Abstract
Objective: To evaluate the long-term outcomes of surgical treatment for popliteal artery entrapment syndrome (PAES).
Materials and Methods: This study was undertaken from a retrospective review of case notes of patients treated for PAES between August 1974 and July 2013. We examined patients’ characteristics and surgical procedures, and evaluated long-term outcomes including clinical symptoms and graft or native artery patency.
Results: Twenty-nine limbs (24 patients, mean age: 32 years) underwent surgery. Popliteal arteries were occluded (n = 18) stenosed (n = 7) and normal (n = 4). Twenty-five limbs required both revasularization (interposition [n = 24] and bypass surgery [n = 1]) and myotomy. Four limbs were treated solely with myotomy. During the long-term follow-up period, three limbs required reoperation. The overall primary graft and native popliteal artery patency rates at one and 5 years were 96.3% and 91.9%, respectively.
Conclusion: The treatment of PAES with myotomy and selective revascularization achieves good short- and long-term outcomes. The use of an interposition vein graft reconstruction is associated with minimal morbidity and good long-term patency.
Keywords: popliteal artery entrapment syndrome, intermittent claudication, medial head of the gastrocnemius muscle, autogenous vein graft
Introduction
Popliteal artery entrapment syndrome (PAES) is a compression syndrome of the popliteal artery caused by musculotendinous structures in the popliteal fossa that was first described in 1879 by Stuart.1) Various anatomic forms are related to either an anomalous course of the popliteal artery, the anomalous development of the medial head of the gastrocnemius muscle (MHGM) or the presence of an accessory ligament, resulting in ischemia and vascular claudication.2) The vast majority of reported cases have occurred in males, with over half of the patients being affected before reaching 30 years of age. PAES is responsible for symptoms in 60% of young patients with ischemic pain.3)
Recurrent popliteal artery compression causes intimal damage, thrombosis,4) distal embolization, poststenotic dilatation and true aneurysm formation,5) leading to irreversible ischemic damage and eventual limb loss. Therefore, making a prompt and accurate diagnosis and providing effective treatment is required to prevent the development of severe irreparable vascular lesions, particularly because the condition generally involves younger patients.
The aim of this study was to review our clinical experiences of PAES and to evaluate the long-term outcomes of our surgical treatments.
Patients and Methods
Between August 1974 and July 2013, 29 limbs in 24 patients with PAES were admitted to Tokyo Medical and Dental University Hospital and treated surgically. All surveys and consent forms were approved by the Institutional Review Board of Tokyo Medical and Dental University Hospital. Written informed consent was obtained from all subjects. All limbs were affected by vascular ischemia and categorized according to Rutherford classification6) based on symptoms and clinical presentation at the time of the surgical procedures. The diagnosis of PAES was made using various modalities. The ankle-brachial pressure index (ABI) was calculated at rest. Duplex ultrasonography (DUS), computed tomography (CT) and magnetic resonance imaging (MRI) were performed, all of which can be used to diagnose PAES, and CT and MRI are more useful to demonstrate the popliteal artery and musculotendinous anatomy and identify other pathologies than US. Conventional angiography was performed however, recently, CT angiography was also used, which is as effective as conventional angiography with less invasiveness.
Surgical management requires releasing extrinsic compression and preserving or restoring the arterial flow. The surgical approach included a posterior S-shaped incision in the popliteal fossa in most cases.7) We preferred the posterior approach in order to inspect the origin of MHGM. If the arterial damage is minimal, myotomy of the MHGM or any abnormal musculotendinous slips may suffice.8) In the presence of occlusion or stenosis, revascularization of the affected popliteal artery in addition to musculotendinous section is required.9) Conventionally, revascularization is performed via the interposition of the affected popliteal artery with a reversed saphenous vein graft. When the affected segment was extended beyond the adductor canal or down to the popliteal trifurcation on preoperative images, bypass surgery was performed through a medial approach. Long-term follow-up data were collected to assess postoperative symptoms and evaluate the graft or native popliteal artery patency rates. Graft patency was evaluated every 3 months during the first postoperative period and every 6 months thereafter using interviews, physical examinations, ABI and DUS. If there were abnormal findings on these noninvasive tests, CT scanning was performed to confirm the graft patency.
The data are expressed as the mean ± SD (standard deviation). The statistical analyses were performed using the Stat View™ version 5 software program (Abacus Concept Inc., Berkley, California, USA). The cumulative patency rates were determined according to the Kaplan-Meier method.
Results
Patient characteristics (Table 1)
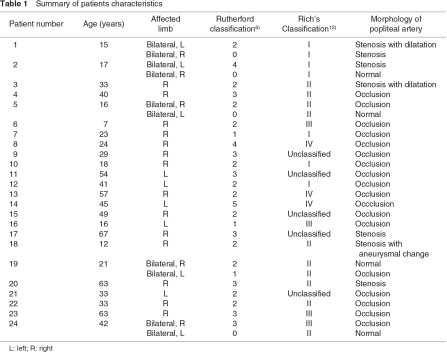
During the study period, 29 limbs in 24 patients with PAES were treated surgically. Eighteen patients were male and 6 patients were female. The mean age at the time of the surgical procedures was 32 ± 17 years (range, 7–67 years). The mean duration of symptoms before surgical treatment was 37.5 months (range, 2–216 months). The affected side of the limb was the right side in 19 limbs and the left side in 10 limbs. Five patients (20%) had bilateral PAES and were treated on both sides. The chief complaints were as follows: asymptomatic (Rutherford category 0) in four limbs, intermittent claudication (category 1–3) in 22 limbs, rest pain (category 4) in two limbs and a foot ulcer (category 5) in one limb. All four asymptomatic patients were with bilateral PAES. Among the patients with claudication, three limbs exhibited sudden onset of symptoms.
The mean preoperative ABI value at rest was 0.83 ± 0.15 (range, 0.60–1.07). The diagnosis of PAES was made using DUS and/or CT and/or MRI and/or conventional angiography. According to the radiologic findings, four popliteal arteries were intact, seven popliteal arteries were stenotic and 18 popliteal arteries were occluded. Among the seven stenotic popliteal arteries, three exhibited poststenotic dilatation and/or aneurysmal changes. Furthermore, four limbs demonstrated distal embolization of the crural artery in the radiologic findings. The PAES cases were classified according to Rich’s classification10) (Table 2) based on the radiologic and intraoperative findings: type I in seven limbs, type II in 10 limbs, type III in four limbs, type IV in three limbs and unclassified in five limbs.

Surgical management
To be free from compression, all but one procedure had been undertaken by myotomy of the MHGM or abnormal musculotendinous slips via a posterior approach. Remaining one limb, which had been previously treated with musculotendinous section at other hospital, was treated with a revascularization procedure only due to occlusion of the prior interposed vein graft. Among the 28 limbs treated with myotomy, four without arterial damage were managed solely with myotomy. Revascularization procedures were performed in 25 limbs. Twenty-four limbs were treated with interposition of the damaged popliteal artery with saphenous vein grafting via a posterior approach. In most cases of a posterior approach, the contralateral great saphenous vein was harvested in the supine position, and the patient’s position was changed to prone for the revascularization procedure. The one revascularization case that had already been treated at another hospital with the interposition of the popliteal artery and musculotendinous section was treated with a superficial femoral artery – below the knee popliteal artery bypass with autogenous vein grafting (Table 3).
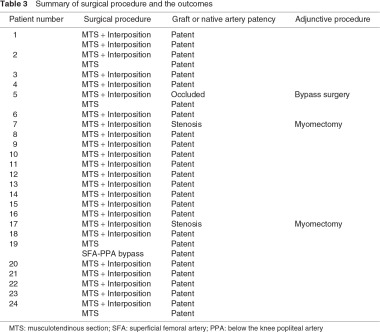
Early postoperative period
There were no severe morbidities, including nerve palsy, in the early postoperative period. In addition, there were no mortalities or amputations, and the foot ulcer healed in approximately two months. The mean postoperative ABI value was 1.00 ± 0.10 (range, 1.10–0.90).
Long-term follow-up
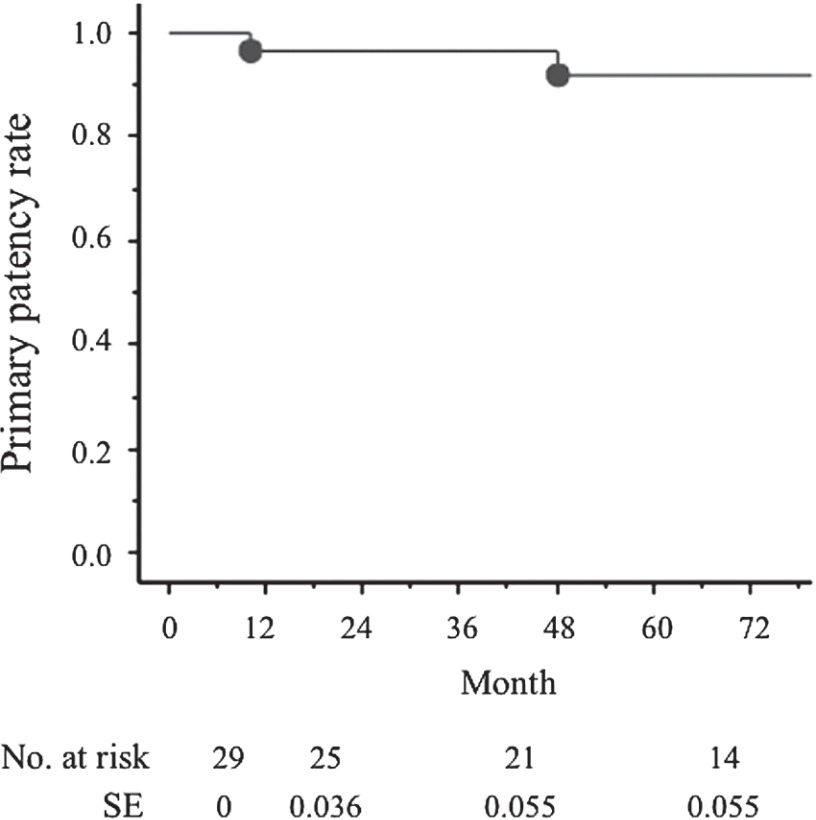
During the long-term follow-up period (mean, 68 months; range, 1–120 months), three limbs required reoperation. Two limbs developed stenosis of the interposed vein graft due to hypertrophy of the remnant musculotendinous slips, and further resection of the musculotendinous slips was performed at 10 and 40 months after the previous operation, respectively. One limb exhibited occlusion of the interposed graft at 84 postoperative months and was treated with a superficial femoral artery – below the knee popliteal artery bypass with autogenous vein grafting. The overall primary graft and native popliteal artery patency rates at one and five years were 96.3% and 91.9%, respectively (Fig. 1).
Fig. 1.
Primary graft or native popliteal artery patency rates. There are 96.3% and 91.9% at 1 and 5 years, respectively. SE: standard error.
Discussion
The clinical diagnosis of PAES relies on the recognition of a history of calf claudication in young and often athletic individuals that is sometimes accompanied by paresthesia of the foot. Although a positive stress test consisted of an ABI drop of greater than 0.50 by active plantar flexion is used for diagnostic screening,11) it is not a reliable diagnostic test12) and was not performed in this study. The symptoms of PAES occur due to arterial wall degeneration, which depends on the degree of compression, the magnitude of the forces exerted on the popliteal artery and the duration of compression.13) Left untreated, the compression mechanism frequently results in the deterioration of the popliteal artery, which may progress to eventual occlusion.14) Sinha, et al.15) reported that PAES occludes the popliteal artery in 24% of patients and changes the popliteal artery to poststenotic dilatation or aneurysmal formation in 13.5% of patients. In our cases, 62% of the affected popliteal arteries were occluded, and 10.3% of the popliteal arteries had changed to poststenotic and/or aneurysm formation. This is why the patients in the study group may suffer from compression of the popliteal artery during long-term periods. Intermittent claudication is the most common presenting symptom. However, PAES results in acute limb ischemia in 11% of patients because distal emboli may develop as a consequence of focal thrombus formation at the site of entrapment or from popliteal aneurysm formation.15,16) In our cases, three limbs exhibited the sudden onset of symptoms due to the formation of distal emboli, and four limbs demonstrated radiographically distal emboli. Therefore, the sudden onset of severe disabling claudication in a young adult without atherosclerotic risk factors is highly suggestive of popliteal artery occlusion due to entrapment. Furthermore, in patients with PAES, it is helpful to apply diagnostic procedures on the contralateral side, as entrapment occurs bilaterally in 25% to 80% of patients.7)
The primary objective of surgical treatment for PAES is to free the patient from symptoms. A further objective is to avoid damage to the arterial wall from the formation of arterial thrombi and prevent peripheral embolization. Various treatment approaches for PAES have so far been described. Surgical options include thrombectomy and/or endarterectomy with patch angioplasty, an autogenous vein bypass graft or segmental arterial resection with graft interposition using autogenous or prosthetic grafts.17) Interposition of vein grafting outperforms thromboendarterectomy and vein patching for stenosis, with a complication rate of 16.7% compared to 45.5% (P <0.01).18) The patency rate of bypass surgery using vein grafts has been reported to be 57%–65% over a period of 8–10 years.19,20) Levien, et al.17) reported a case series of 66 limbs that underwent musculotendinous section and 16 limbs that underwent segmental replacement of the occluded popliteal artery with a reversed saphenous vein. All limbs in the solely musculotendinous section group remained with a patent popliteal artery during the follow-up period (median, 3.9 years). The limbs treated with interposition with vein grafts also showed no graft occlusion (median, 4.2 years). On the other hand, di Marzo, et al.20) reported that 15 limbs that underwent revascularization procedures exhibited a patency rate of 65% (at a mean follow-up of 107 ± 8 months). The revascularization procedures consisted of nine interposition procedures with vein grafts and six bypass surgeries with vein grafts. Kim, et al.21) also reported that bypass surgery is associated with a worse patency rate than interposition procedures (5-year, 30.0% versus 85.9%; P = 0.15). Comparing these reports, it can be presumed that short interposition vein grafting has a better patency rate than long bypass vein grafting. In our revascularization group, most patients underwent interposition procedures, and revascularization procedures for PAES should be performed using interposition of the popliteal artery with autogenous vein grafts when possible. In three failure cases of primary graft patency, two grafts were occluded due to hypertrophy of the remnant musculotendinous slips. Therefore, the total resection not section of the abnormal musculotendinous slips might be performed for long-term graft patency. Therefore, most cases have been treated by the resection of musculotendinous slips in our institute.
There are several limitations to this study, the foremost being the small sample size. Although only a small number of patients were enrolled in the current study, our results demonstrated that the outcomes of surgical treatment for PAES are effective and feasible. As PAES is not a common disease, these limitations will need to be examined in the future with further data collection.
Conclusion
The treatment for PAES consists of releasing the popliteal artery from compression and preserving the popliteal arterial flow. The total resection of the abnormal musculotendinous slips might be more effective to be free from the compression. If a revascularization procedure is required, then the interposition of the affected popliteal artery should be performed with autologous vein grafting via a posterior approach to obtain long-term graft patency.
Disclosure Statement
Igari and the other co-authors have no conflicts of interest to declare.
References
- Stuart TP. Note on a Variation in the Course of the Popliteal Artery. J Anat Physiol 1879; 13: 162. [PMC free article] [PubMed] [Google Scholar]
- Molinaro V, Pagilasso E, Varetto G, et al. Popliteal artery entrapment syndrome in a young girl: case report of a rare finding. Ann Vasc Surg 2012; 26: 572.e5-9 [DOI] [PubMed] [Google Scholar]
- Collins PS, McDonald PT, Lim RC. Popliteal artery entrapment: an evolving syndrome. J Vasc Surg 1989; 10: 484-9; discussion 489-90 [DOI] [PubMed] [Google Scholar]
- Iwai T, Konno S, Soga K, et al. Diagnostic and pathological considerations in the popliteal artery entrapment syndrome. J Cardiovasc Surg (Torino) 1983; 24: 243-9 [PubMed] [Google Scholar]
- López Garcia D, Arranz MA, Tagarro S, et al. Bilateral popliteal aneurysm as a result of vascular type IV entrapment in a young patient: a report of an exceptional case. J Vasc Surg 2007; 46: 1047-50 [DOI] [PubMed] [Google Scholar]
- Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26: 517-38 [DOI] [PubMed] [Google Scholar]
- Ruppert V, Verrel F, Geppert SN, et al. Results of perioperative measurements of ankle-brachial index in popliteal artery entrapment syndrome. J Vasc Surg 2004; 39: 758-62 [DOI] [PubMed] [Google Scholar]
- Ohara N, Miyata T, Oshiro H, et al. Surgical treatment for popliteal artery entrapment syndrome. Cardiovasc Surg 2001; 9: 141-4 [DOI] [PubMed] [Google Scholar]
- Levien LJ. Popliteal artery entrapment syndrome. Semin Vasc Surg 2003; 16: 223-31 [DOI] [PubMed] [Google Scholar]
- Rich NM, Collins GJ, McDonald PT, et al. Popliteal vascular entrapment. Its increasing interest. Arch Surg 1979; 114: 1377-84 [DOI] [PubMed] [Google Scholar]
- Turnipseed WD. Popliteal entrapment syndrome. J Vasc Surg 2002; 35: 910-5 [DOI] [PubMed] [Google Scholar]
- Servello M. Clinical syndrome of anomalous position of the popliteal artery. Differentiation from juvenile arteriopathy. Circulation 1962; 26: 885-90 [DOI] [PubMed] [Google Scholar]
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48: 61S-65S; discussion 65S [DOI] [PubMed] [Google Scholar]
- di Marzo L, Cavallaro A, Sciacca V, et al. Natural history of entrapment of the popliteal artery. J Am Coll Surg 1994; 178: 553-6 [PubMed] [Google Scholar]
- Sinha S, Houghton J, Holt PJ, et al. Popliteal entrapment syndrome. J Vasc Surg 2012; 55: 252-62.e30 [DOI] [PubMed] [Google Scholar]
- Darling RC, Buckley CJ, Abbott WM, et al. Intermittent claudication in young athletes: popliteal artery entrapment syndrome. J Trauma 1974; 14: 543-52 [DOI] [PubMed] [Google Scholar]
- Levien LJ, Veller MG. Popliteal artery entrapment syndrome: more common than previously recognized. J Vasc Surg 1999; 30: 587-98 [DOI] [PubMed] [Google Scholar]
- Hoelting T, Schuermann G, Allenberg JR. Entrapment of the popliteal artery and its surgical management in a 20-year period. Br J Surg 1997; 84: 338-41 [PubMed] [Google Scholar]
- Andrilopoulos V, Papcharalambous G. The popliteal artery entrapment syndrome. Vasc Endovasc Surg 1999; 33: 357-65 [Google Scholar]
- Marzo L, Cavallaro A, Mingoli A, et al. Popliteal artery entrapment syndrome: the role of early diagnosis and treatment. Surgery 1997; 122: 26-31 [DOI] [PubMed] [Google Scholar]
- Kim SY, Min SK, Ahn S, et al. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55: 90-7 [DOI] [PubMed] [Google Scholar]