Abstract
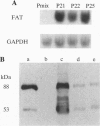
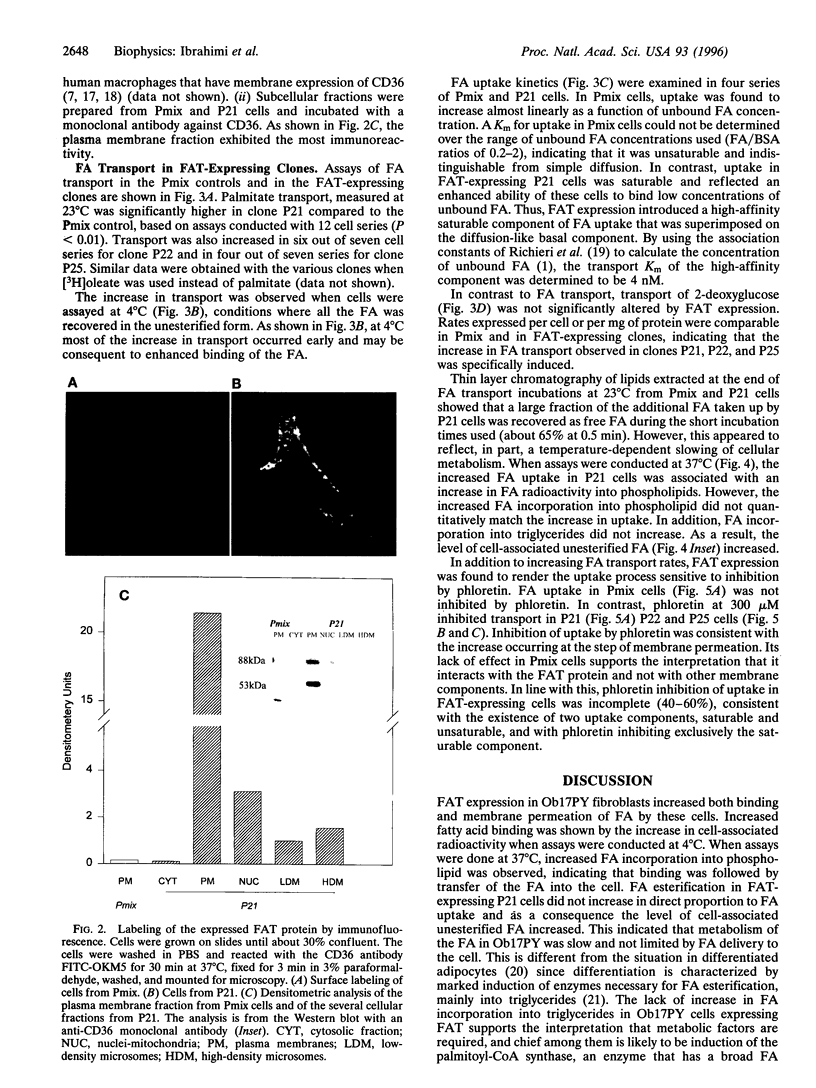
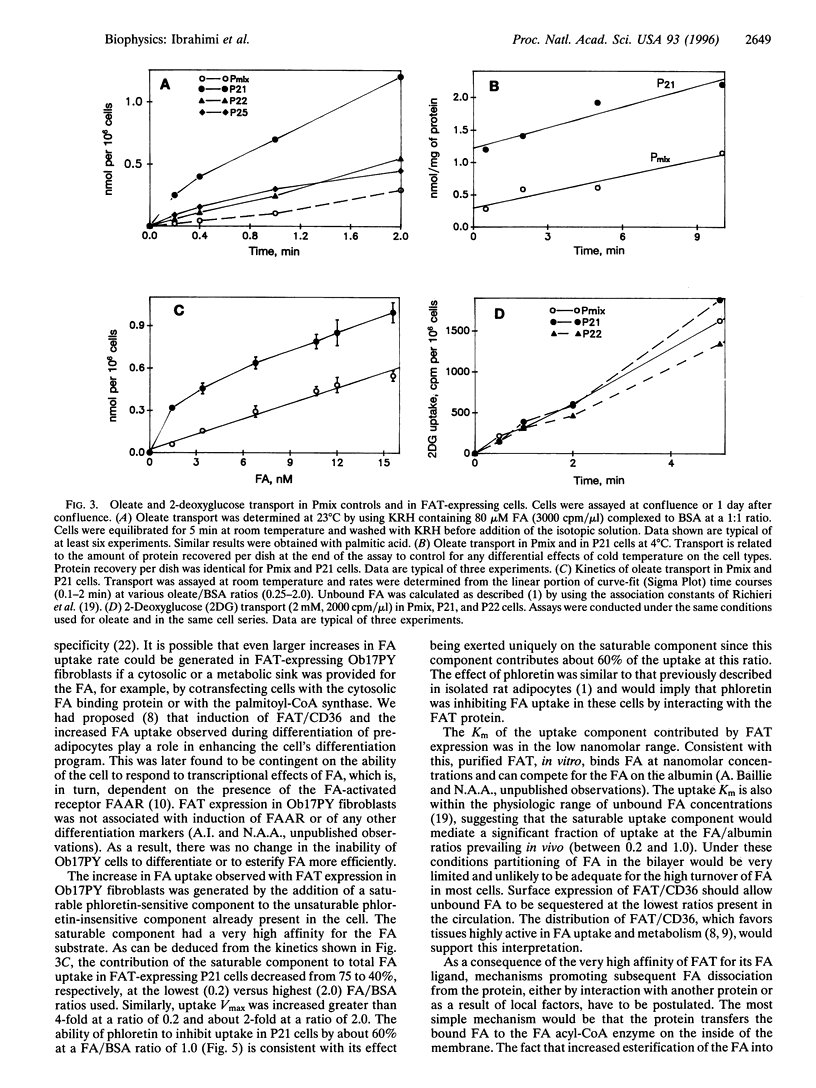
An adipocyte membrane glycoprotein, (FAT), homologous to human CD36, has been previously implicated in the binding/transport of long-chain fatty acids. It bound reactive derivatives of long-chain fatty acids and binding was specific and associated with significant inhibition of fatty acid uptake. Tissue distribution of the protein and regulation of its expression were also consistent with its postulated role. In this report, we have examined the effects of FAT expression on rates and properties of fatty acid uptake by Ob17PY fibroblasts lacking the protein. Three clones (P21, P22, and P25) were selected based on FAT mRNA and protein levels. Cell surface labeling could be demonstrated with the anti-CD36 antibody FITC-OKM5. In line with this, the major fraction of immunoreactive FAT was associated with the plasma membrane fraction. Assays of oleate and/or palmitate uptake demonstrated higher rates in the three FAT-expressing clones, compared to cells transfected with the empty vector. Clone P21, which had the highest protein levels on Western blots, exhibited the largest increase in transport rates. Fatty acid uptake in FAT-expressing P21 cells reflected two components, a phloretin-sensitive high-affinity saturable component with a Km of 0.004 microM and a basal phloretin-insensitive component that was a linear function of unbound fatty acid. P21 cells incorporated more exogenous fatty acid into phospholipids, indicating that binding of fatty acids was followed by their transfer into the cell and that both processes were increased by FAT expression. The data support the interpretation that FAT/CD36 functions as a high-affinity membrane receptor/transporter for long-chain fatty acids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Forest C., Regen D. M., Barnella U. S., Melki S. A. Metabolism of oleic acid in differentiating BFC-1 preadipose cells. Am J Physiol. 1991 Jul;261(1 Pt 1):E76–E86. doi: 10.1152/ajpendo.1991.261.1.E76. [DOI] [PubMed] [Google Scholar]
- Abumrad N. A., Park J. H., Park C. R. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984 Jul 25;259(14):8945–8953. [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993 Aug 25;268(24):17665–17668. [PubMed] [Google Scholar]
- Amri E. Z., Bonino F., Ailhaud G., Abumrad N. A., Grimaldi P. A. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem. 1995 Feb 3;270(5):2367–2371. doi: 10.1074/jbc.270.5.2367. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Greenwalt D. E., Lipsky R. H., Ockenhouse C. F., Ikeda H., Tandon N. N., Jamieson G. A. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992 Sep 1;80(5):1105–1115. [PubMed] [Google Scholar]
- Grimaldi P. A., Knobel S. M., Whitesell R. R., Abumrad N. A. Induction of aP2 gene expression by nonmetabolized long-chain fatty acids. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10930–10934. doi: 10.1073/pnas.89.22.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P., Czerucka D., Rassoulzadegan M., Cuzin F., Ailhaud G. ob17 cells transformed by the middle-T-only gene of polyoma virus differentiate in vitro and in vivo into adipose cells. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5440–5444. doi: 10.1073/pnas.81.17.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon C. M., Abumrad N. A. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol. 1993 Apr;133(1):43–49. doi: 10.1007/BF00231876. [DOI] [PubMed] [Google Scholar]
- Harmon C. M., Luce P., Beth A. H., Abumrad N. A. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J Membr Biol. 1991 May;121(3):261–268. doi: 10.1007/BF01951559. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melki S. A., Abumrad N. A. Glycerolipid synthesis in isolated adipocytes: substrate dependence and influence of norepinephrine. J Lipid Res. 1992 May;33(5):669–678. [PubMed] [Google Scholar]
- Oquendo P., Hundt E., Lawler J., Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989 Jul 14;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Pearce S. F., Wu J., Silverstein R. L. A carboxyl terminal truncation mutant of CD36 is secreted and binds thrombospondin: evidence for a single transmembrane domain. Blood. 1994 Jul 15;84(2):384–389. [PubMed] [Google Scholar]
- Richieri G. V., Anel A., Kleinfeld A. M. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993 Jul 27;32(29):7574–7580. doi: 10.1021/bi00080a032. [DOI] [PubMed] [Google Scholar]
- Schaffer J. E., Lodish H. F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994 Nov 4;79(3):427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Schwieterman W., Sorrentino D., Potter B. J., Rand J., Kiang C. L., Stump D., Berk P. D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc Natl Acad Sci U S A. 1988 Jan;85(2):359–363. doi: 10.1073/pnas.85.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Stump D. D., Zhou S. L., Berk P. D. Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am J Physiol. 1993 Nov;265(5 Pt 1):G894–G902. doi: 10.1152/ajpgi.1993.265.5.G894. [DOI] [PubMed] [Google Scholar]
- Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988 Nov 18;242(4881):1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Trezzini C., Jungi T. W., Spycher M. O., Maly F. E., Rao P. Human monocytes CD36 and CD16 are signaling molecules. Evidence from studies using antibody-induced chemiluminescence as a tool to probe signal transduction. Immunology. 1990 Sep;71(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Trigatti B. L., Mangroo D., Gerber G. E. Photoaffinity labeling and fatty acid permeation in 3T3-L1 adipocytes. J Biol Chem. 1991 Nov 25;266(33):22621–22625. [PubMed] [Google Scholar]
- Van Nieuwenhoven F. A., Verstijnen C. P., Abumrad N. A., Willemsen P. H., Van Eys G. J., Van der Vusse G. J., Glatz J. F. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem Biophys Res Commun. 1995 Feb 15;207(2):747–752. doi: 10.1006/bbrc.1995.1250. [DOI] [PubMed] [Google Scholar]