Abstract
The rodent malaria parasite Plasmodium chabaudi chabaudi shares many features with human malaria species, including P. falciparum, and is the in vivo model of choice for many aspects of malaria research in the mammalian host, from sequestration of parasitized erythrocytes, to antigenic variation and host immunity and immunopathology. this protocol describes an optimized method for the transformation of mature blood-stage P.c. chabaudi and a description of a vector that targets efficient, single crossover integration into the P.c. chabaudi genome. Transformed lines are reproducibly generated and selected within 4–20 d, and show stable long-term protein expression even in the absence of drug selection. this protocol, therefore, provides the scientific community with a robust and reproducible method to generate transformed P.c. chabaudi parasites expressing fluorescent, bioluminescent and model antigens that can be used in vivo to dissect many of the fundamental principles of malaria infection.
INTRODUCTION
Although much is known about the parasite species that cause human malaria and widespread disease, efforts to control malaria still remain far from the development of an effective panel of new anti-malarial drugs or a vaccine. Obvious ethical limitations have severely limited research aimed at the rational design of malaria therapeutics and at an understanding of the complex host-parasite interactions in humans. Animal models of malaria have, therefore, proven to be extremely important, and rodent models are foremost in providing significant insights into parasite genetics and biology and host immunity.
Infections with the rodent malaria parasite Plasmodium chabaudi chabaudi in mice share many characteristics with P. falciparum infection in humans: erythrocytes infected with mature P.c. chabaudi parasite stages rosette, sequester and withdraw from the peripheral circulation. Furthermore, primary P.c. chabaudi infections follow a chronic and recrudescing course, during which the parasite undergoes antigenic variation; mice that have recovered from a primary infection are immune to re-infection with a homologous strain, but they can be susceptible to infection with a heterologous strain of P.c. chabaudi (reviewed in refs. 1,2). Thus, P.c. chabaudi can be regarded as a very useful model for studying sequestration of parasitized erythrocytes, the potential role of antigenic variation in chronic infection, the pathology that ensues after infection, and the various aspects of innate and acquired host immunity, which can lead to protection or immunopathology. In addition, the availability of genetically distinct strains of P.c. chabaudi with different virulence, growth and rates of sexual differentiation has allowed studies that have revealed unexpected aspects of the parasite’s evolution and reproductive strategies3-5.
Transfection of malaria parasites has proven to be an essential and powerful tool in elucidating aspects of parasite biology, particularly during the stages of the parasite’s lifecycle in the mosquito and, subsequently, in the mammalian host, during the invasion of red blood cells by merozoites and during the intra-erythrocytic development of parasites. The rodent malaria parasites, P. berghei and P. yoelii, have proven to be especially informative in such studies. High-efficiency transformation in these parasites relies on the fact that they display a strong preference for invasion and development within reticulocytes, and that infected reticulocytes do not undergo schizogony (rupture of mature parasite-infected erythrocytes) under the relatively static conditions of in vitro culture. Thus, developmentally arrested schizonts can be purified in high numbers from culture and mechanically ruptured to release free merozoites, the optimal stage for transfection, immediately before electroporation (for a detailed description, see ref. 6).
Although P.c. chabaudi shares many characteristics with P. falciparum, and offers many advantages over both P. berghei and P. yoelii as the in vivo rodent malaria model, research has been hampered by the lack of efficient transfection technology for this parasite. Obstacles to the development of efficient protocols include the fact that P.c. chabaudi has a slower proliferation rate in the face of an effective host immune response than P. berghei as well as the technical limitations in obtaining high numbers of merozoites. Mature stages of P.c. chabaudi within erythrocytes sequester in deep tissues in vivo and cannot be isolated from the circulation and, unlike P. berghei and P. yoelii, rupture during schizogony in vitro. Previously, we described the generation of the first P.c. chabaudi transgenic parasite line expressing the green fluorescent protein using unpurified mature blood-stage parasites from the fast-growing P.c. chabaudi line AJ5. However, the protocol used was exceptionally laborious, requiring intensive monitoring to detect patent infections of transformed parasites in mice before immune clearance. Furthermore, the transformation efficiency obtained using this protocol was insufficient for routine laboratory use, or for the reliable modification of less-virulent or slower-growing P.c. chabaudi lines, including the AS strain—the parasite of choice for studying the immunology of the host-parasite interaction.
Here, we describe an optimized protocol for transformation of blood-stage P.c. chabaudi parasites (Fig. 1a). The efficiency of transformation has been markedly improved by the development of in vivo and in vitro protocols for the production of large numbers of merozoites, the design of a transfection vector that specifically targets nonessential regions of the P.c. chabaudi genome and the use of mice lacking an effective immune response for selection of transformed parasites. Fluorescent parasites that we have recently generated using this protocol have proven to be powerful tools in analyses of antimalarial immune responses in mice7,8, and can be used for in vivo imaging of parasite sequestration, cellular interactions and parasite-associated pathology; they are also useful for the rapid discrimination of parasite strains in co-infection studies. We anticipate, therefore, that this important technological advance will open the way for the creation of a custom-made range of new fluorescent, bioluminescent and model antigen–expressing P.c. chabaudi lines that will have wide-ranging applications in many aspects of in vivo malaria research.
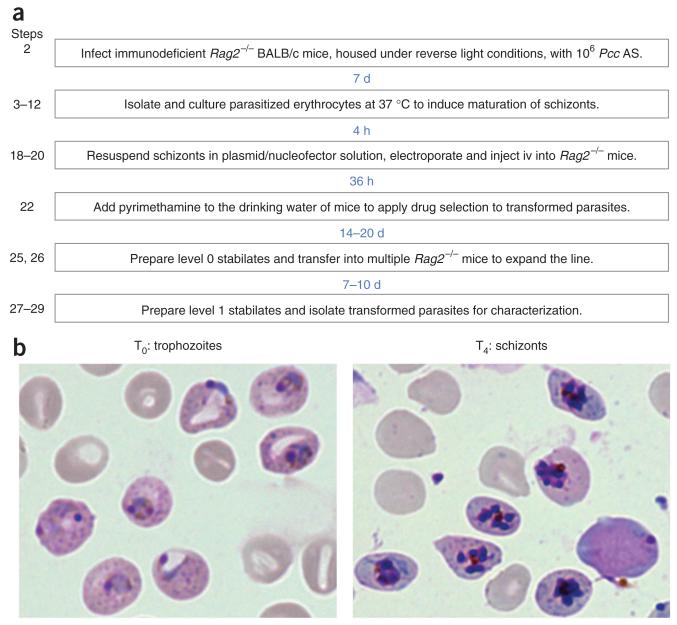
Figure 1. Overview of the generation of transformed P.c. chabaudi.
(a) Flowchart detailing each step in the generation of transformed P.c. chabaudi, and elapsed time between each step. (b) Representative images of P.c. chabaudi following isolation from donor mice (T0: trophozoites) and after 4 h of in vitro culture (T4: schizonts).
MATERIALS
REAGENTS
Parasites
We have generated transfected lines of P.c. chabaudi AS (Pcc AS) and P.c. chabaudi AJ (Pcc AJ). Other P.c. chabaudi strains may also be transfected. Pcc AS can be obtained from the WHO Registry of Standard Malaria Parasites, University of Edinburgh. We use stabilates that are no more than ten passages from mosquito transmission ! CAUTION More virulent strains of P.c. chabaudi may cause unacceptably severe disease at high parasitemia in mice.
Mice
Animals are bred under specific pathogen-free conditions at The National Institute for Medical Research (UK). They may be purchased from Harlan or Jackson laboratories. We routinely use wild-type BALB/c mice or immuno-compromised Rag2−/− BALB/c mice (between 6 and 8 weeks of age, and at ~20 g body weight). Mice are housed under reverse light conditions (light 1900–0700 hours/dark 0700–1900 hours) at 22 °C and 50% relative humidity, and they have continuous access to food and water ! CAUTION All experiments involving animals must be performed according to local and national guidelines and regulations ! CAUTION Host diet can affect the reproducibility of rodent malaria infections (e.g., a 4-aminobenzoic acid (PABA)-fortified diet may be required).
General reagents
BSA (Sigma-Aldrich, cat. no. A8022)
Compressed gas mix: 5% O2, 7% CO2, 88% N2 (Messer UK, UN number 1956)
Distilled water
DMSO (Sigma-Aldrich, cat. no. D5879)
Ethanol (analytical reagent grade, diluted to 70% in distilled H2O; Fisher Scientific UK, cat. no. E/0650DF/17)
FBS Gold (PAA Laboratories, cat. no. A15-649)
Giemsa’s stain solution (BDH (VWR), cat. no. 352603R)
Glucose (BDH (VWR), cat. no. 101174Y)
Glycerol (analytical reagent grade; Merck, cat. no. 356350)
Heparin sodium (mucous) injection B.P. (5,000 IU ml−1; LEO Laboratories)
HCl (BDH, cat. no. 101254H)
HEPES (Invitrogen, cat. no. 15630)
Hoechst 33342 (Molecular Probes, cat. no. H1399)
KCl (Sigma, cat. no. P3911)
KH2PO4 (BDH, cat. no. 102034B)
l-glutamine (Invitrogen, cat. no. 25030)
β-Mercaptoethanol (Invitrogen, cat. no. 31350)
MgSO4·7H2O (Sigma, cat. no. M1880)
NaCl (Sigma, cat. no. S7653)
Na2HPO4 (BDH, cat. no. 102494C)
Methanol (analytical reagent grade; Fisher Scientific UK, cat. no. M/3900/17)
Pentobarbitone sodium (Pentoject; Animal Care, cat. no. XVD132) ! CAUTION This is a biohazard and should be stored in a secure area.
PBS (pH 7.2; Invitrogen, cat. no. 20012)
Pyrimethamine (Vetranal; Sigma-Aldrich, cat. no. 46706)
RPMI 1640 (+ l-glutamine; Invitrogen, cat. no. 21875)
Sodium pyruvate (Sigma-Aldrich, cat. no. 58636)
Basic Parasite Nucleofector Kit 2 (Lonza, cat. no. VMI-1021)
Plasmodipur filter (EuroProxima)
EQUIPMENT
Graduated Pasteur pipette (1 ml, sterile; Fisher Scientific, cat. no. FB55340)
Micro test tubes (1.5 ml; Eppendorf, cat. no. 0030 120.086)
Polypropylene centrifuge tubes (15 and 50 ml; Corning, cat. nos. 430766 and 430291, respectively)
Polystyrene Falcon tissue culture flask (50 ml (25 cm2), nonvented; BD, cat. no. 353014)
U-100 insulin (1 ml syringe, no deadspace; Dispomed, cat. no. 22002)
Needle (27 G × 0.5 inch; BD Microlance 3; BD, cat. no. 300635)
Microscope slides (ground, frosted; VWR, cat. no. 631-1560)
Glass cover slips (18 mm × 18 mm; Menzel Glaser, cat. no. MNJ-350-010K)
Cryogenic vials (1.2 ml; Corning Incorporated, cat. no. 430487)
Bench-top centrifuge (Sigma 2-16 KCH with rotors 12148 and 11192 or equivalent; SciQuip UK) ▲ CRITICAL The centrifuge must be able to operate at 37 °C.
Bench-top microcentrifuge (Heraeus Fresco 17 or equivalent; Thermo Scientific)
Heated water bath (Grant JB2 or equivalent; Grant Instruments)
Heated incubator (Gallenkamp IR700SG or equivalent; Sanyo)
Improved Neubauer cell counting chamber (depth 0.1 mm; Hawksley, cat. no. AC1000)
Nucleofector device (Lonza, cat. no. AAD-1001)
Light microscope with oil-immersion ×100 objective
Fluorescent microscope with oil-immersion ×100 objective and suitable filter sets (Zeiss Axioplan 2 or equivalent; Zeiss)
Infrared heat lamp
Mouse restrainer
LSR II cytometer (Becton Dickinson)
REAGENT SETUP
Giemsa’s phosphate buffer
(10×) Na2HPO4 (0.21 M) and 0.044 M KH2PO4 in dH2O (pH 7.2). Store at room temperature (18–24 °C) for up to 6 months.
Giemsa’s working solution
20% (vol/vol) Giemsa’s stain in 1× Giemsa’s phosphate buffer. ▲ CRITICAL Prepare a fresh solution before use.
Krebs buffer
(4.5×) Na2HPO4 (0.125 M) in dH2O and 2% (vol/vol) HCl (pH 7.4). Store at room temperature for up to 6 months.
Krebs saline + glucose
NaCl (0.1 M), 4.6 mM KCl, 1.2 mM MgSO4·7H2O heparin sodium (sterile). in 1× Krebs buffer and 0.2% (wt/vol) glucose (sterile). Store in a sterile container at 4 °C for up to 6 months.
KSGH
Krebs saline, glucose (KSG), and 25 IU ml−1 Store in a sterile container at 4 °C for up to 6 months.
Buffered RPMI
RPMI 1640, 6 mM HEPES and 2 mM l-glutamine (sterile). ▲ CRITICAL Prepare a fresh solution before use.
Complete RPMI
RPMI 1640, 10% (vol/vol) FBS, 6 mM HEPES, 2 mM l-glutamine, 0.5 mM sodium pyruvate and 50 μM β-mercaptoethanol (sterile). ▲ CRITICAL Prepare a fresh solution before use.
Fluorescence-activated cell sorting buffer
PBS, 1% (wt/vol) BSA, and 2 mM EDTA. Store sterile at 4 °C for up to 6 months.
Basic parasite Nucleofector solution
Add the Nucleofector supplement to the Nucleofector solution. It is stable for 3 months at 4 °C.
Pyrimethamine 100× stock solution
Pyrimethamine (7 mg ml−1) in DMSO, vortexed until completely dissolved. Store at 4 °C for 1 month or −80 °C for long term.
Pyrimethamine drinking water
Pyrimethamine (70 g ml−1) in acidified drinking water (pH 3.5–5.0; make fresh). Requires changing weekly. ▲ CRITICAL Pyrimethamine is light sensitive. Use black drinking bottles (or foil-wrap clear drinking bottles) to protect from light.
DNA plasmid preparation
Linearized DNA construct (2.5 μg) dissolved in 5 μl TE buffer or distilled water (stored at −20 °C) is used for transfection (see Step 18). These constructs contain the Toxoplasma gondii DHFR selectable marker (Tg-dhfr), target sites for integration into the P.c. chabaudi genome and a reporter gene expressed under the control of the constitutive promoter ef-1α. Details of the construction of vectors for transfection of rodent malaria parasites are described in Franke-Fayard et al.9. To generate a vector that introduces the gene encoding mCherry into the P.c. chabaudi SSu-rRNA locus on chromosomes 5, the PF0017 plasmid vector9 was modified as follows: (i) A KpnI-ApaI fragment of the P. berghei SSu-rRNA target region of pL0017 was excised and replaced with the corresponding 704-bp region of P.c. chabaudi chromosome 5 SSu-rRNA that was amplified from Pcc AS genomic DNA using the primers; GACTGGTACCAGTAGTCATATGCTTGTCTC and CAATGGGCCCTATAGTTAAAAGTACGACGAGGC (restriction sites underlined); (ii) The BamH1-Not1 fragment of PF0017 encoding green fluorescent protein was excised and replaced with a customized synthetic gene (Genscript USA) encoding mCherry.
Donor mice infection
Donor Rag2−/− BALB/c mice are infected with at least 106 parasites, maintained through routine weekly passage from frozen stocks (see Box 1). A high inoculum is administered to grow parasites rapidly to a high level of parasitemia, minimizing damage to parasites and anemia in mice. This maximizes the quality and yield of donor parasites, which should be isolated at between 40% and 50% parasitemia following the monitoring and enumeration of thin blood smears (see Box 2). In this way, one donor mouse will provide ~5 × 108 parasitized erythrocytes.
Box 1. ROUTINE PASSAGE OF P.c. chabaudi.
Routine passage of P.c. chabaudi is performed in wild-type BALB/c mice housed under reverse light conditions.
On the morning of day 0 of the pass, infect three BALB/c mice with an i.p. injection of an ascending number of trophozoite-stage parasites: 104, 105 and 106.
On the morning of day 7 post-infection, collect 12 μl of blood from the tail tip of each of the three BALB/c pass mice, mix with 36 μl KSGH and place on ice.
Prepare a thin blood smear and enumerate parasites for each pass mouse, selecting a single mouse (on the ascending curve of infection, with a parasitemia in the range of 5–20%) as the donor for passage.
Determine erythrocyte concentration, after dilution in KSGH, using a cell counting chamber, and calculate the number of parasitized erythrocytes (pE) per ml of solution.
Perform serial dilutions, using KSG as diluent, to give the following concentrations: 105, 106 and 107 pE per ml.
Inject 100 μl of each concentration of pE i.p. into a single BALB/c mouse, giving three BALB/c mice infected with an ascending number of trophozoite-stage parasites: 104, 105 and 106.
-
Repeat Steps 2–7 every 7 d. Thus, on any given day, there is a pass mouse on the ascending curve of infection that can be used to initiate experimental infections.
! CAUTION At every 4 weeks, routine passage is initiated with a cryopreserved stock of P.c. chabaudi, so that parasites are never more than four passages from the original line.
Box 2. ENUMERATION OF P.c. chabaudi IN THIN BLOOD SMEARS.
Collect a drop of blood (approximately 2–5 μl) from the tail tip and place on a microscope slide.
Prepare a thin blood smear using the edge of a second slide and allow to air-dry.
Fix the smear by rinsing for several seconds in methanol, air-dry and stain with Giemsa’s working solution for 25 min.
Remove the stain under running tap water and dedifferentiate the smear by washing for a further 30 s.
Air-dry the slide and observe under a light microscope using an oil-immersion ×100 objective.
Enumerate the number of parasitized erythrocytes in a field of view of ~200 erythrocytes, distributed evenly in a monolayer.
Recipient mice infection
Recipient Rag2−/− BALB/c mice are infected with 2.5 × 107 transfected P.c. chabaudi schizonts.
PROCEDURE
Infection of donor mice with P.c. chabaudi ● TIMING ~7 d
1| Acclimatize donor Rag2−/− BALB/c mice to reverse light conditions for at least 1 week before infection. P.c. chabaudi infections in mice kept under reverse light conditions will undergo synchronous schizogony at 1200–1400 hours.
! CAUTION All experiments involving animals must be performed according to local and national guidelines and regulations.
2| Inject donor mice intraperitoneally (i.p.) with at least 106 parasites (obtained from BALB/c pass mice; see Box 1). Monitor the infection daily on thin blood films (see Box 2).
▲ CRITICAL STEP A high inoculum must be administered to grow parasites rapidly to a high level of parasitemia, minimizing damage to parasites and preventing anemia in mice.
! CAUTION Daily monitoring of parasitemia is essential to avoid unacceptable levels of disease severity.
■ PAUSE POINT The time taken to reach 40–50% parasitemia will depend on the initial inoculum. Rag2−/− BALB/c mice infected with 106 Pcc AS in our laboratory will reach 40–50% parasitemia by day 7 post infection.
? TROUBLESHOOTING
P.c. chabaudi isolation and culture ● TIMING ~1.5 h
3| On the morning of transfection, when parasitemia is between 40% and 50% in donor mice, isolate parasites at the late trophozoite stage; in our laboratory, this is carried out at 1100 hours. To do so, euthanize donor mice with an i.p. injection of 50 μl of Pentoject (or equivalent), and monitor them until respiration ceases.
? TROUBLESHOOTING
4| Flood the fur of the mouse with 70% (vol/vol) ethanol and move into a sterile hood.
5| Dissect to expose the thoracic cavity. Transfer 200 μl of prewarmed KSGH into the cavity, cut the aorta and collect the blood with a sterile Pasteur pipette. Alternatively, collect blood into a prewarmed syringe containing KSGH by cardiac puncture.
▲ CRITICAL STEP All media (KSGH, buffered RPMI and complete RPMI) should be prepared aseptically and should be prewarmed to 37 °C. All equipments (bench-top centrifuge, water bath and incubator) should be maintained at 37 °C before starting the transfection procedure and all disposable plastics (centrifuge tubes and tissue culture flasks) should be prewarmed to 37 °C. Failure to maintain the parasitized erythrocytes at 37 °C may result in delayed or arrested parasite maturation.
! CAUTION Respiration and responses to external stimuli must have ceased before dissecting donor mice. Mice should be exsanguinated quickly, while the heart is still beating, to maximize yield of parasitized erythrocytes.
6| Transfer the collected blood to a 15-ml centrifuge tube, placed in a water bath or beaker of water maintained at 37 °C, and pool the blood from all donor mice.
7| Measure the total volume of collected blood and, for every 1 ml of blood, add 10 ml of prewarmed buffered RPMI. The blood can be transferred into 50-ml centrifuge tubes for large volumes. Pellet the erythrocytes by centrifugation at 400g for 5 min at 37 °C.
8| Aspirate the supernatant and gently bring the cells back into suspension in 10 ml of prewarmed buffered RPMI for each 1 ml of starting blood volume. Centrifuge at 400g for 5 min at 37 °C.
9| Repeat Step 8 to wash the erythrocytes a second time.
10| Aspirate the supernatant and gently bring the cells back into suspension in the appropriate volume of prewarmed complete RPMI to bring the level of hematocrit to 3.0%.
▲ CRITICAL STEP Assuming that the donor mice are not yet anemic from the infection, hematocrit is likely to be ~50%. Therefore, if x ml (x represents any volume) of blood is collected in total, the erythrocytes would require the addition of (50/3.0) × x ml of prewarmed complete RPMI to dilute to 3.0% hematocrit. The level of hematocrit can easily be measured, for example, on a Hematospin, in all donor mice on the day of transfection.
11| Remove a 100-μl sample of cell suspension and transfer it to a 1.5-ml micro test tube for analysis (this is the T0 sample used in Step 13).
12| Transfer the erythrocytes into 50-ml (25 cm2) nonvented tissue culture flasks (10 ml of cell suspension per flask), gas the flasks gently for 30 s with a 5% O2, 7% CO2 and 88% N2 gas mixture and seal. Culture parasitized erythrocytes at 37 °C for maturation of schizonts (Fig. 1b).
! CAUTION Once the first donor mouse is exsanguinated, work must be performed quickly to minimize the time between isolation of parasites and their culture for maturation. We recommend that P.c. chabaudi culture techniques are practiced before transfection is attempted.
P.c. chabaudi schizont maturation ● TIMING ~4 h
13| To calculate the total number of erythrocytes, transfer 10 μl of cell suspension from the T0 sample to a second 1.5-ml micro test tube and dilute with the addition of 190 μl PBS. Transfer 10 μl of diluted cell suspension to a cell counting chamber and enumerate the number of erythrocytes in a 1 mm2 area. Calculate the total number of erythrocytes isolated as follows:
No. of erythrocytes × 20 (dilution factor) × 104 × x ml (total volume of cell suspension)
14| To calculate the proportion of parasitized erythrocytes, spin the remaining 90 μl of cell suspension from the T0 sample in a bench-top microcentrifuge at 2,000g for 2 min at 4 °C. Carefully aspirate the entire supernatant and measure the volume of the cell pellet. Resuspend the cells in a volume of FBS equal to that of the cell pellet to re-establish hematocrit at 50%. Prepare a thin blood smear as standard (see Box 2), and enumerate the percentage of parasitized erythrocytes and the percentage of schizonts.
15| At 2 h (T2), 3 h (T3) and 4 h (T4) after culture, remove a further 100 μl sample of cell suspension from each tissue culture flask and transfer to a 1.5-ml micro test tube for analysis. Gas the flasks gently (as in Step 12) and seal before returning them to 37 °C. Repeat Steps 13 and 14 with each new sample to monitor the maturation of P.c. chabaudi schizonts.
▲ CRITICAL STEP At T0, the mean percentage of schizonts should be 1–3%; after 4 h of culture at 37 °C, the mean percentage of schizonts is expected to be ~50%. The information collected from Steps 13 to 15 will accurately allow for the enumeration of schizonts throughout the maturation culture. Figure 2 shows the return we would expect from one donor mouse at the end of the culture.
Figure 2. Expected return following culture of parasitized erythrocytes from one P.c. chabaudi–infected mouse.
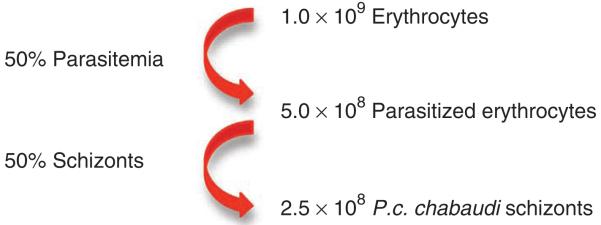
▲ CRITICAL STEP After 4 h of culture, we observe a decrease in the percentage of schizonts, presumably because of in vitro rupture of schizonts and release of merozoites. Therefore, the culture should be stopped at the peak of maturation to maximize schizont yield.
! CAUTION Enumeration of total erythrocytes, the percentage of parasitized erythrocytes and the percentage of schizonts, will routinely require 1 h of work. Therefore, the maturation culture will have progressed a further 1 h from the sampling time point. Thus, if the expected rate of maturation is observed at T2 and T3, we stop the culture at T4, remove a sample for retrospective analysis of the endpoint and estimate the number of schizonts in the culture on the basis of our observations and their expected frequency.
? TROUBLESHOOTING
Transfection of P.c. chabaudi schizonts ● TIMING 1–2 h
16| At the end of the culture, bring the erythrocytes back into suspension by gently shaking the tissue culture flask, and transfer the cell suspension into 15 ml centrifuge tubes (or 50 ml tubes for large volumes). Pellet the cells by centrifugation at 400g for 5 min at 37 °C, and aspirate the supernatant.
17| Resuspend erythrocytes in the appropriate volume of prewarmed complete RPMI to give a cell concentration of 2.5 × 107 schizonts per 1 ml of the medium. For each sample to be transfected, transfer 1 ml of cell suspension to a 1.5-ml micro test tube and pellet by centrifugation at 400g for 5 min at 37 °C. Aspirate the supernatant and proceed immediately to Step 18.
▲ CRITICAL STEP Perform transfections in duplicate for every vector, generating two separate lines of transformed parasites, which requires two controls. The first control (C1) consists of nontransfected schizonts under drug selection; it is used to determine the efficacy of pyrimethamine action. The second control (C2) includes mock-transfected schizonts without drug selection; it is used to determine the viability of matured and electroporated schizonts. Thus, for transfection of P.c. chabaudi with x vectors (x represents any number), you would require x × 2 samples + 2 control samples.
18| Mix 2–5 μg of linearized plasmid in 10 μl TE buffer with 100 μl basic parasite Nucleofector solution and transfer to a single sample tube containing 2.5 × 107 schizonts from Step 17. Gently resuspend the cells and transfer to a cuvette (supplied with the Basic Parasite Nucleofector Kit). Place the cuvette into the Nucleofector device and electroporate schizonts using program U-033.
19| Immediately following electroporation, supplement the cell suspension with 100 μl of prewarmed complete RPMI and inject intravenously (i.v.) into a single recipient Rag2−/− BALB/c mouse immobilized on a mouse restrainer.
▲ CRITICAL STEP Mice should be warmed under an infrared heat lamp for 5–10 min to dilate the tail veins. The heat source should not be closer than 30 cm from the top of the cage and mice must be monitored for signs of heat exhaustion or distress.
▲ CRITICAL STEP The tail should be wiped with 70% (vol/vol) ethanol immediately before injection using a 1-ml syringe with a 27-G, 0.5-inch needle. There must be no air bubbles or large particles in the cell suspension, and mice should be monitored for 2 h post injection for signs of embolism.
! CAUTION The maximum volume that can be injected i.v. into a mouse is 10% of total blood volume, the latter estimated as 2 ml for a 20 g mouse. Therefore, the cell suspension volume must not exceed 200 μl.
20| Repeat Steps 18–19 to generate duplicate lines of parasites transformed with each vector. In addition, for control C1, resuspend one sample of 2.5 × 107 schizonts in 100-μl Basic Parasite Nucleofector Solution (with no plasmid), supplement with 100-μl prewarmed complete RPMI and inject i.v. into a single recipient mouse. For control C2, resuspend one sample of 2.5 × 107 schizonts in 100 μl Basic Parasite Nucleofector Solution (with no plasmid), transfer to a cuvette and electroporate on program U-033, supplement with 100 μl prewarmed complete RPMI and inject i.v. into a single recipient mouse.
21| Transfection of P.c. chabaudi and injection into recipient mice would normally be complete by 1800 hours. The day of transfection is termed day 0. House recipient mice under reverse light conditions, with open access to food and standard drinking water.
Drug selection of transformed P.c. chabaudi ● TIMING 4-20 d
22| At 1000 hours on day 2, immediately before the second cycle of schizogony following transformation, prepare blood smears from all recipient mice for enumeration of parasites (see Box 2). Place recipient mice infected with transfected parasites and control C1 onto acidified drinking water supplemented with pyrimethamine. Control C2 remains on standard drinking water.
23| Monitor parasitemia daily in thin blood smears from day 5 onward.
! CAUTION Intravenous injection of 2.5 × 107 P.c. chabaudi schizonts results in a rapid and high level of parasitemia. The mouse infected with control C2 (without drug selection) should show a substantial increase in parasitemia between days 2 and 5; accordingly, it should be killed before the onset of clinical signs of disease.
▲ CRITICAL STEP The expected prepatent period for recipient mice infected with 2.5 × 107 transformed P.c. chabaudi schizonts under drug selection is 8–14 d, with a mean of 10.6 d post-transfection. In our laboratory, the reproducibility of generating transfected lines, using suitable vectors, is 100% (n = 18).
? TROUBLESHOOTING
24| Once transfected P.c. chabaudi lines reach a parasitemia of between 20% and 40%, terminally anesthetize recipient mice and exsanguinate, as detailed in Steps 4–5. Maintain the blood on ice for use in Steps 25–26.
? TROUBLESHOOTING
25| Expand each transformed line by injecting i.p. a further three recipient Rag2−/− BALB/c mice, which have been on acidified drinking water supplemented with pyrimethamine for 24 h, with 106 parasites (from Step 24).
26| Process the remaining blood (from Step 24) for cryopreservation of parasites (see Box 3), to generate level 0 stabilates for each transformed line.
Box 3. CRYOPRESERVATION OF P.c. chabaudi.
Euthanize mice at between 20% and 40% parasitemia and collect the blood from the heart into 200 μl KSGH.
Measure the total volume of collected blood, add 10% (vol/vol) glycerol and carefully mix.
Transfer 200 μl into cryogenic vials and snap-freeze the parasitized erythrocytes in liquid nitrogen or on dry ice.
-
Cryopreserved P.c. chabaudi parasites are fragile and should be stored for the long term in liquid nitrogen. Storage at −80 °C will rapidly reduce viability after 1–3 years.
! CAUTION P.c. chabaudi parasites are most reliably cryopreserved at the ring stage, before the onset of DNA replication. Trophozoites can also be cryopreserved but with potentially reduced recovery.
Characterizing transformed P.c. chabaudi ● TIMING – 5 d
27| Following expansion of transfected P.c. chabaudi lines (Step 25), monitor parasitemia from day 3 in the recipient mice until parasitemia reaches 20–40%. Terminally anesthetize recipient mice and exsanguinate, as in Steps 4–5, pool the blood and maintain on ice for use in Step 28.
28| Transformed P.c. chabaudi can be characterized in several ways: use option A to identify parasitized erythrocytes by fluorescence microscopy, option B to identify parasitized erythrocytes by flow cytometry or option C to prepare parasite pellets that may be used to extract protein and genomic DNA for genotypic analysis.
- Identify parasitized erythrocytes by fluorescence microscopy
- If transformed parasites are fluorescently tagged, place 6 μl of blood under a cover slip on a glass slide and detect fluorescence with a fluorescence microscope (Fig. 3a).
- Preparation of parasitized erythrocyte pellets
- Transfer 200 μl of infected blood to a micro test tube and dilute with the addition of an equal volume of PBS.
- Pellet the erythrocytes by centrifugation in a microcentrifuge at 2,000g for 2 min at 4 °C, aspirate the supernatant and wash the cells three times with 400 μl PBS.
- Aspirate the supernatant, snap-freeze the cell pellet on dry ice and store long term at −20 °C for DNA extraction10, or snap-freeze in liquid nitrogen and store long term at −80 °C for protein extraction.
Figure 3. Analysis of mCherry expression in transformed P.c. chabaudi.
(a) Immunofluorescent images of transformed mCherry-expressing P.c. chabaudi AS (mCherryPcc AS) and wild-type P.c. chabaudi AS (Pcc AS), demonstrating mCherry expression (red) and Hoechst labeling of DNA (blue) in the mosquito midgut (oocyst) and in each stage of the erythrocytic cycle in the mouse (ring, trophozoite, schizont). The top row shows an overlay of mCherry, Hoechst and bright-field images; the bottom row shows the mCherry image only. (b) Flow cytometry analysis of blood from mCherryPcc AS–infected mice, demonstrating mCherry expression (x axis) and Hoechst labeling of DNA (y axis). Three distinct populations of parasitized erythrocytes are gated based on their labeling with Hoechst, and the mean fluorescence intensity (MFI) of mCherry expression is shown for each population. This demonstrates that expression of the transgene increases with increasing DNA content and, therefore, parasite biomass (see Box 4 for details of flow cytometry analysis of blood). (c) Flow cytometry analysis of blood from uninfected (left), wild-type Pcc AS–infected (center) and transformed mCherryPcc AS–infected (right) mice, demonstrating mCherry expression (x axis) and Hoechst labeling of DNA (y axis). The MFI of mCherry expression in the Hoechst-positive population is shown above each graph. This demonstrates that all parasites (Hoechst-positive cells) are expressing the mCherry transgene (see Box 4 for details of flow cytometry analysis of blood). All animal experiments were carried out according to institutional guidelines (National Institute for Medical Research Ethical Review Panel) and UK Home Office regulations.
Box 4. FLOW CYTOMETRY ANALYSIS OF P.c. chabaudi–INFECTED BLOOD.
Withdraw 25 μl of blood from the tail tip and mix with 5 μl KSGH.
Dilute the blood in the ratio of 1:3 with PBS and deplete leukocytes by passing the suspension, via gravity flow, through a prewetted Plasmodipur filter (EuroProxima).
Wash the filter with 5 ml PBS and centrifuge the total effluent at 340g for 5 min at 4 °C.
Aspirate the supernatant and resuspend the erythrocyte pellet in fluorescence-activated cell sorting (FACS) buffer in a volume 10× that of the pellet.
Enumerate the number of erythrocytes on a cell counting chamber and, together with the parasitemia, calculate the number of parasitized erythrocytes (pEs). Adjust the cell suspension to 5 × 107 pE per 1 ml FACS buffer.
Transfer 100 μl cell suspension (5 × 106 pE) to a 5-ml FACS tube, add 100 μl Hoechst solution (Hoechst 33342 at 10 μg ml−1 in FACS buffer) to the cells and incubate in the dark for 10 min on ice.
Pellet the cells by centrifugation at 340g for 5 min at 4 °C and wash two times with 400 μl FACS buffer.
Following the final wash, resuspend the cells in 400 μl FACS buffer and acquire on an LSR II cytometer (Becton Dickinson) fitted with appropriate filter sets.
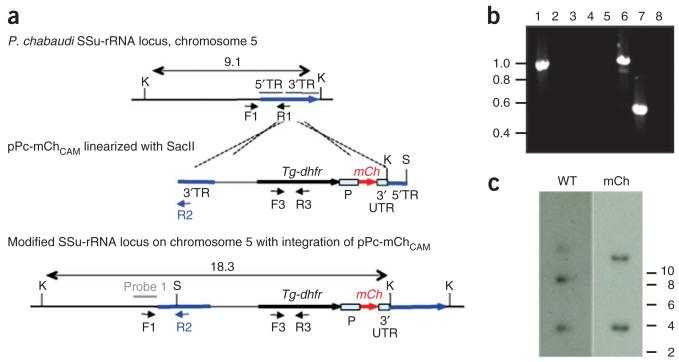
Figure 4. An example of vector design and genotypic analysis of transformed P.c. chabaudi parasites.
Parasites were transfected with plasmid pPc-mChCAM that is designed to achieve targeted integration into the small subunit ribosomal RNA (SSu-rRNA) loci on P.c. chabaudi chromosomes 5 (C5) or 6 (C6). (a) The wild-type (WT) integration locus, plasmid construct and resultant genetically modified locus after vector integration by single crossover recombination. The vector contains the gene encoding the drug-selectable marker, Tg-dhfr, the fluorescent marker mCherry (mCh) between the constitutive EF1α promoter (P), 3′ untranslated sequence (3′ UTR) and target sequences (TR) for integration. The construct was linearized with SacII (S) before transfection. (b) PCR analysis of genomic DNA showing correct integration of pPc-mChCAM into the SSu-rRNA locus on P.c. chabaudi C5. Primers C5-F1 (5′-GTAAATAAAAGCACTACTAATAAGTGTG-3′) and C6-F1 (5′-TGTAAATAAAAGCACTACTAATAAGTG-3′) are designed to anneal specifically to upstream regions of SSu-rRNA on P.c. chabaudi C5 or 6, respectively. Primer R1 (5′-TGGAGCCCTGTGATGATTC-3′) anneals with C5 and 6 SSu-rRNA sequences. Primer R2 (5′-AAGTAACGAGAAACCCAGTC-3′) anneals within the 38-bp region of pPc-mChCAM target sequence that differs significantly from the P.c. chabaudi WT SSu-rRNA sequence. Verification of the 5′ integration site in P.c. chabaudi C5; C5-F1/R2 amplify a product in mCherry-expressing P.c. chabaudi AS (mCherryPcc AS; lane 1) but not in wild-type P.c. chabaudi AS (Pcc AS; lane 2) DNA; C6-F1/R2 does not amplify a product in mCherryPcc AS (lane 3) or wild-type Pcc AS (lane 4) DNA. Parasites containing WT C5 SSu-rRNA sequences are not present; C5-F1/R1 amplify a product in mCherryPcc AS (lane 6) but not in wild-type Pcc AS (lane 5) DNA. Tg-dhfr is present in the genome of mCherryPcc AS (lane 7) but not in wild-type Pcc AS (lane 8) parasites (amplification with primers F3 (5′-ATGCATAAACCGGTGTGTCT-3′) and R3 (5′-CTACACGCGTGATGTACAGG-3′)). (c) Southern blot analysis of restricted genomic DNA to show integration of pPc-mChCAM. A probe designed against the upstream region of WT P.c. chabaudi C5 and C6 SSu-rRNA recognizes Kpn1 (K)-restriction bands of 9.1 and 4 kb, respectively. Integration of pPc-mChCAM into the C5 SSu-rRNA locus through single crossover recombination leads to shift in size from 9.1 to 18.3 kb.
29| Process the remaining blood for cryopreservation of parasites (see Box 3) to generate level 1 stabilates for each transformed line.
? TROUBLESHOOTING
Step 2: Parasitemia in donor mice is not at the expected level at day 7 post-infection. This could be because mice were infected with the wrong number of parasites or the parasites had low viability. Do not proceed with the transfection procedure; infect new donor Rag2−/− BALB/c mice with a high dose of parasites isolated during the ascending curve of infection.
Step 3: Parasites are not at the late trophozoite stage of development. This could be because donor mice were not fully acclimatized to reverse light conditions or the timing of the light/dark cycles are not optimized. Do not proceed with the transfection procedure; confirm that the light/dark cycle used provides late trophozoite-stage parasites at the expected time of day, and infect new donor Rag2−/− BALB/c mice.
Step 15: The number of schizonts is not increasing during culture. This could be because parasite maturation is delayed or has arrested because of not being maintained at 37 °C during isolation or culture or because of inhibitory culture conditions (e.g., incorrect gas mixture in the culture flask, pH of the medium or batch variation in the FBS). Extend the culture beyond 4 h and continue to monitor development; proceed with the transfection procedure only if significant schizont maturation is observed.
Step 23: (i) Parasites are observed growing in control C1 after drug selection. This could be because the drug has reduced efficacy. Make fresh stock pyrimethamine and fresh pyrimethamine drinking water, and administer to control C1 and transfected parasites; monitor the activity of the new drug in control C1. (ii) Parasites do not grow in control C2. This may be because of reduced viability of mature parasites following culture and electroporation. Continue to monitor for growth of transfected parasites and repeat transfection procedure if parasites fail to grow. However, include an additional control (C3); a recipient mouse injected with 2.5 × 107 schizonts, as for control C1, but with no drug selection. This will determine whether loss of viability occurs during culture or during electroporation.
Step 24: (i) Transfected parasites do not grow. This could be because the construct is not integrated into the parasite genome or because the site of integration, or gene product itself, has lethal effects. Check whether the sequence of the construct is correct and consider a new target site for integration. (ii) Transformed parasite lines grow at a reduced rate compared with wild-type parasites. This could be because the site of integration, or gene product itself, leads to perturbed replication or because the construct is not integrated, but exists as episomes. Continue with the expansion and characterization of transformed lines and check for integration into the parasite genome.
● TIMING
Steps 1 and 2, Infection of donor mice with P.c. chabaudi: ~7 d
Steps 3–12, P.c. chabaudi isolation and culture: ~1.5 h
Steps 13–15, P.c. chabaudi schizont maturation: ~4 h
Steps 16–21, Transfection of P.c. chabaudi schizonts: 1–2 h
Steps 22–26, Drug selection of transformed P.c. chabaudi: 14–20 d
Steps 27–29, Characterizing transformed P.c. chabaudi: 1–5 d
ANTICIPATED RESULTS
The optimization of the parasite isolation, culture, transfection and selection methods, together with the design of suitable vectors that specifically target nonessential regions of the P.c. chabaudi genome (Fig. 4a), has led to the development of a protocol tailored to the generation of transformed P.c. chabaudi. From a single donor Rag2−/− BALB/c mouse, it is possible to produce 2.5 × 108 schizonts, which can generate five transformed parasite lines (Fig. 1). The mean pre-patent period following transfection is 10.6 d and, using suitable vectors, the reproducibility of successful transfection is 100%. Further, in our laboratory, we have observed long-term stability of the integrated gene; for example, after sequential passage of Pcc AS parasites transformed with the red fluorescent protein mCherry (mCherryPcc AS; Fig. 3) into six recipient mice over a 49-d period in the absence of drug selection, all parasites maintained mCherry expression (P.J.S. and W.J., unpublished observations). Using the protocol described here, we have generated Pcc AS lines expressing a range of fluorescent, bioluminescent and model antigens that are being used in vivo to dissect many of the fundamental principles of malaria infection.
ACKNOWLEDGMENTS
We thank B. Franke-Fayard (Leiden University Medical Centre, The Netherlands) for the kind gift of plasmid pL0017. This work was supported by the Medical Research Council (MRC; reference U117584248) and the Wellcome Trust (048684). P.S. is the recipient of a fellowship from the Leverhulme Trust, and J. Lawton is funded by an MRC PhD studentship.
Footnotes
COMPETING FINANCIAL INTERESTS: The authors declare no competing financial interests.
References
- 1.Langhorne J, Quin SJ, Sanni LA. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. Chem. Immunol. 2002;80:204–228. doi: 10.1159/000058845. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson MM, Riley EM. Innate immunity to malaria. Nat. Rev. Immunol. 2004;4:169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 3.Reece SE, Duncan AB, West SA, Read AF. Host cell preference and variable transmission strategies in malaria parasites. Proc. Biol. Sci. 2005;272:511–517. doi: 10.1098/rspb.2004.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Roode JC, et al. Virulence and competitive ability in genetically diverse malaria infections. Proc. Natl. Acad. Sci. USA. 2005;102:7624–7628. doi: 10.1073/pnas.0500078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reece SE, Thompson J. Transformation of the rodent malaria parasite Plasmodium chabaudi and generation of a stable fluorescent line PcGFPCON. Malar. J. 2008;7:183. doi: 10.1186/1475-2875-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 2006;1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 7.Sponaas AM, et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114:5522–5531. doi: 10.1182/blood-2009-04-217489. [DOI] [PubMed] [Google Scholar]
- 8.Belyaev NN, et al. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat. Immunol. 2010;11:477–485. doi: 10.1038/ni.1869. [DOI] [PubMed] [Google Scholar]
- 9.Franke-Fayard B, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Menard R, Janse C. Gene targeting in malaria parasites. Methods. 1997;13:148–157. doi: 10.1006/meth.1997.0507. [DOI] [PubMed] [Google Scholar]