Abstract
Objectives: This study was evaluating the outcomes of endovascular aneurysm repair (EVAR) using the endowedge technique (EnT) and/or snorkel technique (SnT) for abdominal aortic aneurysms (AAAs).
Materials and Methods: The patients treated with EnT and/or SnT were retrospectively reviewed between January 2010 and June 2013. All patients underwent EVAR under general anesthesia. Bilateral femoral arterial access was obtained through bilateral femoral cut-down to place the stent graft mainbody, and brachial arterial access was obtained percutaneously to perform the EnT and/or SnT.
Results: Three patients were treated with unilateral EnT, 1 with unilateral SnT, two with bilateral SnT, and two with combined EnT/SnT. A total of 12 renal arteries was attempted to preserve, and could be successfully performed by these techniques in 11 renal arteries. After complete deployment of the endograft, intraoperative angiography showed no type Ia EL. During the median follow-up of 11 months (range: 2–22 months), no deaths nor aneurysm enlargement occurred, and all treated renal arteries were patent without further intervention.
Conclusions: Our findings suggest that the management of AAAs by EVAR with EnT and/or SnT could achieve an adequate proximal seal, and preserve renal artery perfusion in patients with unfavorable neck anatomy.
Keywords: juxtarenal aortic aneurysm, endovascular aneurysm repair, endowedge technique, snorkel technique
Introduction
Endovascular aneurysm repair (EVAR) is a widely used technique for the treatment of infrarenal abdominal aortic aneurysms (AAAs). The proximal landing zone is one of the limiting factors for EVAR, and represents the single most important factor that determines the success or failure of the treatment.1) The management of juxtarenal aortic aneurysms (JAA) with EVAR remains controversial because of the high risk associated with the procedure. Several techniques have been proposed to ensure secure proximal fixation to increase the applicability of EVAR. Fenestrated and branched endografts (FBE) can preserve the perfusion of branch vessels, with promising early-term and mid-term results.2,3) However, the strict anatomical requirements, high costs and long manufacturing delays limit the applicability of the technique, and these devices are not available except limited facilities in Japan. Therefore, some alternative treatments have also been proposed.
The Gore Excluder endoprosthesis (W. L. Gore and Associates, Flagstaff, Arizona, USA) is characteristic in that its proximal 4 mm are scalloped. The endowedge technique is used to wedge this scalloped configuration of the proximal 4 mm of the Excluder endoprosthesis against renal angioplasty balloons, which can provide an additional 4 mm of juxtarenal proximal sealing without interruption of the renal artery perfusion.4) The snorkel technique was originally described by Greenberg5) as an adjunctive procedure involving branch vessel stenting during intentional endograft coverage of the vessel origin to maintain branch perfusion. This technique could also achieve an additional proximal sealing zone. Both techniques involve a renal artery intervention (angioplasty balloon or stent) to achieve additional juxtarenal sealing zone. The present study describes our preliminary experience with these two adjunctive techniques during EVAR, and investigated the effects of challenging neck anatomy on the early- and intermediate-term outcomes of EVAR using these techniques.
Patients and Methods
Patient selection
The data for 98 consecutive patients who underwent EVAR in the Department of Vascular Surgery, Tokyo Medical and Dental University, between January 2010 and June 2013 were reviewed retrospectively. In the same period, we performed open surgery for 140 cases of AAAs and iliac artery aneurysms. All patients provided their informed consent, and approval was obtained from our Institutional Review Board for a retrospective review of the patients’ medical records and images. Among these patients, eight had undergone elective EVAR using bifurcated stent-grafts in conjunction with the endowedge technique (EnT) and/or snorkel technique (SnT) in the renal arteries for AAAs with challenging neck anatomy, which were defined as aneurysms involving the juxtarenal aorta that would require suprarenal clamping to tailor a proximal anastomosis during open repair, and cases with a massive mural thrombus in the proximal neck. The selection criteria for endovascular repair included an age older than 75 years or an age <75 years in patients with severe cardiac, pulmonary or renal dysfunction and/or a hostile abdomen. Only patients with planned intentional coverage of renal arteries with the main body endograft, and ballooning for bail-out and/or parallel adjacent placement of snorkel stents were included in this analysis. Patients with infected or mycotic AAAs, and those with ruptured AAAs, were also excluded from this analysis. Additional information, including the demographic data, surgical technique and outcome variables, was obtained through a review of the inpatient and outpatient clinical records. Prior to performing the endovascular technique, contrast-enhanced computed tomography angiography (CTA) was used to evaluate the location and size of the aneurysm and to plan the surgical approach.
Surgical techniques
All patients underwent endovascular repair under general anesthesia. Bilateral femoral arterial access was obtained through bilateral femoral cut-down in the usual manner, and guidewires were placed in the aorta. Uni- or bilateral brachial arterial access was obtained percutaneously to cannulate the guidewires into the target vessels.
The endowedge and snorkel techniques
For both techniques, a 4–6 Fr guiding sheath (Parent Plus™; Medikit Co., Ltd., Tokyo, Japan) was inserted via the brachial artery, and the target renal artery was cannulated using a 0.035-inch guidewire and a 4 Fr catheter. Once cannulated, the sheath was advanced coaxially into the target artery. From the femoral access sites, the main body endograft was advanced and positioned in the usual fashion. During the EnT, a renal angioplasty balloon was placed into the target renal artery via a brachial access sheath, and the Excluder prosthesis was positioned just distal to the balloon. The balloon was inflated to nominal pressure, and the endograft was deployed by slowly pulling on the deployment cord to release the first 2 to 3 cm of the endograft, and then advancing the device upward against the inflated renal balloon, and completing the deployment (Fig. 1A and 1B).6) We performed the EnT only for the cases of AAAs treated with the Excluder prosthesis.
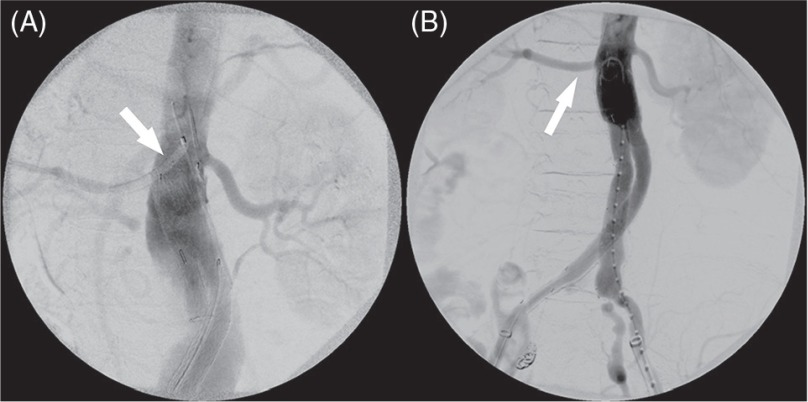
Fig. 1.
The endowedge technique. (A) Renal angioplasty balloon was placed into the target artery via brachial sheath (white arrow), and the Excluder prosthesis was positioned just distal to the balloon. (B) After the deployment of mainbody with endowedege technique, the target renal artery (white arrow) was patent.
During the SnT, the endograft main body was first deployed over the ostium of the lower renal artery. The snorkel stent was inserted into the brachial access sheath, and positioned at the site where the proximal end of the renal stent was outside the endograft. The brachial sheath was then pulled back, and the chimney stent was deployed.7) Thereafter, simultaneous balloon molding was performed using a compliant aortic balloon in the endograft and reinflation of the balloon of the renal stent (Fig. 2A and 2B). We used bare stent for SnT because of availability to insurance in Japan. Finally, endograft limbs and possible extensions were deployed, and completion angiography was performed to assess the patency of the repair and target artery, and to detect possible endoleaks (EL). We chose the SnT for the cases of AAAs, which had relatively shorter proximal neck than the cases with EnT. We could perform the SnT with any mainbody endoprosthesis.
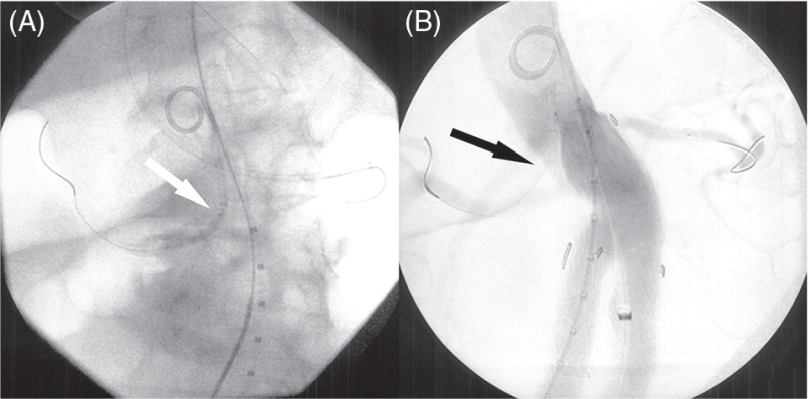
Fig. 2.
The snorkel technique. (A) Snorkel stent (white arrow) was positioned to the target vessel through brachial access sheath. (B) Simultaneous ballooning of compliant aortic balloon in the endograft and the balloon of the stent. The target vessel was patent with stent (black arrow).
Postoperative management
After the procedure, all patients underwent surveillance for at least 24 h in the intensive care unit. In the absence of specific contraindication, a contrast- enhanced CTA scan of the entire aorta was performed before discharge, usually within 5–10 postoperative days. During the follow-up period, CTA was performed at 3, 6 and 12 months and then biannually after the surgery. We investigated the aneurysm diameter, the endograft and target renal artery patency, and the presence of ELs and stent graft migration with CTA.
Results
Patient demographics
The demographic information for the study population is reported in Table 1. EVAR with EnT and/or SnT was offered to eight (seven male) consecutive patients. The median age at intervention was 76.5 years (range: 64–84 years), with a median aneurysm size of 56 mm (range: 33–85 mm). The comorbidities of this high physiological risk cohort included hypertension (seven patients), chronic obstructive pulmonary disease (five patients), malignant disease (two oral cancer patients), chronic heart failure (two patients), cerebrovascular disease (2 patients), coronary arterial disease and a hostile abdomen (one patient each). The preoperative estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula,8) and the median eGFR was 59.5 mL/min/1.73 m2 (range: 43.4–97.1 mL/min/1.73 m2). The indications for EnT were short proximal neck and/or massive mural thrombus in the proximal neck (range: 7–20 mm; median, 10 mm), and the indication for SnT was a short proximal neck (range: 3–14 mm; median, 4.5 mm) in six renal arteries of four patients. The median neck diameter was 20.6 mm (range: 18–24.4 mm).
Intraoperative management
The perioperative outcomes are shown in Table 2. The median length of the operation was 171 minutes (range: 107–511 minutes), and the median intraoperative blood loss was 235 mL (range: 100–2204 mL). A median of 105 mL of contrast agent was used (range: 100–200 mL). The aortic repairs were performed using an Excluder bifurcated endograft (W. L. Gore and Associates, Flagstaff, Arizona, USA) in six patients and an Endologic Powerlink bifurcated graft (Endologix, Inc., Irvine, California, USA) in two patients. Unilateral EnT was performed in 3 patients, and unilateral SnT for the renal artery was performed in one patient. Bilateral SnT for the renal artery was performed in 2 patients, and unilateral EnT and unilateral SnT for the renal artery were performed in two patients. We attempted to preserve 12 renal arteries, and could successfully perform eleven of them. One snorkel stent migrated, and a further approach failed due to the loss of wire access; however, the renal artery was patent by intraoperative angiography. This migrated stent was revealed in an endograft limb, then the stent was pinned to endograft wall by endoluminal ballooning. The renal artery stents used were between 4–6 mm in diameter and 15–19 mm in length. The renal angioplasty balloons were between 4–5 mm in diameter and 40 mm in length. After complete deployment of the endograft, intraoperative angiography showed no type Ia EL in all eight patients.
Postoperative management
The postoperative outcomes and follow-up data appear in Tables 2 and 3. The 30-day and in-hospital mortality rate was 0%, and one early postoperative complication of pneumonia occurred; however, the patient recovered with conservative treatment. The postoperative CTA showed a type II EL in one patient, but no type I EL was documented. During the median follow-up of 11 months (range: 2–22 months), no deaths occurred. Concerning the aneurysm morphology, there was one type II EL detected during the six-month CTA, which persisted from postoperative period. We considered that it was minor, and no reintervention was performed. The aneurysm size (± 5 mm) decreased in three patients, and stayed the same in five patients. No aneurysm became enlarged or ruptured during the follow-up period. All treated renal arteries were patent without assisted procedures, and no infarcts were detected by postoperative CTA. The mid-term evaluation of the renal function showed a median eGFR of 61.9 mL/min/1.73 m2 (range: 45.4–76.4 mL/min/1.73 m2).

Discussion
A severe limitation of EVAR is the need for an adequate proximal sealing zone to achieve durable and effective exclusion of complex AAAs.9) Recent improvements of FBE have the best track record of durable success,10) but the use of such devices mandates highly precise planning, a manufacturing delay of 6 to 12 weeks, and a high cost, because the devices are customized for each patient’s anatomy. Therefore, FBE use is limited to a few investigational centers, and most departments cannot treat JAAs endovascularly.
Even though the open operative management of AAAs with challenging neck anatomy remains the gold standard, the therapeutic efficacy and safety of endovascular treatments have been reported. The EnT has many features that make it an attractive alternative to FBE. The technique is performed with standard, commercialized endografts, the renal artery can be protected during the deployment of endografts by the angioplasty balloon, and the scallop on the graft can be wedged into alignment during deployment.11) However, the minimum amount of seal required for successful sustained exclusion of aneurysms with the EnT remains controversial. Minion, et al.4) reported that the shortest neck in patients treated with EnT alone was 9 mm. When interpreting our results, the shortest neck in patients treated with EnT alone was 7 mm; therefore, we suggest that EVAR with the EnT might successfully exclude AAAs with necks ≥7 mm.
The SnT has been used to preserve visceral artery perfusion, including renal artery perfusion, in EVAR.12,13) The advantage of the SnT is that the ready availability of conventional endograft components and peripheral stents has enabled the use of this technique for both urgent symptomatic and emergency ruptured aneurysms. Although it could be advocated that the SnT represents an easy and effective alternative to FBE,14) the SnT remains a technical challenge. The durations of the procedure and fluoroscopy times are reasonable, but the approach is complex, as demonstrated by the observation of several complications during and after the procedure.14) The main question with the SnT is whether proximal sealing of the repair can be safely achieved. The mechanism responsible for forming a seal around the chimney stents by closure of the so-called ‘gutters’ around the chimney stent is likely multifactorial.12) The ‘gutters’ located between the aorta, the stents and the endograft might also be the origin of type Ia ELs. In our study, all EVAR with the EnT were with proximal necks ≥7 mm, and excluded AAAs without type Ia ELs. Therefore, the EnT might be more suitable for the patients with necks ≥7 mm than the SnT. Longer-term follow-up will be necessary to verify that the proximal fixation remains stable and without complications, including type Ia ELs. In our series, there were no type Ia ELs, nor any aneurysm growth during the follow-up period. Furthermore, most of previous reports of the SnT used covered stents.15) Even though we used bare stent for SnT because of availability to insurance in Japan; however, we achieved the good results for exclusion of AAAs without ELs using bare stents. These issues have challenged vascular surgeons to attempt the same approaches for the management of AAAs with challenging neck anatomy in the absence of available devices for FBE.
It is important to recognize that our sample size is too small to determine which variables were associated with successful sealing. Furthermore, the late patency and stability of the chimney stent remains in question. Our median follow-up was 10 months, but patients treated for AAAs can expect to live much longer. Therefore, longer follow-up is needed to determine the durability of this procedure. However, given the steady progression of endograft technology and the fact that patients prefer less invasive treatment, some derivative of these EnT and SnT techniques will likely prevail and extend the option of undergoing EVAR to essentially all patients being treated for AAAs with challenging neck anatomy.
Conclusion
We have been able to consistently achieve an adequate proximal seal, and preserve the renal artery perfusion, in patients with unfavorable neck anatomy using commercially available devices. Although careful and longer-term follow-up is needed to determine the long-term durability of these techniques, including the preservation of graft fixation, seal and the target vessel patency, these cases suggest that the EnT and SnT are technically feasible and result in the successful exclusion of the aneurysm, and that they can be performed with low morbidity and mortality even in patients with significant comorbidities, who are deemed unfit for open repair.
Disclosure Statement
Dr. Igari and the other co-authors have no conflicts of interest to declare.
References
- Abbruzzese TA, Kwolek CJ, Brewster DC, et al. Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): an anatomic and device-specific analysis. J Vasc Surg 2008; 48: 19-28 [DOI] [PubMed] [Google Scholar]
- Greenberg RK, Sternbergh WC, Makaroun M, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg 2009; 50: 730-7.e1 [DOI] [PubMed] [Google Scholar]
- O’Neill S, Greenberg RK, Haddad F, et al. A prospective analysis of fenestrated endovascular grafting: intermediate-term outcomes. Eur J Vasc Endovasc Surg 2006; 32: 115-23 [DOI] [PubMed] [Google Scholar]
- Minion DJ, Yancey A, Patterson DE, et al. The endowedge and kilt techniques to achieve additional juxtarenal seal during deployment of the Gore Excluder endoprosthesis. Ann Vasc Surg 2006; 20: 472-7 [DOI] [PubMed] [Google Scholar]
- Greenberg RK, Clair D, Srivastava S, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair. J Vasc Surg 2003; 38: 990-6 [DOI] [PubMed] [Google Scholar]
- Ghouri MA, Dougherty KG, Krajcer Z. Technical tips for endovascular treatment of abdominal aortic aneurysms with challenging infrarenal neck anatomy using the Excluder endoprosthesis. J Endovasc Ther 2010; 17: 705-11 [DOI] [PubMed] [Google Scholar]
- Bruen KJ, Feezor RJ, Daniels MJ, et al. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J Vasc Surg 2011; 53: 895-904; discussion 904-5 [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donas KP, Torsello G, Austermann M, et al. Use of abdominal chimney grafts is feasible and safe: short-term results. J Endovasc Ther 2010; 17: 589-93 [DOI] [PubMed] [Google Scholar]
- Haulon S, Amiot S, Magnan PE, et al. An analysis of the French multicentre experience of fenestrated aortic endografts: medium-term outcomes. Ann Surg 2010; 251: 357-62 [DOI] [PubMed] [Google Scholar]
- Minion DJ, Rodriguez CC, Moore EM, et al. Technique of slow deployment of Gore Excluder endograft improves accuracy of placement. J Vasc Surg 2006; 43: 852-4 [DOI] [PubMed] [Google Scholar]
- Ohrlander T, Sonesson B, Ivancev K, et al. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther 2008; 15: 427-32 [DOI] [PubMed] [Google Scholar]
- Allaqaband S, Jan MF, Bajwa T. “The chimney graft” —a simple technique for endovascular repair of complex juxtarenal abdominal aortic aneurysms in no-option patients. Catheter Cardiovasc Interv 2010; 75: 1111-5 [DOI] [PubMed] [Google Scholar]
- Coscas R, Kobeiter H, Desgranges P, et al. Technical aspects, current indications, and results of chimney grafts for juxtarenal aortic aneurysms. J Vasc Surg 2011; 53: 1520-7 [DOI] [PubMed] [Google Scholar]
- Lee JT, Greenberg JI, Dalman RL. Early experience with the snorkel technique for juxtarenal aneurysms. J Vasc Surg 2012; 55: 935-46; discussion 945-6 [DOI] [PubMed] [Google Scholar]