Abstract
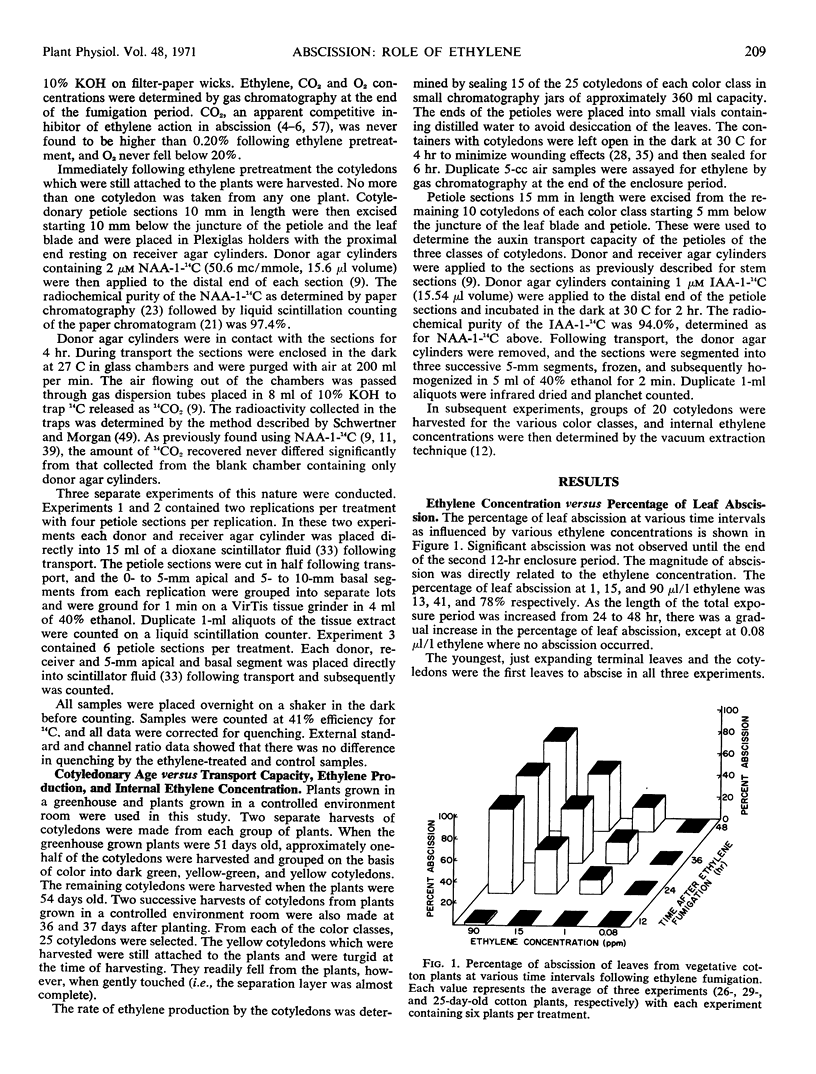
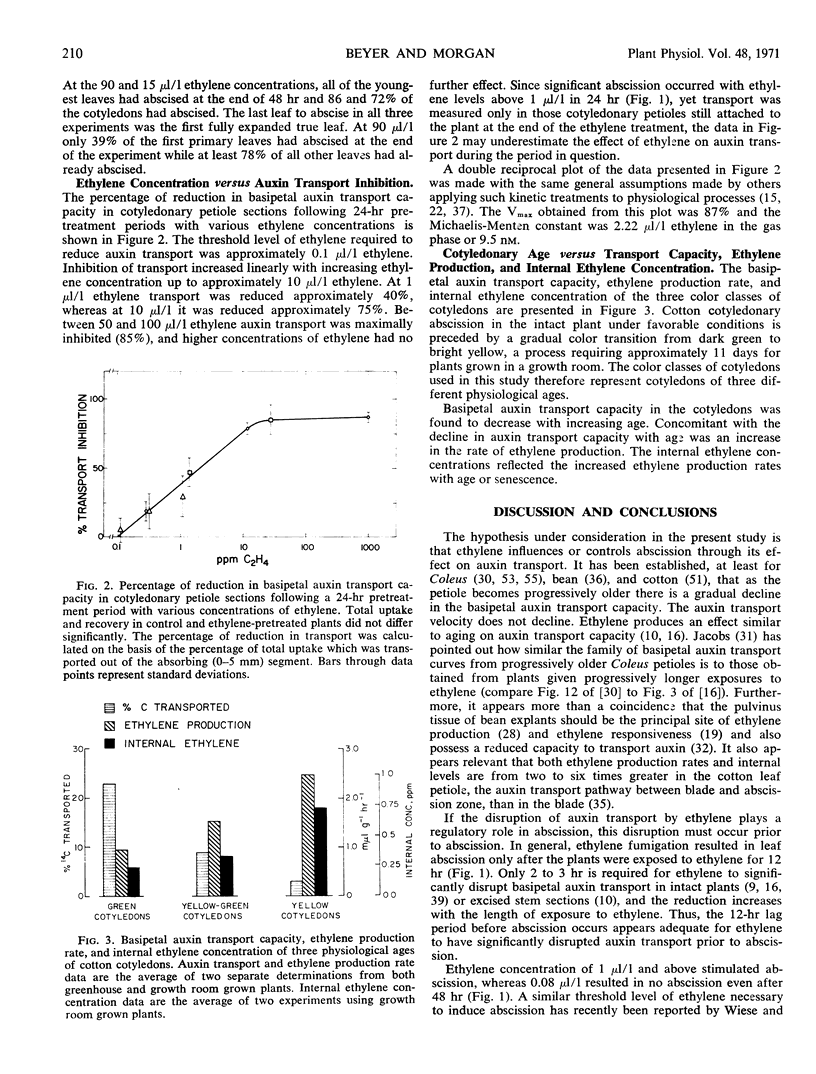
The role of ethylene-mediated reduction of auxin transport in natural and ethylene-induced leaf abscission was studied in the cotton (Gossypium hirsutum L., cv. Stoneville 213) cotyledonary leaf system. The threshold level of ethylene required to cause abscission of intact leaves was between 0.08 and 1 μl/l with abscission generally occurring 12 to 24 hours following ethylene fumigation. The threshold level of ethylene required to reduce the auxin transport capacity in the cotyle-donary petiole paralleled that required for stimulation of abscission. In plants where cotyledons are allowed to senesce naturally there is a decline in auxin transport capacity of petioles and increase in ethylene synthesis of cotyledons. The visible senescence process which precedes abscission requires up to 11 days, and increases in ethylene production rates and internal levels were detected well before abscission. Ethylene production rates for entire cotyledons rose to 2.5 mμ1 g−1 hr−1 and internal levels of 0.7 μl/l were observed. These levels appear to be high enough to cause the observed decline in auxin transport capacity. These findings, along with those of others, indicate that ethylene has several roles in abscission control (e.g., transport modification, enzyme induction, enzyme secretion). The data indicate that ethylene modification of auxin transport participates in both natural abscission and abscission hastened by exogenous ethylene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Abscission: role of cellulase. Plant Physiol. 1969 Mar;44(3):447–452. doi: 10.1104/pp.44.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Gahagan H. E. Abscission: the role of ethylene, ethylene analogues, carbon dioxide, and oxygen. Plant Physiol. 1968 Aug;43(8):1255–1258. doi: 10.1104/pp.43.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Holm R. E. Enhancement of RNA synthesis, protein synthesis, and abscission by ethylene. Plant Physiol. 1966 Oct;41(8):1337–1342. doi: 10.1104/pp.41.8.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Holm R. E., Gahagan H. E. Abscission: the role of aging. Plant Physiol. 1967 Oct;42(10):1351–1356. doi: 10.1104/pp.42.10.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B. Role of RNA and protein synthesis in abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1577–1586. [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. A method for determining the concentration of ethylene in the gas phase of vegetative plant tissues. Plant Physiol. 1970 Aug;46(2):352–354. doi: 10.1104/pp.46.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Effect of ethylene on the uptake, distribution, and metabolism of indoleacetic Acid-1-C and -2-C and naphthaleneacetic Acid-1-C. Plant Physiol. 1970 Jul;46(1):157–162. doi: 10.1104/pp.46.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Ethylene modification of an auxin pulse in cotton stem sections. Plant Physiol. 1969 Dec;44(12):1690–1694. doi: 10.1104/pp.44.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Inhibition of polar auxin transport by ethylene. Plant Physiol. 1967 Sep;42(9):1224–1228. doi: 10.1104/pp.42.9.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P. Ethylene, plant senescence and abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1503–1511. [PMC free article] [PubMed] [Google Scholar]
- CAMPA P. P., SCIACCA A., CONDORELLI M., POY C. [Right ventricular prevalence in the lamb: comparison of the electrocardiographic aspects and weight relations between the two ventricles]. Boll Soc Ital Biol Sper. 1959 Dec 31;35:1957–1960. [PubMed] [Google Scholar]
- Cooper W. C., Rasmussen G. K., Rogers B. J., Reece P. C., Henry W. H. Control of abscission in agricultural crops and its physiological basis. Plant Physiol. 1968 Sep;43(9 Pt B):1560–1576. [PMC free article] [PubMed] [Google Scholar]
- Dela Fuente R. K., Leopold A. C. Senescence processes in leaf abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1496–1502. [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Andreae W. A., Ysselstein M. W. Studies on 3-Indoleacetic Acid Metabolism. II. Some Products of the Metabolism of Exogenous Indoleacetic Acid in Plant Tissues. Plant Physiol. 1956 May;31(3):231–235. doi: 10.1104/pp.31.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Osborne D. J. Ethylene, the natural regulator of leaf abscission. Nature. 1970 Mar 14;225(5237):1019–1022. doi: 10.1038/2251019a0. [DOI] [PubMed] [Google Scholar]
- Jacobs W. P. Hormonal regulation of leaf abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1480–1495. [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. P., McCready C. C., Osborne D. J. Transport of the Auxin 2,4-Dichlorophenoxyacetic Acid Through Absiccion Zones, Pulvini, and Petioles of Phaseolus vulgaris. Plant Physiol. 1966 Apr;41(4):725–730. doi: 10.1104/pp.41.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. W., Hall W. C. Indoleacetic Acid Oxidizing Enzyme & Inhibitors from Light-Grown Cotton. Plant Physiol. 1963 Jul;38(4):365–370. doi: 10.1104/pp.38.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertner H. A., Morgan P. W. Role of IAA-Oxidase in Abscission Control in Cotton. Plant Physiol. 1966 Nov;41(9):1513–1519. doi: 10.1104/pp.41.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos J. G., Ernest L. C., Henry E. W. Effect of ethylene and gibberellic Acid on auxin synthesis in plant tissues. Plant Physiol. 1967 Dec;42(12):1803–1806. doi: 10.1104/pp.42.12.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen H., Jacobs W. P. Transport and metabolism of indole-3-acetic Acid in coleus petiole segments of increasing age. Plant Physiol. 1969 Aug;44(8):1157–1162. doi: 10.1104/pp.44.8.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese M. V., Devay J. E. Growth Regulator Changes in Cotton Associated with Defoliation Caused by Verticillium albo-atrum. Plant Physiol. 1970 Mar;45(3):304–309. doi: 10.1104/pp.45.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]



