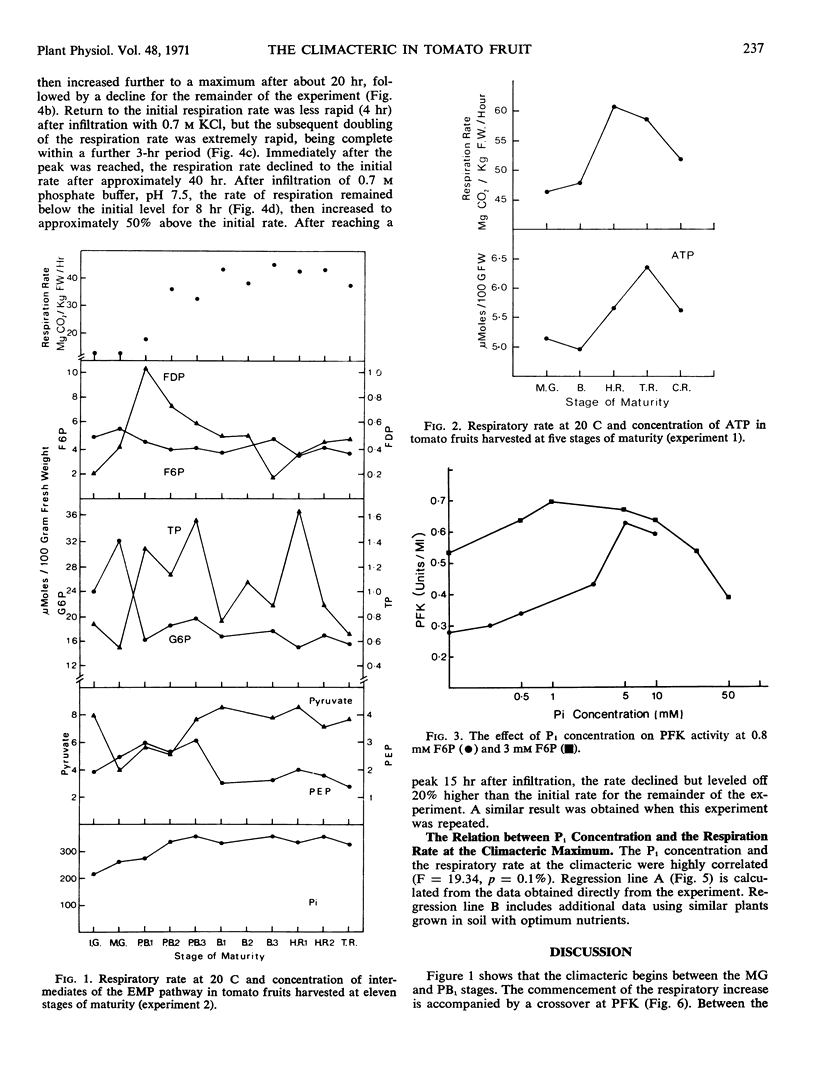
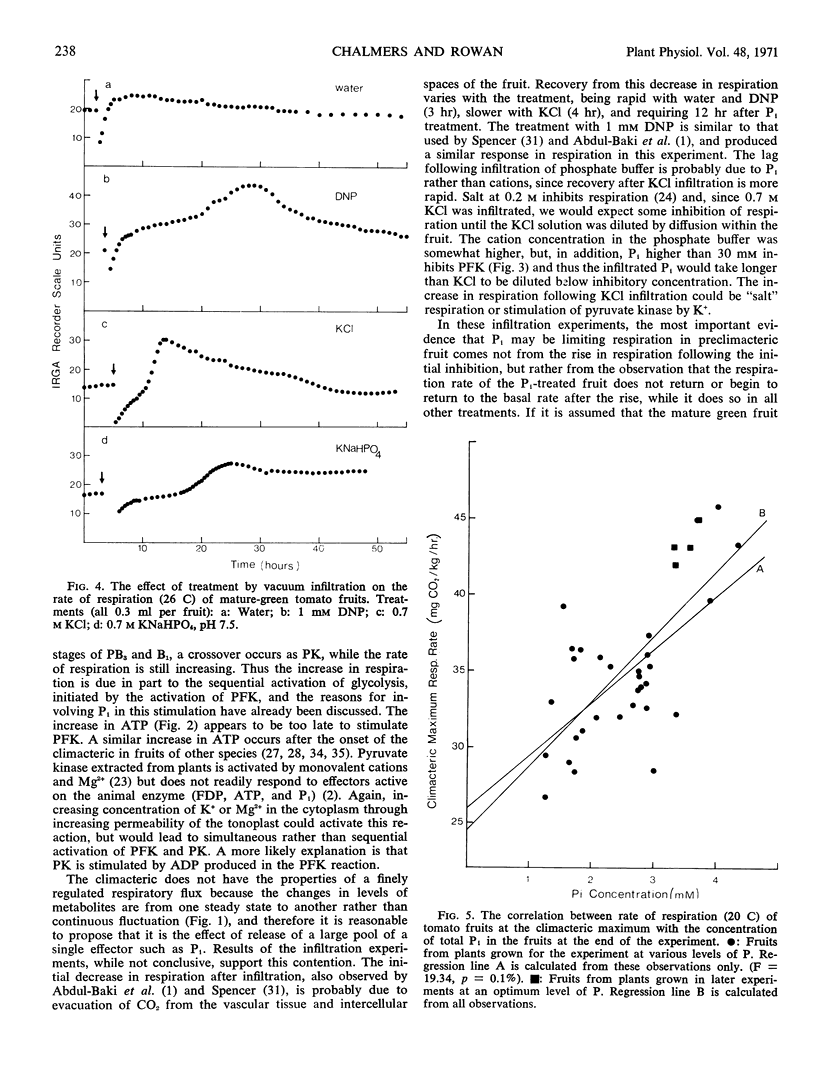
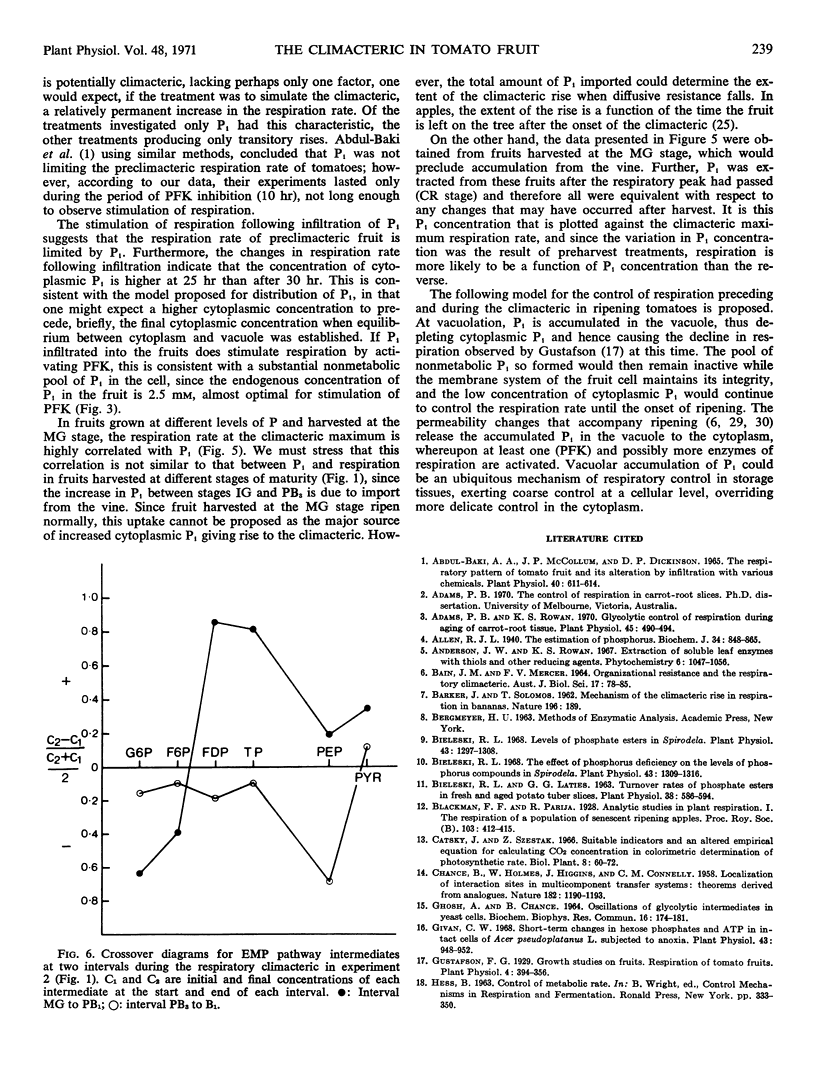
Abstract
Phosphofructokinase is identified as the regulator reaction activated at the onset of the climacteric rise in respiration of the ripening tomato fruit (Lycopersicon esculentum Mill). The concentration of ATP in the fruit increases to a maximum value after the climacteric peak of respiration is past. Orthophosphate is proposed as the most probable activator of phosphofructokinase in the ripening fruit.
Fifteen hours after infiltrating tomato fruit with orthophosphate, the rate of respiration increased and remained high until the end of the experiment, 45 hours after infiltration. In experiments where tomato plants were grown at various nutrient levels of P, the rate of respiration when fruit harvested at the mature-green stage reached the respiratory climacteric was correlated with the concentration of orthophosphate in the fruit at the end of the experiment. These results are consistent with the hypothesis that stimulation of phosphofructokinase through increasing concentration of orthophosphate in the cytoplasm of the fruit contributes to the climacteric rise in respiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A., McCollum J. P., Dickinson D. B. The Respiratory Pattern in the Tomato Fruit and its Alteration by Infiltration with Various Chemicals. Plant Physiol. 1965 Jul;40(4):611–614. doi: 10.1104/pp.40.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. B., Rowan K. S. Glycolytic control of respiration during aging of carrot root tissue. Plant Physiol. 1970 Apr;45(4):490–494. doi: 10.1104/pp.45.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L. Effect of phosphorus deficiency on levels of phosphorus compounds in spirodela. Plant Physiol. 1968 Aug;43(8):1309–1316. doi: 10.1104/pp.43.8.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L., Laties G. G. Turnover Rates of Phosphate Esters in Fresh and Aged Slices of Potato Tuber Tissue. Plant Physiol. 1963 Sep;38(5):586–594. doi: 10.1104/pp.38.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L. Levels of phosphate esters in spirodela. Plant Physiol. 1968 Aug;43(8):1297–1308. doi: 10.1104/pp.43.8.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Chance B. Oscillations of glycolytic intermediates in yeast cells. Biochem Biophys Res Commun. 1964 Jun 1;16(2):174–181. doi: 10.1016/0006-291x(64)90357-2. [DOI] [PubMed] [Google Scholar]
- Givan C. V. Short-term Changes in Hexose Phosphates and ATP in Intact Cells of Acer pseudoplatanus L. Subjected to Anoxia. Plant Physiol. 1968 Jun;43(6):948–952. doi: 10.1104/pp.43.6.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson F. G. GROWTH STUDIES ON FRUITS. RESPIRATION OF TOMATO FRUITS. Plant Physiol. 1929 Jul;4(3):349–356. doi: 10.1104/pp.4.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. The regulation of pea-seed phosphofructokinase by 6-phosphogluconate, 3-phosphoglycerate, 2-phosphoglycerate and phosphoenolpyruvate. Biochim Biophys Acta. 1970 Jun;208(3):360–367. doi: 10.1016/0304-4165(70)90207-2. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. The regulation of pea-seed phosphofructokinase by phosphoenolpyruvate. Biochem J. 1969 Nov;115(3):481–487. doi: 10.1042/bj1150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughman B. C. Uptake and Utilization of Phosphate Associated With Respiratory Changes in Potato Tuber Slices. Plant Physiol. 1960 Jul;35(4):418–424. doi: 10.1104/pp.35.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Evans H. J. The Influence of Salts on Pyruvate Kinase from Tissues of Higher Plants. Plant Physiol. 1957 Jul;32(4):346–354. doi: 10.1104/pp.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER M. S. Ethylene metabolism in tomato fruit. III. Effect of 2,4-dinitrophenol on respiration, ethylene evolution, and ripening. Can J Biochem Physiol. 1959 Jan;37(1):53–59. [PubMed] [Google Scholar]
- Sacher J. A. Permeability Characteristics and Amino Acid Incorporation during Senescence (Ripening) of Banana Tissue. Plant Physiol. 1966 Apr;41(4):701–708. doi: 10.1104/pp.41.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. E., Biale J. B. Phosphorylation in avocado fruit slices in relation to the respiratory climacteric. Plant Physiol. 1967 Oct;42(10):1357–1362. doi: 10.1104/pp.42.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]


