total midsagital area of corpus callosum, the major interconnection between the two hemispheres that facilitates intercortical communication, was smaller in maltreated children [119]. Smaller cerebral volumes were significantly associated with earlier onset of PTSD trauma and negatively associated with duration of abuse [119]. PTSD symptoms of intrusive thoughts, avoidance, hyperarousal and dissociation correlated negatively with intracranial volume and total corpus callosum measures [119]. another study showed smaller brain and cerebral volumes and attenuation of frontal lobe asymmetry in children with maltreatment-related PTSD or subthreshold PTSD compared with archival non-maltreated controls [203].
However, these two previously described studies did not control for low socioeconomic status, which influences brain maturation through ecological variables [119, 207]. In another study which controlled for socioeconomic status, children with maltreatment-related PTSD had smaller intracranial, cerebral and prefrontal cortex, prefrontal cortical white matter, and right temporal lobe volumes and areas of the corpus callosum and its subregions and larger frontal lobe CSF volumes than controls [205]. The total midsagittal area of corpus callosum and middle and posterior regions remained smaller; while right, left, and total lateral ventricles and frontal lobe CSF were proportionally larger than controls, after adjustment for cerebral volume [205]. Brain volumes also positively correlated with age of onset of PTSD trauma and negatively correlated with duration of abuse [205]. The larger lateral ventricles were only seen in maltreated males, suggesting that males are more vulnerable to the neurotoxic effects of childhood maltreatment. Smaller cerebellar volumes were seen in male and female maltreated children with PTSD [204]. Younger age of onset and longer trauma duration were significantly correlated with smaller cerebellum volumes [204]. Smaller cerebellum volumes were also seen in previously institutionized youth [202]. The cerebellum is a complex posterior brain structure that is involved in cognitive functions [208], decision making, reward circuits [209, 210] and the default mode network that is associated with understanding social intentions [211]. Child maltreatment is also associated with adverse effects in individual brain structures that are involved in reward and default network processing. In a large study of 61 medically healthy youth (31 males and 30 females) with chronic PTSD secondary to abuse, who had similar trauma and mental health histories, and 122 healthy non-maltreated controls (62 males and 60 females), the midsagital area of the corpus callosum subregion 7 (splenium) was smaller in both boys and girls with maltreatment-related PTSD compared to their gender-matched comparison subjects [212]. Youth with PTSD did not show the normal age-related increases in the area of the total corpus callosum and its region 7 (splenium) compared to non-maltreated children [212]. This was an important finding for several reasons. The maltreated and control children were not prenatally exposed to substances and had no pregnancy or birth trauma, were psychotropically naïve, and had no history of substance abuse or dependence, thus excluding confounds which are commonly seen in maltreated children [213, 214] and not addressed in exclusion criteria in most neurobiological studies published to date. The axons in the splenium of the corpus callosum myelinate during adolescence and are important to the posterior reward circuits and the posterior default networks [215]. Additionally, clinical symptoms of PTSD intrusive, avoidant, and hyperarousal symptoms, symptoms of childhood dissociation, and child behavioral checklist internalizing T score significantly and negatively correlated with corpus callosum measures [212]. Children with maltreatment-related PTSD had reduced fractional anisotropy values on diffusion tensor imaging brain scans of white matter, indicating less myelin integrity in the medial and posterior corpus, a region which contains interhemispheric projections from brain structures involved in circuits that mediate emotional and memory processing, core disturbances associated with trauma history [216]. Smaller corpus callosum area measures were seen in another anatomical magnetic resonance imaging brain study of neglected children with psychiatric disorders compared to nonmaltreated children with psychiatric disorders, suggesting that smaller corpus callosum measures may be a consequence of maltreatment [217].
Furthermore, areas of executive function show evidence of adverse brain development in children with maltreatment-related PTSD. Decreased N-acetylaspartate (NAA) concentrations are associated with increased metabolism and loss of neurons [218]. For example, brain NAA levels decrease when someone has neuronal loss such as a stroke. A preliminary investigation suggested that maltreated children and adolescents with PTSD demonstrated lower NAA/creatine ratios in the medial prefrontal cortex compared to sociodemographically matched controls [219]. These findings suggest neuronal loss in the medial prefrontal cortex, an executive brain region, in pediatric maltreatment-related PTSD. Another group found that decreased left ventral and left inferior prefrontal gray matter volumes in maltreated children with PTSD symptoms negatively correlated with bedtime salivary cortisol levels, further suggesting that early trauma damages executive regions [220]. One functional imaging study of maltreated children and adolescents with PTSD symptoms showed significant decreases in inhibitory processes compared to nonmaltreated controls [221], and another showed impaired cognitive control in adopted children with histories of maltreatment who were formerly raised in foster care [222]. These investigations strongly suggest that childhood maltreatment interferes with executive or control circuits, whose dysregulation is an important contributor to adolescent and adult mental health and substance use disorders. Thus, childhood trauma can have detrimental effects on the brain networks that establish an individual's ability to think, and regulate their sense of self, motivations, and behaviors.
Another important contributor to memory and the default mode network is the hippocampus. Unlike findings in adult PTSD, where several studies reported hippocampal atrophy [223], maltreated children and adolescents with PTSD or subthreshold PTSD showed no anatomical differences in limbic (hippocampal or amygdala) structures cross-sectionally [119, 203, 205] or longitudinally [224]. However, investigators have demonstrated functional brain differences in the amygdala and hippocampus of maltreated youth compared to non-maltreated children [222, 225]. One study suggests that hippocampal atrophy may be a latent developmental effect of childhood maltreatment [226].
Child maltreatment is also associated with adverse development of brain reward regions involved in recognizing emotions and social cognition such as the superior temporal gyrus [206] and the orbital frontal cortex [227]. In carefully screened young adult subjects, those with a history of verbal abuse and no other forms of maltreatment had reduced fractional anisotropy on diffusion tensor imaging brain scans of white matter in the arcuate fasciculus in left superior temporal gyrus, the cingulum bundle by the posterior tail of the left hippocampus, and the left body of the fornix, indicating decreased integrity in these language neural pathways[228]. Furthermore, fractional anisotropy values negatively correlated with verbal abuse experiences [228]. In healthy adult women, a history of sexual abuse was specifically associated with hippocampal, corpus callosum, or frontal cortex reductions if it occurred during specific developmental age periods, indicating vulnerable windows for the brain effects of child trauma [217].
Functional neuroimaging studies of adults with PTSD related to childhood maltreatment have shown decreased levels of executive and attentional function as reflected by decreased activation in the dorsal control networks with corresponding increased activation of the amygdala and hippocampus and other structures of the affective emotional networks during emotional challenge tasks; suggesting dorsal control network deficits in adult PTSD secondary to childhood trauma [229-231]. Research using functional neuroimaging of children and adolescents exposed to maltreatment has shown similar executive, attentional, and affective emotional dysregulation [221, 222, 232-234]. Further, previously institutionalized children demonstrated decreased prefrontal white matter microstructural organization on measures of diffusion tensor imaging that was associated with neurocognitive deficits in spatial planning and a visual learning and memory task compared to non-neglected controls [235].
In another study of healthy adult women with a history of childhood sexual abuse, investigators found higher T2 relaxation time (an indirect index of resting blood volume) in the cerebellar vermis than in non-maltreated women, which correlated strongly with Limbic System Checklist ratings of temporal lobe epilepsy and their frequency of substance use [236]. In studies of carefully characterized healthy adults who only experienced corporal punishment without other forms of maltreatment, T2 relaxation times were increased in dopamine-rich brain decision making and reward regions (caudate and putamen, dorsolateral prefrontal cortex, substantia nigra, thalamus and accumbens)[237]. In the latter study, regional T2 relaxation times were significantly associated with increased use of drugs and alcohol. These studies provide further evidence that specific types of abuse and neglect contribute to the intergenerational cycle of emotional and behavioral problems as well as addiction by their detrimental impact on the brain.
Genetic factors interact with childhood trauma to influence brain structure and function. For example, adults with the Val66MET polymorphism of brain-derived neurotrophic factor (BDNF) who had a history of child maltreatment had a greater likelihood of depressive disorders and a smaller hippocampus, highlighting a child trauma x polymorphism interaction for hippocampal volume reductions [238]. Epigenetic factors also play a role in brain function. In a genome-wide study of promoter methylation in individuals with severe abuse during childhood trauma, decreased promoter transcriptional activity associated with decreased hippocampal expression of the Alsin variants were seen [239]. Adults who were maltreated as children and committed suicide showed similar hypermethylation in the nerve growth factor-induced protein A binding site within a glucocorticoid receptor variant that was associated with decreased glucocorticoid receptor expression in the hippocampus [101]. Mice without the expression of the Alsin gene exhibited more anxiety compared to wild type mice [240].
Furthermore, there are gender x maltreatment effects on brain development. Gender differences were demonstrated using anatomical MRI. Maltreated boys with PTSD had smaller cerebral volumes and larger lateral ventricular volumes than maltreated girls with PTSD, even though the two groups had similar trauma, mental health histories, and IQ [212]. In addition, research has shown a relationship between the type of trauma and gender, as neglect has been shown to have a strong association with smaller corpus callosum size in boys, while sexual abuse was strongly linked to decreased corpus callosum size in girls [217]. In a functional MRI study comparing maltreated youth with PTSD symptoms to non-maltreated youth during performance on an emotional oddball task, left precuneus/posterior middle cingulate hypoactivation to fear versus calm or scrambled face targets were seen in maltreated versus control males and may represent dysfunction and less resilience in attentional networks in maltreated males[241]. These findings were not seen in maltreated females with PTSD symptoms and gender by maltreatment effects were not attributable to demographic, clinical, or maltreatment parameters, suggesting that maltreated males may be dedicating significant functional neural resources to processing affective stimuli in lieu of cognitive processes, which may lead to impulsive decision-making during states of fear emotion and thus less resilience in maltreated males [241].
Thus, the data to date strongly suggests that childhood trauma is associated with adverse brain development in multiple brain regions that negatively impact emotional and behavioral regulation, motivation, and cognitive function. Molecular aging may contribute to these mechanisms and lead to a premature aging but less than optimal brain maturation process in traumatized children as they become adults.
v. Clinical practice application
Understanding the biological effects of maltreatment provide important information that can be used in practice. The first approach is to ensure a safe environment for the child and adolescent patient. It is unlikely that any treatment or buffering of the biological stress systems will occur if a child continues to live in an extremely adverse environment. As outlined in this volume , evidence based interventions show promise in treating traumatized children. Similar interventions have not only shown promise in treating traumatized adults but have showed that the treatment “heals” some of the dysregulations we reviewed in neurobiological stress systems. Many of victims of trauma have sleep problems [242, 243]. In a longitudinal investigation of sexually abused girls, self-reported sleep disturbances were seen approximately 10 years post abuse disclosure, compared to control girls, and were related to current depression, PTSD and revictimization [244]. It was recently demonstrated that sleep has a restorative function; as during sleep potentially neurotoxic waste products (e.g., beta-amyloid) are cleared from the brain [245]. Furthermore, inhibition of catecholamines enhance this waste removal [245]. Therefore, a first step in helping patients with trauma symptoms is to address any sleep hygiene issues they may have, with behavioral methods and/or medications if needed.
A review of recent functional MRI studies demonstrated that cognitive-behavioral therapy can alleviate dysregulation of the fear response and negative emotions associated with anxiety disorders and predict treatment outcomes [246]. Furthermore, greater left amygdala activity on fMRI in pediatric patients with non-trauma related anxiety was associated with greater improvement in anxiety symptoms post treatment [247]. Evidenced-based trauma therapies likely work by changing brain responses. However, studies of brain function and trauma in pediatric patients are in their infancy. Psychopharmacological treatments that target biological stress systems are beyond the scope of this article [248, 249]. Cognitive-behavioral therapy is the treatment of choice with the most evidenced-based studies suggesting its effects in children. Most child psychiatrists start with sleep hygiene, then a selective serotonin reuptake inhibitor for symptoms of anxiety and depression or an adrenergic agent such as clonidine, propranolol, and guanfacine to down-regulate biological stress systems, if trauma-focus cognitive behavioral therapy and improved sleep have less than optimal results. Other medications are used to treat co-morbid disorders such as attention deficit hyperactivity disorder. Because some childhood traumas (child maltreatment) are associated with family histories of mood and anxiety disorders, clinicians should be careful with anti-psychotic drugs as they may be more likely to cause tardive dyskinesia in these child victims [248]. Although medications are useful for treating certain symptoms, double blind studies are needed. It is likely that with further imaging and genetic studies, specific treatments and medication regimens may be tailored for specific PTSD symptoms and individuals in the future. However, the biological effects of PTSD and its treatment continue to be understudied.
vi. Important tools for practice
Understanding the biological effects of maltreatment provides important tools one can useful in practice. Besides self-report instruments to study changes in moods, emotions, and behaviors during psychotherapeutic and/or pharmacological treatments, sleep can be objectively measured using bio-markers such as actographs, which are non-invasive digital monitoring devices that can be worn on the wrist and measure daily activity, sleep awakenings, and sleep efficiency. Other bio-markers that can be used to monitor and tailor treatment and help prevent the negative biological effects of trauma are monitoring measures of heart rate, body mass index, and post-trauma and follow-up salivary cortisol and amylase concentrations as these measures predict PTSD [250], and decreasing these levels during treatment may predict remission.
vii. Future directions
The largest contributor to childhood trauma in the USA is family dysfunction; as almost half of child onset mental disorders and about a third of adult onset mental disorders are preceded by child abuse and neglect, and family dysfunction [251]. While the mental disorders found in maltreating parents and child victims are serious, they are amenable to prevention and treatment.
Given how detrimental childhood trauma is to an individual's development, more efforts and social resources are needed for prevention. Studies show that child maltreatment is amendable to primary prevention. Child maltreatment prevention programs, such as home visiting [252] during an expectant mother's first pregnancy, aimed at addressing mental health and parenting concerns of high-risk new mothers, show great promise in preventing child abuse and neglect. For example, home visiting of an expectant mother by nurses for low-income, at-risk families has been a well replicated strategy for preventing child abuse and neglect[253, 254]. Several studies of this Nurse-Family Partnership Program have demonstrated: improved grade-point averages and achievement test scores in math and reading in grades 1 through 3 in children during their age 9 follow-up assessment [255]; decreased internalizing symptoms and decreased rates of tobacco, alcohol, and marijuana use along with improved reading and math scores at age 12 follow-up assessment [256]; and decreased antisocial behaviors at age 19 follow-up assessment [257]. However, a when a parent becomes maltreating, even an intensive program of home visitation by nurses in addition to standard treatment is not enough to prevent recidivism of physical abuse and neglect [258]. Maltreated children in foster care showed less self-destructive behavior, substance use, and total risk behavior problem standardized scores and higher grades than maltreated children who were reunified with their biological families when interviewed during their 6th year follow-up[259]. These studies indicate that there are two opportunities to break the cycle of maltreatment. The first opportunity is during the expectant parent's first pregnancy, to aid in fostering a loving and caring environment for the parent/child dyad, and to avoid the neurobiological consequences of childhood maltreatment. These types of early prevention programs are cost effective [260]. However, if this cannot be done, the second opportunity is to treat victims of maltreatment after the fact.
There are important evidence-based interventions (e.g., trauma-focused cognitive behavior therapy) for the treatment of PTSD and depression [261] and for antisocial behaviors in youth with early trauma (e.g., multisystemic therapy [262]). Many of these interventions are in other articles within this issue. However, we do not know the long term effectiveness of these programs. We do not have enough professionals trained in evidence-based practices to treat all child victims, and some youth and their families are simply non-complaint with these treatments. Therefore, we still need to understand the neurobiological consequences of chronic stress on a child's developing brain and body so that we can treat the adverse medical and mental health outcomes of early life stress [263]. A society that places its focus on an infrastructure of primary prevention would be choosing the less costly option for victims and for itself.
viii. Conclusions
We outlined how childhood trauma has detrimental consequences on the biological stress systems, and cognitive and brain development. Trauma in childhood is costly for its victims and for society. Resilience is not a common outcome of childhood trauma. In a longitudinal study of individuals who had experienced abuse and neglect during childhood, only 22% of those who had been abused or neglected achieved resiliency based on a comprehensive assessment of healthy adult functioning, by the time they reached young adulthood [264]. Females who grew up in poverty and were not maltreated were more likely to show resilience [264]. While we have some important evidenced-based treatments for child victims, it is in our interest to put in place a national infrastructure of primary child trauma prevention as the less costly option for future victims and for society. Understanding the neurobiological consequences of child trauma will assist in treating child and adult victims, who tend to be more treatment refractory and may have a different endophenotype, than individuals with medical (including mental health) disorders who do not have such histories. Nevertheless, more work is needed to understand the neurobiological consequences of chronic stress on a child's developing brain and body so that we can treat the adverse medical and mental health outcomes of early life stress in those cases (e.g., warfare, natural disasters, child maltreatment) where prevention and effective early intervention may not occur. Such understanding of the neurobiology and genetic influences of child trauma on child development will lead to novel and effective approaches to treatments (e.g., personalized medicine).
Key Points.
Trauma in childhood is a grave psychosocial, medical, and public policy problem that has serious consequences for its victims and for society.
Chronic interpersonal violence in children is common worldwide.
Developmental traumatology, the systemic investigation of the psychiatric and psychobiological effects of chronic overwhelming stress on the developing child, provides a framework and principles when empirically examining the neurobiological effects of pediatric trauma.
Despite the widespread prevalence of childhood trauma, less is known about trauma's biological effects in children as compared to adults with child trauma histories; and even less is known about how these pediatric mechanisms underlie trauma's short-term and long-term medical and mental health consequences.
Glossary
- PTSD
posttraumatic stress disorder
- PTSS
posttraumatic stress symptoms
- GABA
gamma-amino butyric acid
- LHPA
limbic-hypothalamic-pituitary-adrenal
- SNS
Sympathetic nervous system
- HPA
hypothalamic pituitary axis
- LHPA
Limbic-Hypothalamic-Pituitary-Adrenal
- CRH
corticotrophin releasing hormone
- CRF
corticotrophin releasing factor
- ACTH
adrenocorticotrophic hormone
- CNS
Central nervous system
- mPFC
medial prefrontal cortex
- CSF
cerebrospinal fluid
- SNPs
Single nucleotide polymorphisms
- AVP
arginine vasopressin
- BDNF
brain-derived neurotropic factor
- PFC
Prefrontal cortex
- LC
locus coeruleus
- NET
norepinephrine transporter
- BDNF
brain-derived neurotropic factor
- NSCAW
National Survey of Child and Adolescent Well-Being
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition Text Revision. American Psychiatric Press; Washington D.C: 2000. [Google Scholar]
- 2.American Psychiatric Association . Trauma- and Stressor-Related Disorders, in Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 3.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. American Journal of Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 4.Ford JD, Stockton P, Kaltman S, Green BL. Disorders of Extreme Stress (DESNOS) Symptoms Are Associated With Type and Severity of Interpersonal Trauma Exposure in a Sample of Healthy Young Women. Journal of Interpersonal Violence. 2006;21(11):1399–1416. doi: 10.1177/0886260506292992. [DOI] [PubMed] [Google Scholar]
- 5.Carrion VG, Weems CF, Ray RD, Reiss AL. Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;41:166–173. doi: 10.1097/00004583-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of General Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Copeland W, Keeler G, Angold A, Costello E. Traumatic Events and Posttraumatic Stress in Childhood. Archives of General Psychiatry. 2007;64:577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- 8.Luntz BK, Widom CS. Antisocial Personality Disorder in Abused and Neglected Children Grown Up. American Journal of Psychiatry. 1994;151:670–674. doi: 10.1176/ajp.151.5.670. [DOI] [PubMed] [Google Scholar]
- 9.Clark DB, Lesnick L, Hegedus A. Trauma and other stressors in adolescent alcohol dependence and abuse. Journal of the American Academy of Child and Adolescent Psychiatry. 1997:1744–1751. doi: 10.1097/00004583-199712000-00023. [DOI] [PubMed] [Google Scholar]
- 10.De Bellis MD. Developmental Traumatology: A contributory mechanism for alcohol and substance use disorders Special Review in Psychoneuroendocrinology. 2001;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- 11.Dube SR, Miller JW, Brown DW, Giles WH, Felitti VJ, Dong M, Anda RF. Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. Journal of Adolescent Health. 2006;38(4):444e1–10. doi: 10.1016/j.jadohealth.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, household dysfunction and the risk of illicit drug use: The adverse childhood experiences Study. Pediatrics. 2003;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 13.De Bellis MD. Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13(3):539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- 14.Kirsch I, Lynn SJ, Vigorito M. The Role of Cognition in Classical and Operant Conditioning. Journal of Clinical Psychology. 2004;60(4):369–392. doi: 10.1002/jclp.10251. [DOI] [PubMed] [Google Scholar]
- 15.Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Osso B, Nestadt G, Allen A, Hollander E. Serotonin-Norepinephrine Reuptake Inhibitors in the Treatment of Obsessive-Compulsive Disorder: A Critical Review. Journal of Clinical Psychiatry. 2006;67:600–610. doi: 10.4088/jcp.v67n0411. [DOI] [PubMed] [Google Scholar]
- 17.Pagel JF. In: The neuropharmacology of nightmares, in Sleep and Sleep Disorders: A Neuropsychopharmacological Approach. Lader M, Cardinali DP, Pandi-Perumal SR, editors. Springer Science; New York NY: 2006. pp. 243–250. [Google Scholar]
- 18.Argyropoulos SV, Nutt DJ. Anhedonia revisited: Is there a role for dopamine-targeting drugs for depression? Journal of Psychopharmacology. 2013;27:869–877. doi: 10.1177/0269881113494104. [DOI] [PubMed] [Google Scholar]
- 19.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 20.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 22.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 23.LeDoux J. Fear and the brain: where have we been, and where are we going? . Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 24.Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic Mechanisms Of Posttraumatic-Stress-Disorder. Archives Of General Psychiatry. 1993;50(4):294–306. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- 25.Cannon WB. The wisdom of the body. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 26.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Review. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 27.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 28.De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 29.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuropsychopharmacology & Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10–12 year old children: A population-based study of individual differences. Psychoneuroendocrinology. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/Eveningness, Morning-to-Afternoon Cortisol Ratio, and Antisocial Behavior Problems During Puberty. Developmental Psychology. 2007;43(4):811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schuermeyer TH, Hirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa K, Konishi Y, Matsuda T, Kuriyama M, Konishi K, Yamashita K, Okumura R, Hamanaka D. Development and aging of brain midline structures: Assessment with MR imaging. Radiology. 1989;172:171–177. doi: 10.1148/radiology.172.1.2740500. [DOI] [PubMed] [Google Scholar]
- 34.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress- induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 35.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 37.Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Science USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. Journal of Clinical Endocrinology and Metabolism. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 40.Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The Differential Impacts of Early Physical and Sexual Abuse and Internalizing Problems on Daytime Cortisol Rhythm in School-Aged Children. Child Development. 2010;81(1):252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- 42.De Bellis MD, Baum A, Birmaher B, Keshavan M, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. A.E. Bennett Research Award. Developmental Traumatology: Part I: Biological Stress Systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 43.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development & Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 44.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 45.Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the September 11, 2001, terror attacks. Biological Psychiatry. 2007;61:957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 47.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 48.Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. American Journal of Psychiatry. 1996;153:929–934. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- 49.King JA, Mandansky D, King S, Fletcher KE, Brewer J. Early sexual abuse and low cortisol. Psychiatry and Clinical Neurosciences. 2001;55:71–74. doi: 10.1046/j.1440-1819.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan N. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- 51.Murali R, Chen E. Exposure to Violence and Cardiovascular and Neuroendocrine Measures in Adolescents. Ann Behav Med. 2005;30(2):155–163. doi: 10.1207/s15324796abm3002_8. [DOI] [PubMed] [Google Scholar]
- 52.MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, et al. Cortisol Response to Stress in Female Adolescents Exposed to Childhood Maltreatment: Results of the Youth Mood Project. Biological Psychiatry. 2009;66(1):62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The Dexamethasone/Corticotropin-Releasing Factor Test in Men with Major Depression: Role of Childhood Trauma. Biol Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 55.Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL, LaRowe SD, Timmerman MA, Upadhyaya H, Brady KT. PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology. 2006;31(4):501–509. doi: 10.1016/j.psyneuen.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 57.Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology. 2012;37(3):317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Simon GE, Perlis RH. Personalized Medicine for Depression: Can We Match Patients With Treatments? American Journal of Psychiatry. 2010;167:1445–145. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas LA, De Bellis MD. Pituitary volumes in pediatric maltreatment related PTSD. Biological Psychiatry. 2004;55:752–758. doi: 10.1016/j.biopsych.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 60.Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Research. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 61.Weiss SB, Post RM, Gold PW, Chrousos GP, Sullivan TL, Walker D, Pert A. CRF-Induced Seizures and Behavior: Interaction With Arnygdala Kindling. Brain Research. 1986;372:345–351. doi: 10.1016/0006-8993(86)91142-x. [DOI] [PubMed] [Google Scholar]
- 62.Korbey SM, Heinrichs SC, Leussis MP. Seizure susceptibility and locus ceruleus activation are reduced following environmental enrichment in an animal model of epilepsy. Epilepsy & Behavior. 2008;12:30–38. doi: 10.1016/j.yebeh.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Science USA. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coplan JD, Smith ELP, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Gorman JM, Rosenblum LA. Elevations in Cisternal Cerebrospinal Fluid Corticotropin-Releasing Factor Concentrations in Adult Primates. Biological Psychiatry. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- 65.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 66.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. American Journal of Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam Veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- 68.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of Early Adverse Experiences on Brain Structure and Function: Clinical Implications. Biological Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 69.Yehuda R, Kahana B, Binder-Brynes K, Southwick S, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. American Journal of Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- 70.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22(1):165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann NY Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 72.Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Dev Psychobiol. 2008;50(6):588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elzinga BM, Schmahl C, Vermetten E, van Dyck R, Bremner JD. Higher Cortisol Levels Following Exposure to Traumatic Reminders in Abuse-Related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- 74.Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: Reactivity to peer interactions and altered circadian activity. Developmental Psychobiology. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- 75.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 76.De Bellis MD, Dahl RE, Perel J, Birmaher B, Al-Shabbout M, Williamson DE, Nelson B, Ryan ND. Nocturnal ACTH, cortisol, growth hormone, and prolactin secretion in prepubertal depression. J Am Acad Child Adolesc Psychiatry. 1996;35:1130–1138. doi: 10.1097/00004583-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Dimitriou T, Maser-Gluth C, Remer T. Adrenocortical activity in healthy children is associated with fat mass. American Journal of Clinical Nutrition. 2003;77:731–736. doi: 10.1093/ajcn/77.3.731. [DOI] [PubMed] [Google Scholar]
- 78.Hart J, Gunnar M, Cicchetti D. Altered neuroendocrine activity in maltreated children related to symptoms of depression. Development & Psychopathology. 1996;8:201–214. [Google Scholar]
- 79.Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- 80.Kaufman J. Depressive disorders in maltreated children. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:257–265. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased Adrenocorticotropic Hormone and Cortisol Responses to Stress in Healthy Adults Reporting Significant Childhood Maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropinreleasing factor in transgenic mice: a genetic model of anxiogenic behavior. Journal of Neuroscience. 1994;14(5):2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeYoung C, Cicchetti D, Rogosch FA. Moderation of the association between childhood maltreatment and neuroticism by the corticotropin-releasing hormone receptor 1 gene. Journal of Child Psychology and Psychiatry. 2011;52:898–906. doi: 10.1111/j.1469-7610.2011.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development & Psychopathology. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Lui W, Gillespie CF, Berg T, Evces M, Newport J, et al. Influence of Child Abuse on Adult Depression: Moderation by the Corticotropin-Releasing Hormone Receptor Gene. Archives of General Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Archives of General Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of Childhood Maltreatment with the Corticotropin-Releasing Hormone Receptor Gene: Effects on Hypothalamic-Pituitary-Adrenal Axis Reactivity. Biological Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spijker AT, van Rossum EFC. Glucocorticoid Sensitivity in Mood Disorders. Neuroendocrinology. 2012;95:179–186. doi: 10.1159/000329846. [DOI] [PubMed] [Google Scholar]
- 90.Heim CM, Bradley RG, Deveau TC, Musselman DL, Nemoroff CB, Ressler KJ, Binder EB. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Frontiers in Behavioral Neuroscience. 2009;3:1–10. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. Journal of Biological Chemistry. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 92.Zimmermann P, Brückl T, Nocon A, Pfister H, Binder EB, Uhr M, Lieb R, Moffitt TE, Caspi A, Holsboer F, et al. Interaction of FKBP 5 Gene Variants and Adverse Life Events in Predicting Depression Onset: Results From a 10-Year Prospective Community Study. American Journal of Psychiatry. 2011;168:1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Müller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. European Journal of Neuroscience. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 94.Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, et al. Moderation of Adult Depression by a Polymorphism in the FKBP5 Gene and Childhood Physical Abuse in the General Population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, et al. Association of FKBP5 Polymorphisms and Childhood Abuse With Risk of Posttraumatic Stress Disorder Symptoms in Adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, Enoch MA. Interaction Between FKBP5 and Childhood Trauma and Risk of Aggressive Behavior. Archives of General Psychiatry. 2012;69:62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.White MG, Bogdan R, Fisher PM, Muñoz KE, Williamson D, E., Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. 2012;11(7):869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. The EMBO Journal. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murgatroyd C, Spengler D. Epigenetics of early child development. Frontiers in Psychiatry. 2011;2(16):1–15. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGowan PO. Epigenomic mechanisms of early adversity and HPA dysfunction: considerations for PTSD research. Frontiers in Psychiatry. 2013;4(110):1–6. doi: 10.3389/fpsyt.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGowan PO, Szyf M. The epigenetics of social adversity in early life: Implications for mental health outcomes. Neurobiology of Disease. 2010;39:66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 105.Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38(1):111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 107.Kurata A, Morinobu S, Fuchikami M, Yamamoto S, Yamawaki S. Maternal postpartum learned helplessness (LH) affects maternal care by dams and responses to the LH test in adolescent offspring. Horm Behav. 2009;56:112–120. doi: 10.1016/j.yhbeh.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 108.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OFX, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Neuroscience. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 109.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bick J, Naumova O, Hunter S, Barbot B, Lee M, Luthar SS, Raefski A, Grigorenko EL. Childhood adversity and DNA methylation of genes involved in the hypothalamus-pituitary-adrenal axis and immune system: whole-genome and candidate-gene associations. Development and Psychopathology. 2012;24(4):1417–1425. doi: 10.1017/S0954579412000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeSantis SM, Baker NL, Back SE, Spratt E, Ciolino JD, Moran-Santa Maria M, Dipankar B, Brady KT. Gender differences in the effect of early life trauma on hypothalamic-pituitary-adrenal axis functioning. Depression and Anxiety. 2011;28(5):383–392. doi: 10.1002/da.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seltzer LJ, Ziegler T, Connolly MJ, Prososki AR, Pollak SD. Stress-induced elevation of oxytocin in maltreated children: evolution, neurodevelopment, and social behavior. Child Development. 2013 doi: 10.1111/cdev.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Widom CS. Child abuse, neglect, and violent criminal behavior. American Journal of Psychiatry. 1989;27(2):251–271. [Google Scholar]
- 114.Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Preventive Medicine. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- 115.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social Support and Oxytocin Interact to Suppress Cortisol and Subjective Responses to Psychosocial Stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 116.Eisenberger NI, Taylor SA, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Therarapy. 1999;291(3):999–1007. [PubMed] [Google Scholar]
- 118.Ressler KJ, Bradley RG, Mercer KB, Deveau TC, Smith AK, Gillespie CF, Nemoroff CB, Cubells JF, Binder EB. Polymorphisms in CRHR1 and the Serotonin Transporter Loci: GeneGeneEnvironment Interactions on Depressive Symptoms. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;153B:812–824. doi: 10.1002/ajmg.b.31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Bellis MD, Keshavan M, Clark DB, Casey BJ, Giedd J, Boring AM, Frustaci K, Ryan ND. A.E. Bennett Research Award. Developmental Traumatology, Part II: Brain Development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 120.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Molecular Psychiatry. 2013;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paus T. Mapping brain maturation and cognitive development during adolescence. TRENDS in Cognitive Sciences. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 122.McEwen BS. The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. Psychiatr Clin North Am. 2002;25(2):469–494. doi: 10.1016/s0193-953x(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 123.Edwards E, Harkins K, Wright G, Menn F. Effects of bilateral adrenalectomy on the induction of learned helplessness. Behavioral Neuropsychopharmacology. 1990;3:109–114. [PubMed] [Google Scholar]
- 124.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. Journal of Neuroscience. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simantov R, Blinder E, Ratovitski T, Tauber M, Gabbay M, Porat S. Dopamine induced apoptosis in human neuronal cells: inhibition by nucleic acids antisense to the dopamine transporter. Neuroscience. 1996;74:39–50. doi: 10.1016/0306-4522(96)00102-9. [DOI] [PubMed] [Google Scholar]
- 126.Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. Journal of Maternal-Fetal Medicine. 1997;6:309–313. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 127.Todd RD. Neural development is regulated by classical neuro-transmitters: dopamine D2 receptor stimulation enhances neurite outgrowth. Biological Psychiatry. 1992;31:794–807. doi: 10.1016/0006-3223(92)90311-m. [DOI] [PubMed] [Google Scholar]
- 128.Lauder JM. Neurotransmitters as morphogens. Progress in Brain Research. 1988;73:365–388. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- 129.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. Journal of Neuroscience. 1997;17:2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gould E, Tanapat P, Cameron HA. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Brain Research. Developmental Brain Research. 1997;103:91–3. doi: 10.1016/s0165-3806(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 131.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3168–71. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pizarro JM, Lumley LA, Medina W, Robison CL, Changa WE, Alagappana A, Bah MJ, Dawood MY, Shah JD, Mark B, et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Research. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 133.Zhang L-X, Levine S, Dent G, Zhan G, Xing G, Okimoto D, Gordon MK, Post RM, Smith MA. Maternal deprivation increases cell death in the infant rat brain. Developmental Brain Research. 2002;133:1–11. doi: 10.1016/s0926-6410(01)00118-5. [DOI] [PubMed] [Google Scholar]
- 134.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 135.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, Fox NA, Zeanah CH, Nelson CA. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry. 2012;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Bellis MD, Putnam FW. The Psychobiology of Childhood Maltreatment. Child and Adolescent Psychiatric Clinics of North America. 1994;3(4):663–678. [Google Scholar]
- 138.Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Progress in Brain Research. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- 139.Arnsten AFT. The biology of being frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- 140.De Bellis MD, Lefter L, Trickett PK, Putnam FW. Urinary catecholamine excretion in sexually abused girls. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:320–327. doi: 10.1097/00004583-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 141.Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou C, Hindmarsh P, Bakoula C, Papassotiriou I, Tsiantis J, Chrousos GP. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder after motor vehicle accidents: progressive divergence of noradrenaline and cortisol concentrations over time. Biological Psychiatry. 2007;62:1095–1102. doi: 10.1016/j.biopsych.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 142.Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, Yehuda R, Marmar CR. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Pyschiatry. 2005;57(1):27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 143.Savitz JB, van derMerwe L, Newman TK, Solms M, Stein DJ, Ramesar RS. The relationship between childhood abuse and dissociation. Is it influenced by catechol- O-methyltransferase (COMT) activity? . Int J Neuropsychopharmacol. 2008;11:149–161. doi: 10.1017/S1461145707007900. [DOI] [PubMed] [Google Scholar]
- 144.Lesch KP, Mossner R. Genetically driven variation in serotonin update: is there a link to affective spectrum, neurodevelopmental and neurodegenerative disorders? Biological Psychiatry. 1998;44(3):179–192. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 145.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 147.Lauder JM. Neurotransmitters as growth regulatory signals: Role of receptors and second messengers. Trends in Neuroscience. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 148.Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Frontiers in Neuroendocrinology. 2009;30(4):497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 149.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression and Anxiety. 2000;12(Suppl. 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 150.Goodman M, New A, Siever L. Trauma, genes, and the neurobiology of personality disorders. Annals of the New York Academy of Sciences. 2004;1032:104–116. doi: 10.1196/annals.1314.008. [DOI] [PubMed] [Google Scholar]
- 151.Lesch KP, Heils A. Serotonergic gene transcriptional control regions: Targets for antidepressant drug development? . International Journal of Neuropscyhopharmacology. 2000;3:67–79. doi: 10.1017/S1461145700001747. [DOI] [PubMed] [Google Scholar]
- 152.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 153.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Science USA. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sugden K, Arseneault L, Harrington H, Moffitt TE, Williams B, Caspi A. Serotonin transporter gene moderates the development of emotional problems among children following bullying victimization. The Journal of Child & Adolescent Psychiatry. 2010;49:830–840. doi: 10.1016/j.jaac.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Banny AM, Cicchetti D, Rogosch FA, Oshri A, Crick NR. Vulnerability to depression: A moderated mediation model of the roles of child maltreatment, peer victimization, and serotonin transporter linked polymorphic region genetic variation among children from low socioeconomic status backgrounds. Development & Psychopathology. 2013;25:599–614. doi: 10.1017/S0954579413000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cicchetti D, Rogosch FA. GeneEnvironment interaction and resilience: Effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Development & Psychopathology. 2012;24:411–427. doi: 10.1017/S0954579412000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Munafo MR, Durrant C, Lewis G, Flint J. Gene Environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 158.Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hob J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sadeh N, Javdani S, Verona E. Analysis of monoaminergic genes, childhood abuse, and dimensions of psychopathy. Journal of Abnormal Psychology. 2013;122(1):167–179. doi: 10.1037/a0029866. [DOI] [PubMed] [Google Scholar]
- 161.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annual Review of Neuroscience. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene–environment interaction predicting children's mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 163.Nilsson KW, Sjoberg RL, Wargelius HL, Leppert J, Lindstrom L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- 164.Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J. Genetic and environmental predictors of early alcohol use. Biological Psychiatry. 2007;61(11):1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 165.Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular Psychiatry. 2008;13(3):334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- 166.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 167.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12(12):1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 168.Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bradley B, Davis TA, Wingo AP, Mercer KB, Ressler KJ. Family environment and adult resilience: contributions of positive parenting and the oxytocin receptor gene. Eur J Psychotraumatol. 2013;4 doi: 10.3402/ejpt.v4i0.21659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research review: the role of cytokines in depression in adolescents: a systematic review. Journal of Child Psychology and Psychiatry. 2013;54(8):816–835. doi: 10.1111/jcpp.12080. [DOI] [PubMed] [Google Scholar]
- 172.De Bellis MD, Burke L, Trickett PK, Putnam FW. Antinuclear antibodies and thyroid function in sexually abused girls. Journal of Traumatic Stress. 1996;9(2):369–378. doi: 10.1007/BF02110669. [DOI] [PubMed] [Google Scholar]
- 173.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspectives in Psychiatric Care. 2009;45(4):262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 174.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156B(6):700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain, Behavior, and Immunity. 2008;22(6):994–1003. doi: 10.1016/j.bbi.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular immune response in women with PTSD related to childhood abuse. The American Journal of Psychiatry. 2003;160(9):1705–1707. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- 177.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between Plasma IL-6 Response to Acute Stress and Early-Life Adversity in Healthy Adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse Childhood Experiences and Adult Risk Factors for Age-Related Disease:Depression, Inflammation, and Clustering of Metabolic Risk Markers. Arch Pediatr Adolesc Med. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61(5):611–619. doi: 10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 180.Aber JL, Allen JP, Carlson V, Cicchetti D. In: The effects of maltreatment on development during early childhood: recent studies and their theoretical, clinical, and policy implications, in Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. Carlson V, Cicchetti D, editors. Cambridge University Press; New York: 1989. pp. 579–619. [Google Scholar]
- 181.Carrey NJ, Butter HJ, Persinger MA, Bialik RJ. Physiological and cognitive correlates of child abuse. J Am Acad Child Adolesc Psychiatry. 1995;34(8):1067–1075. doi: 10.1097/00004583-199508000-00017. [DOI] [PubMed] [Google Scholar]
- 182.Culp RE, Watkins RV, Lawrence H, Letts D, Kelly DJ, Rice ML. Maltreated children's language and speech development: abused, neglected, and abused and neglected. First Language. 1991;11:377–389. [Google Scholar]
- 183.Eckenrode J, Laird M, Doris J. School performance and disciplinary problems among abused and neglected children. Developmental Psychology. 1993;29:53–62. [Google Scholar]
- 184.McFadyen RG, Kitson WJH. Language comprehension and expression among adolescents who have experienced childhood physical abuse. Journal of Child Psychiatry and Psychology. 1996;37(5):551–562. doi: 10.1111/j.1469-7610.1996.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 185.Trickett PK, McBride-Chang C. The developmental impact of different forms of child abuse & neglect. Developmental Review. 1995;15:311–337. [Google Scholar]
- 186.Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S. Domestic violence is associated with environmental suppression of IQ in young children. Development and Psychopathology. 2003;15(2):297–311. doi: 10.1017/s0954579403000166. [DOI] [PubMed] [Google Scholar]
- 187.Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- 188.DePrince AP, Weinzierl KM, Combs MD. Executive function performance and trauma exposure in a community sample of children. Child Abuse and Neglect. 2009;33(6):353–361. doi: 10.1016/j.chiabu.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 189.Fishbein D, Warner T, Krebs C, Trevarthen N, Flannery B, Hammond J. Differential relationships between personal and community stressors and children's neurocognitive functioning. Child Maltreatment. 2009;14(4):299–315. doi: 10.1177/1077559508326355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Frenn KA, Loman MM, Gunnar MR. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.De Bellis MD, Hooper S, Spratt EG, Woolley DW. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. Journal of the International Neuropsychological Society. 2009;15:868–878. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Samuelson KW, Krueger CE, Burnett C, Wilson DK. Neuropsychological functioning in children with posttraumatic stress disorder. Child Neuropsychology. 2010;16(2):119–133. doi: 10.1080/09297040903190782. [DOI] [PubMed] [Google Scholar]
- 193.De Bellis MD, Woolley DP, Hooper SR. Neuropsychological findings in pediatric maltreatment: relationship of PTSD, dissociative symptoms, and abuse/neglect indices to neurocognitive outcomes. Child Maltreat. 2013;18(3):171–183. doi: 10.1177/1077559513497420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jaffee SR, Maikovich-Fong AK. Effects of chronic maltreatment and maltreatment timing on children's behavior and cognitive abilities. J Child Psychol Psychiatry. 2011;52(2):184–194. doi: 10.1111/j.1469-7610.2010.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lansford JE, Dodge KE, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatrics & Adolescent Medicine. 2002;156(8):824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mills R, Alati R, O'Callaghan M, Najman JM, Williams GM, Bor W, Strathearn L. Child abuse and neglect and cognitive function at 14 years of age: findings from a birth cohort. Pediatrics. 2011;127(1):4–10. doi: 10.1542/peds.2009-3479. [DOI] [PubMed] [Google Scholar]
- 197.Noll JG, Shenk CE, Yeh MT, Ji J, Putnam FW, Trickett PK. Receptive language and educational attainment for sexually abused females. Pediatrics. 2010;126(3):e615–e622. doi: 10.1542/peds.2010-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Perez C, Widom CS. Childhood victimization and long-term intellectual and academic outcomes. Child Abuse & Neglect. 1994;18(8):617–633. doi: 10.1016/0145-2134(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 199.Strathearn L, Gray PH, O'Callaghan F, Wood DO. Childhood neglect and cognitive development in extremely low birth weight infants: a prospective study. Pediatrics. 2001;108(1):142–151. doi: 10.1542/peds.108.1.142. [DOI] [PubMed] [Google Scholar]
- 200.Trickett PK, McBride-Chang C, Putnam FW. The classroom performance and behavior of sexually abused girls. Development and Psychopathology. 1994;6:183–194. [Google Scholar]
- 201.Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32(23):7917–7925. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry. 2009;66:1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 204.De Bellis M, Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2006;60(7):697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 205.De Bellis MD, Keshavan M, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain Structures in Pediatric Maltreatment-Related Posttraumatic Stress Disorder: A Sociodemographically Matched Study. Biological Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 206.De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, Hall J. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biological Psychiatry. 2002;51:544–552. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- 207.Hackman D, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Riva D, Giorgi C. The cerebellum contributes to higher functions during development. Brain. 2000;123:1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- 209.Bellebaum C, Daum I. Cerebellar involvement in executive control. The Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- 210.Thoma P, Bellebaum C, Koch B, Schwarz M, Daum I. The Cerebellum Is Involved in Reward-based Reversal Learning. Cerebellum. 2008;7:433–443. doi: 10.1007/s12311-008-0046-8. [DOI] [PubMed] [Google Scholar]
- 211.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 212.De Bellis MD, Keshavan MS. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci Biobehav Rev. 2003;27(1-2):103–117. doi: 10.1016/s0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 213.Conners NA, Bradley RH, Mansell LW, Liu JY, Roberts TJ, Burgdorf K, Herrell JM. Children of mothers with serious substance abuse problems: An accumulation of risks. American Journal of Drug and Alcohol Abuse. 2004;30:85–100. doi: 10.1081/ada-120029867. [DOI] [PubMed] [Google Scholar]
- 214.Smith DK, Johnson AB, Pears KC, Fisher PA, DeGarmo DS. Child Maltreatment and Foster Care: Unpacking the Effects of Prenatal and Postnatal Parental Substance Use. Child Maltreatment. 2007;12:150. doi: 10.1177/1077559507300129. [DOI] [PubMed] [Google Scholar]
- 215.Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences USA. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH, Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56(2):80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 218.Prichard JW. MRS of the brain-prospects for clinical application. In: Young IR, Charles HC, editors. MR Spectroscopy: Clinical Applications and Techniques. The Livery House; London: 1996. pp. 1–25. [Google Scholar]
- 219.De Bellis MD, Keshavan MS, Spencer S, Hall J. N-acetylaspartate concentration in the anterior cingulate in maltreated children and adolescents with PTSD. American Journal of Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 220.Carrion VG, Weems CF, Richert K, Hoffman BC, Reiss AL. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biological Psychiatry. 2010;68(5):491–493. doi: 10.1016/j.biopsych.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depress Anxiety. 2008;25(6):514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- 222.Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Smith ME. Bilateral Hippocampal Volume Reduction in Adults With Post-Traumatic Stress Disorder: A Meta-Analysis of Structural MRI Studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 224.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A Pilot Longitudinal Study of Hippocampal Volumes in Pediatric Maltreatment-Related Posttraumatic Stress Disorder. Biological Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 225.Carrion VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. Journal of Pediatric Psychology. 2010;35(5):559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119(3):509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 227.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Choi J, Jeong B, Rohan ML, Polcari A, Teicher MH. Preliminary Evidence for White Matter Tract Abnormalities in Young Adults Exposed to Parental Verbal Abuse. Biological Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bremner JD, Narayan M, Staib L, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine. 2005;35(6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Pitman RK. Regional cerebral blood flow during script-imagery in childhood sexual abuse-related PTSD: a PET investigation. American Journal of Psychiatry. 1999;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 232.De Bellis MD, Hooper SR. Neural substrates for processing task-irrelevant emotional distracters in maltreated adolescents with depressive disorders: a pilot study. Journal of Traumatic Stress. 2012;25(2):198–202. doi: 10.1002/jts.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin J, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev. 2013;84(5):1566–1578. doi: 10.1111/cdev.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 237.Sheu Y-S, Polcari A, Anderson CM, Teicher MH. Harsh corporal punishment is associated with increased T2 relaxation time in dopamine-rich regions. NeuroImage. 2010;53:412–419. doi: 10.1016/j.neuroimage.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Carballedo A, Morris D, Zill P, Fahey C, Reinhold E, Meisenzahl E, Bondy B, Gill M, Möller HJ, Frodl T. Brain-derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am J Med Genet B Neuropsychiatric Genetics. 2013;162:183–190. doi: 10.1002/ajmg.b.32130. [DOI] [PubMed] [Google Scholar]
- 239.Labonté B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, Bureau A, Mechawar N, Szyf M, Meaney MJ, et al. Genome-wide epigenetic regulation by early-life trauma. Archives of General Psychiatry. 2012;69(7):722–731. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Devon RS, Orban PC, Gerrow K, Barbieri MA, Schwab C, Cao LP, Helm JR, Bissada N, Cruz-Aguado R, Davidson TL, et al. ALS2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proceedings of the National Academy of Science USA. 2006;103(25):9595–9600. doi: 10.1073/pnas.0510197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Crozier JC, Wang L, Huettel SA, De Bellis MD. Neural correlates of cognitive and affective processing in maltreated youth with posttraumatic stress symptoms: Does gender matter? Development and Psychopathology. 2014;26:491–513. doi: 10.1017/S095457941400008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pillar G, Malhotra A, Lavie P. Post-traumatic stress disorder and sleep—what a nightmare! Sleep Medicine Reviews. 2000;4(2):183–200. doi: 10.1053/smrv.1999.0095. [DOI] [PubMed] [Google Scholar]
- 243.Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clinical Psychology Review. 2003;23:377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 244.Noll JG, Trickett PK, Susman EJ, Putnam FW. Sleep Disturbances and Childhood Sexual Abuse. Journal of Pediatric Psychology. 2006;31(5):469–480. doi: 10.1093/jpepsy/jsj040. [DOI] [PubMed] [Google Scholar]
- 245.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P. Change the Brain? A Systematic Review of Neuroimaging in Anxiety Disorders. The Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21:114–125. doi: 10.1176/jnp.2009.21.2.114. [DOI] [PubMed] [Google Scholar]
- 247.McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 248.DeBellis MD. Medication Considerations with Maltreated Children. In: Talley F, editor. Handbook for the Treatment of Abused and Neglected Children. Haworth Press Inc; Binghamton NY: 2005. pp. 423–472. [Google Scholar]
- 249.Donnally C. In: Psychopharmacotherapy for Children and Adolescents, in Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. Foa EB, et al., editors. The Guilford Press; New York NY: 2009. p. 568. [Google Scholar]
- 250.Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. Journal of Neuroendocrinology. 2008;20(5):632–638. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 251.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication, I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mikton C, Butchart A. Child maltreatment prevention: a systematic review of reviews. Vol. 87. Bulletin of the World Health Organization; 2009. pp. 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kitzman H, Olds DL, Henderson CR, Jr, Hanks C, Cole R, Tatelbaum R, McConnochie KM, Sidora K, Luckey DW, Shaver D, et al. Effect of Prenatal and Infancy Home Visitation by Nurses on Pregnancy Outcomes, Childhood Injuries, and Repeated Childbearing: A Randomized Controlled Trial. Journal of the American Medical Association. 1997;278:644–652. [PubMed] [Google Scholar]
- 254.Olds DL, Eckenrode J, Henderson CR, Jr, Kitzman H, Powers J, Cole R, Sidora K, Morris P, Pettitt LM, Luckey D. Long-term effects of home visitation on maternal life course and child abuse and neglect: fifteen-year follow- up of a randomized trial. Journal of the American Medical Association. 1997;278(8):637–643. [PubMed] [Google Scholar]
- 255.Olds DL, Kitzman H, Hanks C, Cole R, Anson E, Sidora-Arcoleo K, Luckey DW, Henderson CR, Jr, Holmberg J, Tutt RA, et al. Effects of Nurse Home Visiting on Maternal and Child Functioning: Age-9 Follow-up of a Randomized Trial. Pediatrics. 2007;120:e832. doi: 10.1542/peds.2006-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kitzman H, Olds DL, Cole R, Hanks CA, Anson EA, Arcoleo KJ, Luckey DW, Knudtson MD, Henderson CR, Jr, Holmberg J. Enduring Effects of Prenatal and Infancy Home Visiting by Nurses on Children. Archives of Pediatric and Adolescent Medicine. 2010;164(5):412–418. doi: 10.1001/archpediatrics.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Eckenrode J, Campa M, Luckey DW, Henderson CR, Jr, Cole R, Kitzman H, Anson EA, Sidora-Arcoleo K, Powers J, Olds DL. Long-term Effects of Prenatal and Infancy Nurse Home Visitation on the Life Course of Youths 19-Year Follow-up of a Randomized Trial. Archives of Pediatric and Adolescent Medicine. 2010;164(1):9–15. doi: 10.1001/archpediatrics.2009.240. [DOI] [PubMed] [Google Scholar]
- 258.MacMillan HL, Thomas BH, Jamieson E, Walsh CA, Boyle MH, Shannon HS, Gafni A. Effectiveness of home visitation by public-health nurses in prevention of the recurrence of child physical abuse and neglect: a randomised controlled trial. Lancet. 2005;365:1786–1793. doi: 10.1016/S0140-6736(05)66388-X. [DOI] [PubMed] [Google Scholar]
- 259.Taussig HN, Clyman RB, Landsverk J. Children Who Return Home From Foster Care: A 6-Year Prospective Study of Behavioral Health Outcomes in Adolescence. Pediatrics. 2001;108(1):1–7. doi: 10.1542/peds.108.1.e10. [DOI] [PubMed] [Google Scholar]
- 260.Foster ME, Prinz RJ, Sanders MR, Shapiro CJ. The costs of a public health infrastructure for delivering parenting and family support. Child Youth Serv Rev. 2008;30:493–501. doi: 10.1016/j.childyouth.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cohen JA, Mannarino AP, Deblinger E, Berliner L. In: Cognitive-Behavioral Therapy for Children and Adolescents, in Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. Foa EB, et al., editors. The Guilford Press; New York NY: 2009. pp. 223–244. [Google Scholar]
- 262.Curtis NM, Ronan KR, Borduin CM. Multisystemic Treatment: A Meta-Analysis of Outcome Studies. Journal of Family Psychology. 2004;18(3):411–419. doi: 10.1037/0893-3200.18.3.411. [DOI] [PubMed] [Google Scholar]
- 263.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes if death in adults. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 264.McGloin JM, Widom CS. Resilience among abused and neglected children grown up. Development and Psychopathology. 2001;13(4):1021–1038. doi: 10.1017/s095457940100414x. [DOI] [PubMed] [Google Scholar]