Abstract
The transcription factor neurogenin3 (Ngn3) triggers islet cell differentiation in the developing pancreas. However, little is known about the molecular mechanisms coupling cell cycle exit and differentiation in Ngn3+ islet progenitors. We identified a novel effector of Ngn3 endocrinogenic function, the p21 protein–activated kinase Pak3, known to control neuronal differentiation and implicated in X-linked intellectual disability in humans. We show that Pak3 expression is initiated in Ngn3+ endocrine progenitor cells and next maintained in maturing hormone-expressing cells during pancreas development as well as in adult islet cells. In Pak3-deficient embryos, the proliferation of Ngn3+ progenitors and β-cells is transiently increased concomitantly with an upregulation of Ccnd1. β-Cell differentiation is impaired at E15.5 but resumes at later stages. Pak3-deficient mice do not develop overt diabetes but are glucose intolerant under high-fat diet (HFD). In the intestine, Pak3 is expressed in enteroendocrine cells but is not necessary for their differentiation. Our results indicate that Pak3 is a novel regulator of β-cell differentiation and function. Pak3 acts downstream of Ngn3 to promote cell cycle exit and differentiation in the embryo by a mechanism that might involve repression of Ccnd1. In the adult, Pak3 is required for the proper control of glucose homeostasis under challenging HFD.
Introduction
Understanding the mechanisms controlling the differentiation of pancreatic progenitor cells into highly specialized insulin-secreting β-cells is a major issue for future cell-based therapies for type 1 diabetes. In the last 10–15 years, considerable knowledge has been acquired on the signals and transcriptional regulations promoting β-cell development during mouse embryogenesis (1,2). These findings significantly contributed to the generation of insulin-producing cells from human embryonic stem cells or induced pluripotent stem cells by recapitulating embryonic differentiation programs (3,4). However, to date, the cells that have been produced in vitro are immature (5). These limitations highlight the importance of carrying on basic research to gain a highly detailed knowledge of the differentiation programs giving rise to functional β-cells.
The basic helix-loop-helix transcription factor neurogenin3 (Ngn3/Neurog3) has been identified as the master gene implementing the endocrine program in the mouse embryonic pancreas resulting in islet and β-cell differentiation. Indeed in Ngn3-deficient mice, all endocrine cells fail to develop and mice die of diabetes shortly after birth (6). Conversely, ectopic expression of Ngn3 is sufficient to generate all islet cell types in vivo in mice (7). Several studies support that Ngn3 directly or indirectly activates downstream target genes controlling islet subtype differentiation as well as generic programs (8–13). However, our knowledge of the genetic programs downstream of Ngn3, like those controlling cell cycle exit, migration, and maturation, is only fragmental. Therefore, we have previously performed gene expression profiling of islet cell progenitors to identify novel downstream effectors of Ngn3 (13). Among the candidate genes, we will describe here our findings on the role of the p21 protein (Cdc42/Rac1)–activated kinase 3 (Pak3) in endocrine cell differentiation and glucose homeostasis.
Pak3 is a serine/threonine kinase of the PAK family. Paks play important roles in many cellular processes including cytoskeleton dynamics, cell motility, and cell cycle regulation in brain ontogenesis and neuronal differentiation (14). PAKs are divided into two distinct groups: PAK As include Pak 1–3 and PAK Bs include Pak 4–6. Although they have also been termed PAKs, Pak 4–6 differ significantly in their structural organization and regulation (15). Pak3 is part of group A, the members of which are effectors of the Rho GTPases Rac1 and Cdc42 (16). The mouse Pak3 gene is located on position qF2 on mouse X chromosome and contains 16 coding exons. Pak3 has been previously studied in the brain because of its role in X-linked nonsyndromic intellectual disability (17). Pak3 KO mice are fertile and exhibit a normal life span but have abnormalities in synaptic plasticity and deficiencies in learning and memory (18). In Xenopus laevis, Pak3 has been demonstrated to promote cell cycle exit downstream of neurogenin during primary neurogenesis (19).
The role of Pak3 in the pancreas is unknown so far. However, recent accumulating evidence points to a potential role of Pak1, another member of PAK family group A, in the regulation and maintenance of glucose homeostasis by controlling insulin release from β-cells and skeletal muscle glucose clearance (20–22). In this report, we provide the first evidence that Pak3 regulates endocrine cell differentiation in the mouse embryonic pancreas in part by controlling cell cycle exit and is necessary for glucose homeostasis in the adult.
Research Design and Methods
Mouse Strains
Ngn3+/− and Ngn3eYFP/+ mice were generated in the laboratory (6,23). Pak3 (18) and NeuroD (24) knockout (KO) mice are a gift of Z. Jia (The Hospital for Sick Children) and J. Lee (University of Colorado, Boulder, CO), respectively. BAC-Ins1-mRFP1 mice were generated in collaboration with the Mouse Clinical Institute (Strasbourg, France) (C.M. and G.G., unpublished data) and used for purification of β-cells. All mice were kept on a mixed background, and experiments were approved by the Direction des Services Vétérinaires in compliance with the European legislation on care and use of laboratory animals.
In Situ Hybridization, Immunofluorescence, and Immunohistochemistry on Mouse Tissues
Tissues were fixed in 4% paraformaldehyde, embedded, and frozen using standard methods. Detailed protocols for in situ hybridization, immunofluorescence and immunohistochemistry on frozen sections are available on request. For BrdU detection assays, BrdU (50 mg/kg body weight) was injected into pregnant females 2 and 24 h before they were killed to assess proliferation in embryos or adult mice, respectively. The following antibodies were used: guinea pig (gp) anti-Ngn3 (1:1,000, M. Sander, University of California, San Diego, CA; 1:500, Institut de Génétique et de Biologie Moléculaire et Cellulaire [IGBMC]); gp anti-insulin (1:1,000, Linco), mouse anti-insulin (1:1,000, Sigma-Aldrich); gp anti-glucagon (1:2,000, Linco); rat anti-BrdU (1:10, AbD Serotec); rabbit anti-Pdx1 (1:2,000, C. Wright, Vanderbilt University, Nashville, TN); rabbit anti-Sox9 (1:500, AbCys); gp anti-Insm1 (1:500, C. Birchmeier, Max-Delbrück-Centrum for Molecular Medicine, Berlin, Germany); and secondary antibodies conjugated to DyLight488, DyLight549, and DyLight649 (1:500, Jackson ImmunoResearch). cRNA probes for in situ hybridization specifically recognized Ngn3 and NeuroD1 (6) and Pak3 (transcribed from a 2.2-kb mouse cDNA; IMAGE clone 30060082, which does not contain the alternative exons).
Morphometric Analysis
Quantification was performed on pancreas sections every 50 µm (embryos and newborns) and 2 mm (adults). For nucleic staining, the number of cells was counted manually using ImageJ software. For cytoplasmic staining, the immunopositive area was reported to the total DAPI+ area of pancreas using Metamorph or ImageJ softwares.
Quantitative RT-PCR Analyses
Total RNA was isolated in Tri Reagent (Invitrogen). Total RNA (1 μg) was used for cDNA synthesis using the Transcriptor Reverse Transcriptase (Roche). Quantitative PCR was performed using mouse-specific TaqMan probes recognizing Ngn3 (Mm00437606_s1), Pak3 (Mm00435482_m1, which recognizes all the isoforms), Pak1 (Mm00440612_m1), Pak2 (Mm01170646_m1), Ins1 (Mm01259683_g1), Gcg (Mm01259683_g1), Sst (Mm00436671_m1), Ppy (Mm00435889_m1), Gip (Mm01259683_g1), Sct (Mm00441235_g1), Cck (Mm00446170_m1), Tph1 (Mm00493794_m1), Pax4 (Mm01159036_m1), Arx (Mm00545903_m1), and NeuroD1 (Mm01280117_m1) with Light Cycler 480 Probes Master mix (Roche) on Light Cycler 480 (Roche). Gene expression was normalized to Rplp0 (Mm01974474_gH) expression levels. For the analysis of sorted YFP+ and YFP− cells from Ngn3eYFP/+; Pak3 KO or Pak3 wild-type (WT) E15.5 embryos, RNA was isolated with the NucleoSpin RNA XS kit (Macherey-Nagel) and linearly amplified and converted into cDNA with the NuGen Ovation PICO WTA System (NuGen) according to the manufacturer’s instructions. cDNA (45 ng) was used for one reaction of qPCR. Primers to determine the expression of cell cycle regulators (Ccnd1–3, Ccna2, Ccnb1–2, Ccne1–2, Cdk1, Cdk2, Cdk4, Cdk6; Cdkn1a-c, Cdkn2b, and Cdkn2d) were designed using the Universal Probe Library Centre (Roche), and sequences are available on request.
Statistics
Values are presented as mean ± SD or SEM. P values were determined using the two-tailed Student t test with unequal variance. P < 0.05 was accepted as statistically significant.
Cell Culture, Small Interfering RNA Treatment, and Western Blot
Min6B1 cells were provided by P. Alban (University of Geneva, Geneva, Switzerland) with permission from J.-I. Miyazaki (University of Osaka, Japan) who produced the maternal MIN6 cell line (25) and maintained as previously described (22,26). Cells were harvested in lysis buffer (20 mmol/L Tris-Cl pH 7.5, 2 mmol/L dithiothreitol, 20% glycerol, 400 mmol/L KCl, and protease inhibitors), and lysates were cleared by centrifugation. Proteins present in lysates were resolved by 10% SDS-PAGE and detected by immunoblotting. Membranes were incubated with goat anti-Pak3 antibody (1:200, Santa Cruz Biotechnology) overnight and then with donkey anti-goat antibody conjugated to horseradish peroxidase (1:10,000; Santa Cruz Biotechnology). Bands were visualized by enhanced chemiluminescence (Millipore; Immobilon Western). For Pak3 knockdown experiments, Min6B1 cells were transfected with 66 nmol/L of small interfering RNA (siRNA) oligonucleotides against Pak3 (PAK3 siGENOME SMART pool; Dharmacon) using Lipofectamine2000 (Invitrogen). Cells were harvested for RNA extraction and cell cycle analysis 48 h after transfection.
Mouse Islet Purification
Mouse islet purification was performed as previously described (27). In brief, mice were euthanized and injected with Type V Collagenase solution (Sigma-Aldrich C9263) directly into the common bile duct to perfuse the pancreas. Pancreas was dissected out and digested, and islets were handpicked after several purification steps and kept in culture overnight before harvesting for fluorescence-activated cell sorter (FACS) sorting or RNA isolation.
Preparation of Single-Cell Suspensions and Flow Cytometric Cell Sorting
Dissected pancreata from E15.5 Pak3 WT and Pak3 KO embryos were prepared as previously described (23). In brief, E15.5 pancreata or isolated adult islets were dissociated in 0.05% trypsin at 37°C for 2–10 min. Digestion was inactivated by the addition of FCS, and cells were filtered (70 μmol/L; Falcon 35-2360), centrifuged, and resuspended in Hanks’ balanced salt solution/FCS (2%) or PBS 1×/EGTA 10 mmol/L/FCS 2% for sorting. Flow cytometric sorting was performed on a FACSAria (Becton, Dickinson and Company). Sorted cells were then harvested for RNA extraction. For cell cycle analysis, suspension of Min6B1 cells was stained with propidium iodide (15 μg/mL) and processed on a FACSCalibur (Becton, Dickinson) using Cell Quest Pro acquisition software (version 5.2.1), and results were analyzed with ModFit LT 3.2 software (Verity Software House).
Metabolic Studies
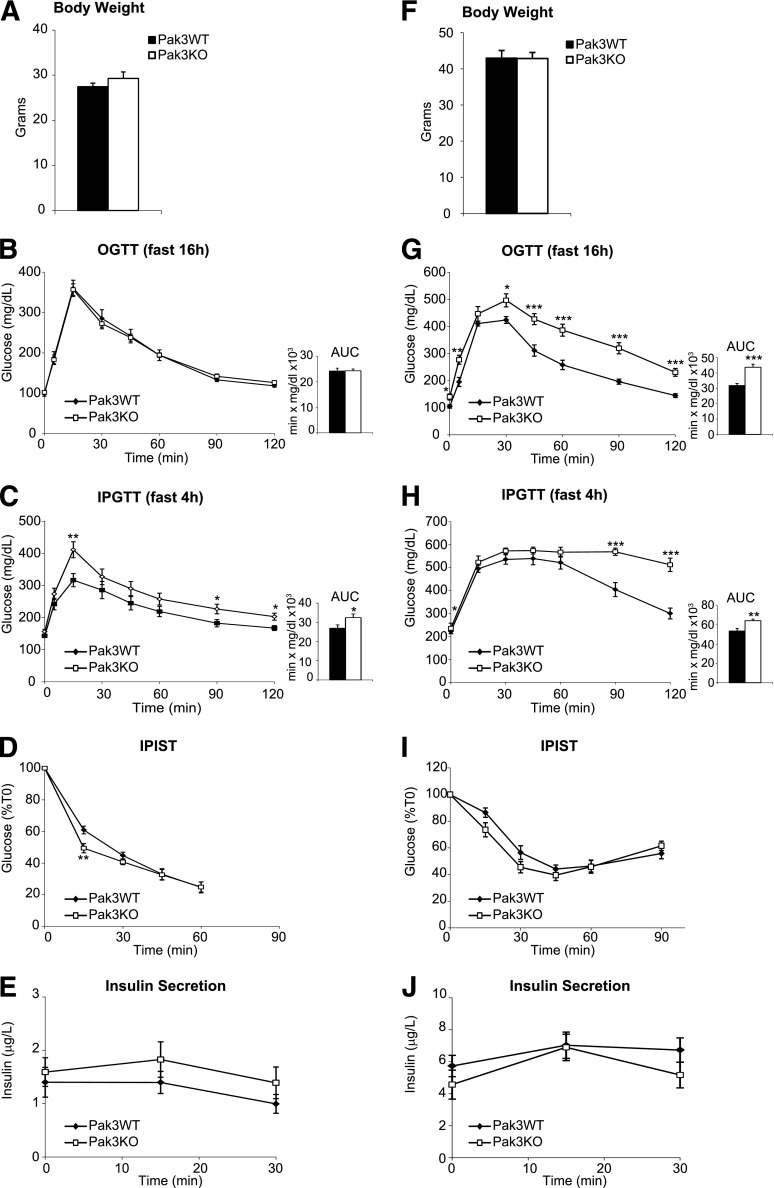
During metabolic studies, mice were fed with normal chow diet (DO3; SAFE) or high-fat diet (HFD) (D12492; Research Diets) from weaning to the time of sacrifice. For oral glucose tolerance test (OGTT), 16 h–fasted, 17-week-old males received glucose by intragastric gavage (2 g/kg body weight of 15% d-glucose). For intraperitoneal glucose tolerance test (IPGTT), 4 h–fasted, 18-week-old males received glucose by intraperitoneal injection (2 g/kg body weight of 15% d-glucose). For OGTT and IPGTT, circulating blood glucose was measured in tail blood at 0, 5, 15, 30, 45, 60, 90, and 120 min using Glucofix Sensor (A. Menarini Diagnostics). For the intraperitoneal insulin sensitivity test, 6 h–fasted, 20-week-old males were given an intraperitoneal injection of human insulin (1 IU/kg, Umuline; Eli Lilly and Company). Circulating blood glucose was measured in tail blood at 0, 15, 30, 45, 60, and 90 min. Insulin secretion was measured in tail blood during IPGTT at 0, 15, and 30 min.
Results
Ngn3-Dependent Expression of Pak3 in Islet Progenitor Cells
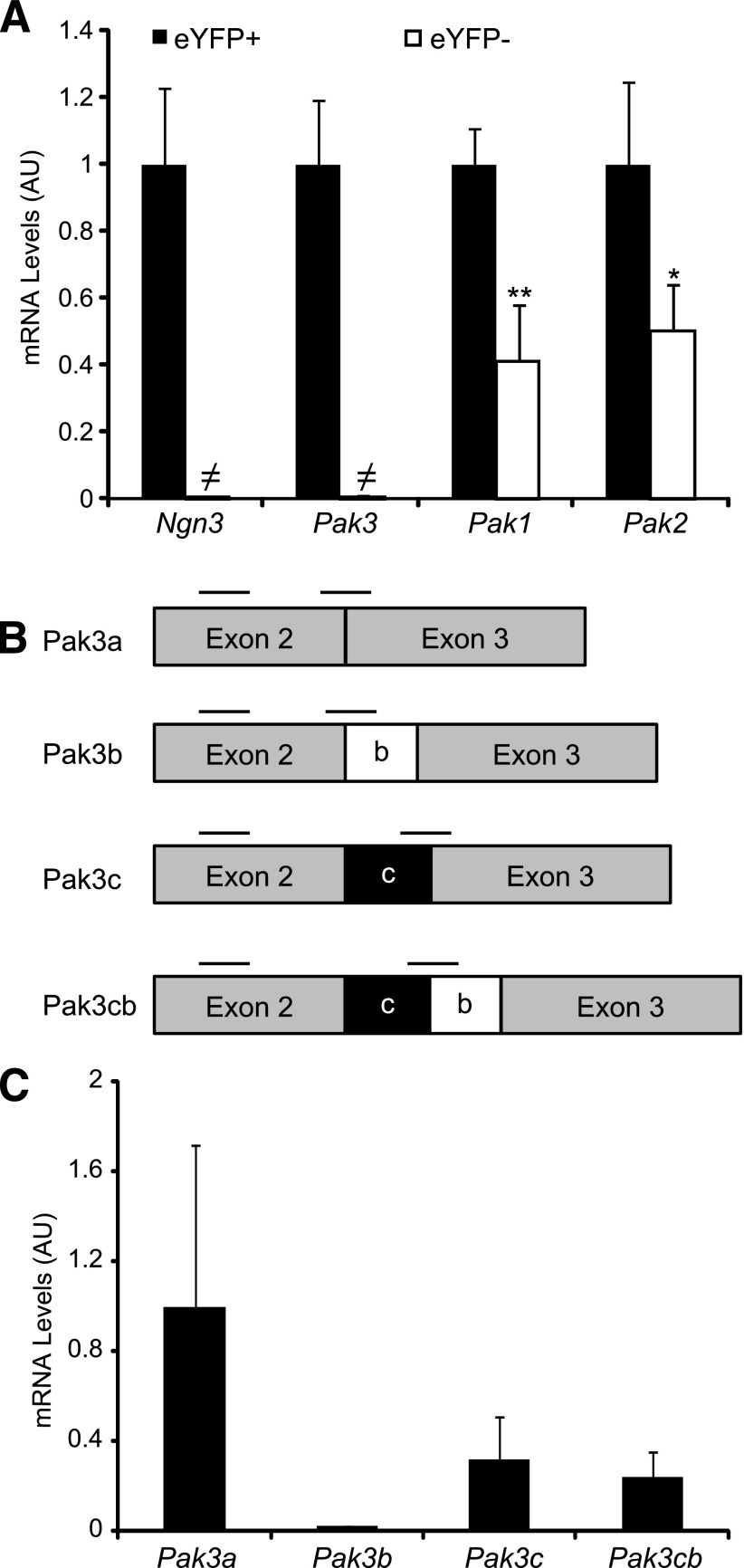
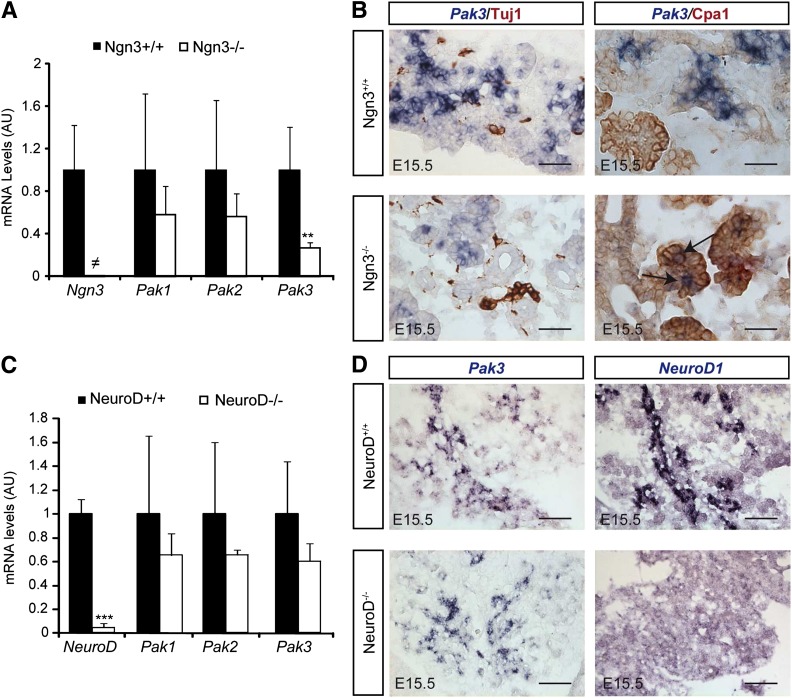
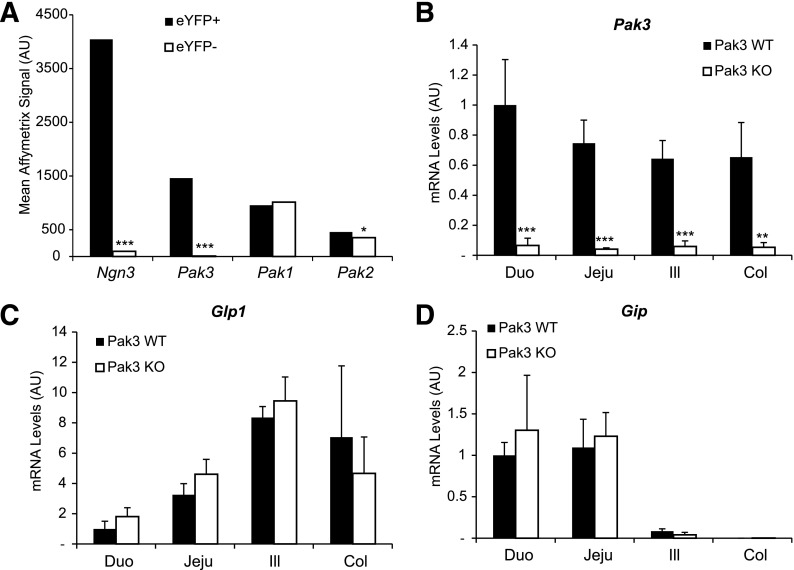
To identify the panel of genes activated specifically in islet progenitor cells, we previously determined the genes differentially expressed in eYFP+ versus eYFP− cells purified from E15.5 Ngn3eYFP/+ pancreata (13,23). Importantly, due to the greater stability of eYFP protein compared with Ngn3, the eYFP+ population includes Ngn3+ progenitor cells as well as their endocrine descendants. Interestingly, we found that the p21 protein–activated kinase Pak3 was enriched 75-fold in eYFP+ cells, demonstrating that this kinase is endocrine specific and might be an effector of Ngn3 function. Real-time quantitative PCR (RT-qPCR) experiments confirmed the specific expression of Pak3 in eYFP+ cells (Fig. 1A), and accordingly Pak3 transcripts (corresponding to all the isoforms) can be found in a subpopulation (∼62%) of Ngn3+ endocrine progenitors (Fig. 2E, arrows). The four splice variants of Pak3 (Fig. 1B) described in the brain (28,29) have been detected by RT-qPCR on E15.5 WT pancreata (Fig. 1C). Pak3 expression is strongly decreased in Ngn3-deficient embryos but is unchanged in mice lacking NeuroD (Fig. 3). However, ∼30% of Pak3 transcripts (Fig. 3A) can still be detected in Ngn3 mutant mice compared with controls due to ectopic expression (see discussion) in developing Cpa1+ acinar cells (Fig. 3B, right column, arrows). In contrast to Pak3, Pak1 and Pak2 transcripts are found both in endocrine and nonendocrine cells (Fig. 1A and Fig. 3). Thus, Pak3 is a novel endocrine-specific gene that relies on Neurog3 but not on NeuroD.
Figure 1.
Expression of Pak3 and its isoforms in mouse embryonic pancreas. A: RT-qPCR on eYFP+ and eYFP− cells sorted from Ngn3eYFP/+ E15.5 embryonic pancreas revealed that Pak3 is expressed in eYFP+ endocrine cells, whereas Pak1 and Pak2 mRNAs are found in both endocrine (eYFP+) and nonendocrine (eYFP−) populations. B: mRNA structure of the four Pak3 mouse transcripts identified in the brain. Black bars represent primer position for RT-PCR; b and c boxes represent the alternative exons. C: RT-PCR revealing the presence of all known Pak3 isoforms in E15.5 WT embryonic pancreas, with Pak3b being less expressed. A and C: Data are represented as mean ± SD of n = 4. AU, arbitrary units. *P < 0.5; **P < 0.01; ≠, not detected.
Figure 2.
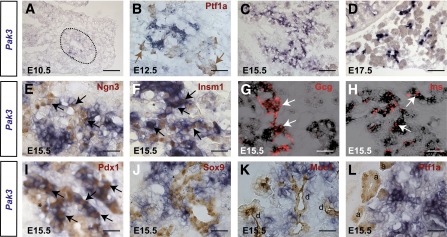
Pak3 is expressed in endocrine progenitors and developing endocrine cells in the embryonic pancreas. A–D: In situ hybridization (blue) for Pak3 mRNA revealed expression throughout pancreas development. Dotted line delineates pancreatic domain. In situ hybridization (blue) for Pak3 mRNA followed by immunohistochemistry (brown) or immunofluorescence (red) for different endocrine (E–I) and exocrine (J–L) markers in E15.5 WT embryos, as well as a multipotent progenitor marker at E12.5 (B). Pak3 transcripts are detected exclusively in the endocrine lineage, both in Ngn3+ endocrine progenitors and their descendant cells (Insm1+ or hormone+). Black and white arrows indicate costaining. Brown arrows point to Ptf1a+ multipotent progenitor tip domain. I: Pdx1 low (light brown, *) marks trunk pancreatic progenitors, and Pdx1 high (dark brown, arrows) marks β-cells. Scale bar, 50 (A–D) and 20 μm (E–L). d, duct; a, acini.
Figure 3.
Pak3 depends on Ngn3 but is independent of NeuroD. A: RT-qPCR for Pak1–3 and Ngn3 on E15.5 pancreata from Ngn3 KO and control littermates revealed a downregulation of Pak3 but not of Pak1 and -2 in the absence of Ngn3. B: In situ hybridization for Pak3 (blue) followed by ICC (brown) for the pan neuronal marker (Tuj1) or the acinar marker (Cpa1) in E15.5 Ngn3-deficient and control embryos showing ectopic expression of Pak3 in acinar cells (arrows). Scale bars, 50 μm. C: RT-qPCR for Pak1–3 and NeuroD1 on E15.5 pancreata from NeuroD KO and control littermates. D: In situ hybridization for Pak3 and NeuroD1 in E15.5 NeuroD-deficient and control embryos. Pak3 expression is independent of NeuroD. Scale bars, 20 μm. A and C: Data are represented as mean ± SD on n = 4. AU, arbitrary units. **P < 0.01; ***P < 0.001; ≠, not detected.
Pak3 Expression Persists in Embryonic and Adult Islet Cells
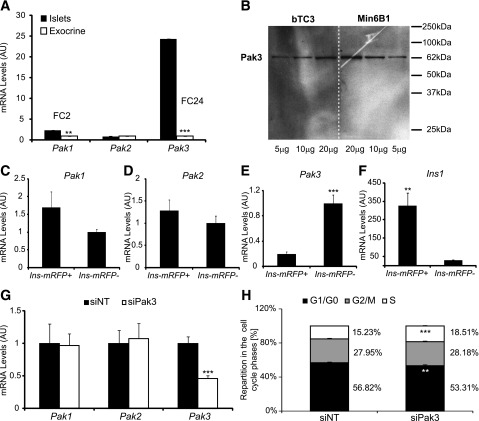
Starting at E10.5, Pak3 transcripts can be detected throughout pancreas development in a typical endocrine pattern (Fig. 2A–D). In the absence of a working anti-Pak3 antibody, we performed in situ hybridization followed by immunohistochemistry for endocrine markers to further characterize Pak3-expressing cells. We found that Pak3 is initiated in islet progenitors (Fig. 2E) but next maintained in more differentiated descendant cells, here stained for Insm1, a direct target of Ngn3 (Fig. 2F), as well as in developing α- (Fig. 2G) and β-cells (Fig. 2H and Pdx1Hi in Fig. 2I). About 80% of Insm1+ cells express Pak3, whereas Pak3 transcripts are found in almost all α- and β-cells at E15.5. In contrast, Pak3 was not found in multipotent progenitors, as supported by the restricted expression in the E10.5 pancreatic bud (Fig. 2A) and as Pak3 seems excluded from Ptf1a+ tip cells (30) (Fig. 2B) at E12.5. Bipotential ductal/endocrine epithelial cord cells (31) (Pdx1Low in Fig. 2I and Sox9+ in Fig. 2J) also seem devoid of Pak3, although it is difficult to completely exclude that a few cells might express the gene. Pak3 is also not expressed in developing acinar (Ptf1a+), ductal (Muc1+), or neuronal (Tuj1+) cells (Fig. 2K–L and Fig. 3B). Next we explored the expression of Pak3 in the adult pancreas by RT-qPCR on purified islets. Pak3 was found strongly enriched (fold change [FC] 24) in islets compared with exocrine tissue, as well as, to a lower extent, Pak1 (FC 2), in contrast to Pak2, which was found equally distributed between both compartments (Fig. 4A). To determine whether the Pak genes were expressed in β-cells, we took advantage of Ins1-mRFP mice generated in the laboratory where the monomeric red fluorescent protein is expressed in β-cells (C.M. and G.G., unpublished observations). Ins+/mRFP+ β-cells were FACS sorted from purified islets. Pak3 transcripts were readily detected in adult mouse β-cells; however, the data suggest that higher levels of Pak3 are found in non–β-islet cells (Fig. 4E). Pak1 and Pak2 were similarly expressed in β versus other islet cells (Fig. 4C and D). In agreement with the presence of Pak3 in β-cells, Pak3 protein is detected as a 62-kDa peptide in the β-cell lines bTC3 and Min6B1 (Fig. 4B). Taken together, these data indicate that Pak3 is expressed first in Ngn3+ endocrine progenitors and next in maturing hormone-positive islet cells as well as in adult islet cells, suggesting that this kinase might regulate pancreatic endocrine cell development and function.
Figure 4.
Pak3 in adult islet cells. A: RT-qPCR for Pak1–3 on purified islets and exocrine tissue from adult CD1 mice (n = 4) revealed strong expression of Pak3 in islets. B: Western blot for Pak3 on protein extracts from bTC3 and Min6B1 β-cell lines. Pak3 protein is detected as a band of 62 kDa that corresponds to the different Pak3 splice variants that display relatively identical electrophoretic migration. C–F: RT-qPCR for Pak1–3 on Ins/mRFP+ sorted cells from adult islets purified from BAC-Ins1-mRFP1 transgenic mice. Ins1 showed as a control of efficient sorting of β-cells. G: RT-qPCR for Pak1–3 on Min6B1 cells treated with nontargeting siRNA (siNT) or Pak3 siRNA (siPak3) for 48 h. H: Cell cycle analyses of Min6B1 cells treated with siNT or siPak3 by flow cytometry showing an increased number of cells in S phase upon Pak3 knockdown in Min6B1 cells. A and C–H: Data are represented as mean ± SD on n = 4. AU, arbitrary units. **P < 0.01; ***P < 0.001.
Pak3 Promotes Cell Cycle Exit and β-Cell Differentiation in the Embryonic Mouse Pancreas
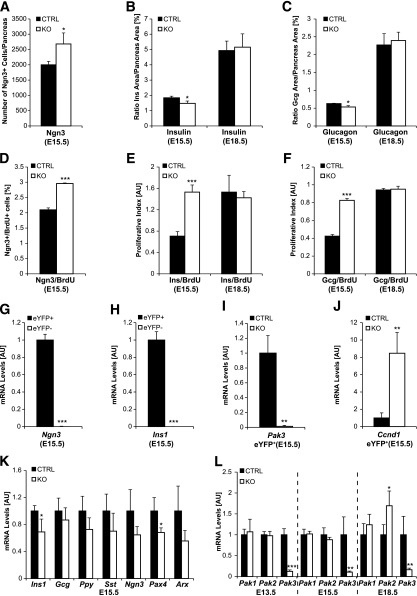
To determine the role of Pak3 in endocrine cell development, we characterized the pancreatic phenotype of Pak3 KO embryos (18). At E15.5, we observed a small but significant decrease in the number of insulin- and glucagon-positive cells (Fig. 5B and C) concomitantly with a 1.25-fold increase of the total number of Ngn3+ progenitor cells (Fig. 5A). Reduction of insulin 1 and Pax4 transcripts (Fig. 5G) further supports impaired β-cell differentiation. The modest reduction in α-cell number was not sufficient to reveal any changes in Arx or Gcg expressions (Fig. 5K). Pak3 expression is initiated in Ngn3+ islet progenitors that are mainly postmitotic (32,33). Accordingly, Pak3 is excluded from dividing cells in the embryonic pancreas (not shown). We speculated that, as it is the case during Xenopus neurogenesis (19), the increased number of Ngn3+ progenitor cells observed in Pak3-deficient embryos might result from a failure to exit the cell cycle. Quantification of Ngn3+/BrdU+ double-positive cells revealed that the number of proliferating Ngn3+ cells is indeed increased in the absence of Pak3 (FC 1.5) (Fig. 5D). Concomitantly, we also observed more dividing insulin- and glucagon-positive cells (FC 2) (Fig. 5E and F). All these observations were confirmed ex vivo, in cultures of embryonic pancreatic explants treated with Pak3 morpholinos (not shown). In line with the augmented proliferation of β-cells at E15.5, knockdown of Pak3 in Min6b1 cells leads to an increase in the population of cells in S phase (Fig. 4G and H). Importantly, expression of the Ccnd1 gene (encoding cyclin D1) was eight times higher in purified YFP+ islet progenitor cells when Pak3 was lacking compared with controls (Fig. 5G–J), whereas the expression of other cyclin, Cdk, or cell cycle inhibitor (including Cdkn1a) genes tested (see research design and methods) was unaffected (data not shown). Neither the proliferation rate nor the number of α- and β-cells was altered at E18.5 (not shown and Fig. 5B, C, E, and F) or in neonates (Supplementary Fig. 1 and not shown for α-cells) in Pak3-deficient mice. Pak2 was upregulated at E18.5, suggesting a potential functional compensation (Fig. 5H). Together these data suggest that during a specific time window of pancreas development, Pak3 is required to control cell cycle exit and proper differentiation of islet cells by a mechanism that could involve the repression of Ccnd1 transcription.
Figure 5.
Increased islet progenitors and β-cell proliferation together with impaired β-cell differentiation in Pak3-deficient embryos. Differentiation (A–C) of endocrine cells was explored at E15.5 and E18.5 in Pak3-deficient (KO) and control mice (CTRL) by immunofluorescence for Ngn3, insulin, and glucagon and quantified. Proliferation (D–F) was assessed and quantified in parallel by evaluating cells in S phase by costaining for BrdU (2-h pulse). The number of Ngn3+ islet progenitor cells increases at E15.5 (A), whereas the number of α- and β-cells is reduced at E15.5, but not at E18.5 (B and C), in Pak3-deficient embryos compared with controls. Ngn3 cells (D) as well as developing α- (F) and β-cells (E) proliferate more in Pak3-deficient embryos at E15.5. G–J: eYFP+ cells were sorted from E15.5 Ngn3eYFP/+ pancreas (littermates) that were either Pak3 KO or heterozygous controls and analyzed by RT-qPCR. Ngn3- and insulin-expressing cells (due to the stability of the eYFP protein) are purified in the eYFP+ population (G and H) and Pak3 is lost as expected in eYFP+ cells from Pak3 KO embryos (I). Transcription of cyclin D1 (Ccnd1) is upregulated in eYFP+ cells from Pak3 KO embryos compared with controls (J). K: RT-qPCR for different endocrine markers in Pak3 KO and control pancreas at E15.5. L: RT-qPCR for Pak1–3 in Pak3 KO and control pancreas from E13.5, E15.5, and E18.5 embryos. For each experiment, three to four control and mutant samples were analyzed. Data are represented as mean ± SD. AU, arbitrary units. B and C: Pancreas area = DAPI area. E and F: AU = number of BrdU/Ins+ or Gcg+ cells/Ins or Gcg area × 10,000. *P < 0.05; **P < 0.01; ***P < 0.001.
Pak3 Is Not Essential for Enteroendocrine Cell Differentiation
Very similar genetic regulatory cascades control pancreatic and intestinal endocrine cell differentiation (34). Like in the pancreas, Ngn3 is essential for endocrine differentiation in the intestine (35,36). Accordingly, we found that Pak3 is strongly enriched when we analyzed the transcriptome in eYFP+ endocrine cells purified from embryonic (not shown) and adult small intestines (FC 159) (Fig. 6A) of Ngn3eYFP/+ mice. To determine if Pak3 is required for the differentiation of enteroendocrine cells, we performed RT-qPCR on 3-week-old intestinal segments from Pak3 WT and Pak3 KO males. We found that Pak3 is expressed from the duodenum to the colon, and, as expected, Pak3 is strongly downregulated in the KO (Fig. 6B). However, no effect of Pak3 deficiency has been observed on mRNA levels of the incretin hormones Glp1 and Gip (Fig. 6C and D) or other markers of differentiated enteroendocrine cells, such as Sct, Cck, and Tph1 (not shown). In contrast to Pak3, Pak1 and Pak2 are expressed in endocrine cells (eYFP+), suggesting that they could functionally compensate for the absence of Pak3, but also in nonendocrine (eYFP−) intestinal cells (Fig. 6A). However neither Pak1 nor Pak2 mRNA levels are affected in Pak3 KO (not shown). These results suggest that despite being strongly expressed in the enteroendocrine lineage, Pak3 is not absolutely required to complete hormonal gene expression and endocrine differentiation in the intestine.
Figure 6.
Pak3 is expressed in enteroendocrine progenitor cells but is not necessary for the differentiation of Glp1- and Gip-expressing L and K cells in the mouse intestine. A: Schematic representation of Affymetrix data comparing expression levels of Ngn3, Pak1, Pak2, and Pak3 in FACS-purified eYFP+ and eYFP− cells from duodenal crypt (n = 3) of Ngn3eYFP/+ adult mice showing specific expression of Pak3 in the enteroendocrine lineage. RT-qPCR for Pak3 (B), Glp1 (C), and Gip (D) on Pak3 WT and Pak3 KO intestinal segments from 3-week-old males (n = 3 for WT and n = 4 for KO). Data are represented as mean ± SD. AU, arbitrary units; Duo, duodenum; Jeju, jejunum; Ill, ileum; Col, colon. *P < 0.05; **P < 0.01; ***P < 0.001.
Impaired Glucose Homeostasis in Pak3-Deficient Mice
The expression of Pak3 in adult islets prompted us to determine the role of this gene in the control of glucose homeostasis, which had not been explored so far in Pak3-deficient mice. We did not detect any gross abnormalities in pancreatic islet size or cellular composition in Pak3-deficient mice (not shown). To explore glucose clearance, we generated cohorts of Pak3-deficient and control adult males and performed metabolic studies under normal diet or HFD (Fig. 7). Mice did not develop overt diabetes, and no difference in body weight (Fig. 7A and F) was observed in any diet between WT and Pak3 KO mice. Under classical diet, glucose homeostasis is mildly perturbed in Pak3-deficient mice, which are slightly glucose intolerant when administrated intraperitoneally but not orally (Fig. 7B and C). However, when we challenged mice with an HFD, basal glucose levels were higher and glucose clearance was clearly impaired in Pak3 KO males compared with controls independently of the route of glucose administration (Fig. 7G and H). In all feeding conditions, we observed a trend for decreased insulin sensitivity in mutant mice (Fig. 7D and I), but insulin secretion appeared unaltered (Fig. 7E and J). α- and β-Cell mass and proliferation were unchanged and we did not observe any compensatory increase of other Paks in KO mice under HFD compared with controls (Supplementary Fig. 2). Taken together, these results suggest that Pak3 is necessary to maintain normal glucose homeostasis, particularly under challenging conditions.
Figure 7.
Impaired glucose homeostasis in Pak3-deficient mice. Exploration of glucose metabolism in adult Pak3 KO (n = 13) and Pak3 WT (n = 13) males under normal diet (A–E) or HFD (F–J). Body weight measure (A and F), OGTT after 16-h fasting (B and G), IPGTT after 4-h fasting (C and H), intraperitoneal insulin sensitivity test (IPIST) after 6-h fasting (D and I), and blood insulin levels during the IPGTT (E and J). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The p21 protein–activated kinase Pak3 was known to be a brain-specific PAK (28), but here we describe for the first time the expression and function of Pak3 in the mouse endocrine pancreas. Our studies provide evidence that, in the embryo, Pak3 expression initiates in Ngn3+ progenitors and promotes cell cycle exit and then quiescence in developing islet cells and thus controls their differentiation. Further supporting the idea that Pak3 is an effector of Ngn3 endocrinogenic function in the mouse embryonic pancreas, Pak3 transcripts were found to be increased (FC 7.2) in a study designed to identify Ngn3 downstream targets by rescuing Ngn3 expression and endocrine differentiation in Ngn3-deficient mice (7). Although these data support that Pak3 is a downstream target of Ngn3, we found that Pak3 expression was not completely lost in Ngn3 KO mice and ectopically expressed in acinar cells. Our hypothesis is that Pak3+/Cpa1+ cells could arise from failed endocrine progenitors, which can adopt an acinar destiny in Ngn3-deficient mice (33). This implies that although Pak3 is specific to the endocrine lineage in the WT context, it could actually be expressed independently of Ngn3 in acinar cells that derive from Ngn3-deficient cells. However, ectopic Pak3 expression is not sufficient to prevent acinar cell proliferation (not shown). Pak3 is not affected in NeuroD KO but is downregulated (FC 1.5) in Rfx6-deficient embryos at E15.5 (J.P. and G.G., in preparation), we suggest that Pak3 lies downstream of Ngn3 and Rfx6 and either upstream of or in parallel with NeuroD.
Several studies have shown that Ngn3 controls cell cycle exit in endocrine progenitors in the embryonic pancreas. The most direct evidence has been provided by the tracing and analysis of Ngn3-deficient cells, which were shown to continue to proliferate (32,33). The cycling-dependent kinase inhibitor 1a (Cdkn1a) has been demonstrated to be an important effector downstream of Ngn3 to inhibit proliferation (32). In the current study, we provide evidence that Pak3 might contribute as well to cell cycle exit and thus maturation of islet cells. Indeed, we observed an increased number of proliferating Ngn3+ endocrine progenitor cells when Pak3 was inactivated. Importantly, transcript levels of the Ccnd1 gene were concomitantly increased (Cdkn1a mRNA amounts were unaffected) in Pak3-deficient endocrine progenitors, demonstrating that Pak3 function in cell cycle control involves the regulation of cell cycle gene transcription. Ccnd1 encodes cyclin D1, a positive regulator of G1/S phase (37). Thus, Ngn3 might control cell cycle withdrawal in a multistep process where G1/S transition is initially stopped by a Pak3-dependent repression of cyclin D1. Second, the cyclin-dependent kinase inhibitor Cdkn1a would reinforce blockade of S phase. A similar mechanism has been recently proposed for the role of Ngn2 in cell cycle arrest during neurogenesis (38). Interestingly, it has also been proposed that lengthening of the G1 phase, which could result from the repression of G1 cyclins such as cyclin D1, might be important for cell fate determination (37). Of note, we observed that Sox9 is downregulated in Ngn3 cells, as expected (39) in Pak3 KO. Thus, failure to shut off Sox9 does not explain the increased proliferation. Importantly, the increased proliferation of Ngn3 cells in Pak3 KO pancreas (2–3%) cannot completely account for the 25% augmentation of the overall number of Ngn3 cells. Our interpretation is that differentiation is blocked and that islet progenitors accumulate. Accordingly, we counted slightly less α- and β-cells, suggesting impaired endocrine differentiation. Notably, we also observed an increased number of proliferating β-cells in the pancreas of Pak3-deficient E15.5 embryos, suggesting that, at this stage, Pak3 promotes maturation of β-cells by repressing proliferation. However, the proliferation observed is not sufficient to compensate for the differentiation defect at this stage. At later embryonic stages, it is known that β-cells re-enter the cell cycle to increase β-cell mass (40). This mechanism seems independent of Pak3 since we did not detect any change in the proliferation of β-cells at E18.5 and β-cell mass was normal. We do believe that these results could indicate that, during a specific developmental window, Pak3 promotes the transition between Ngn3+ islet progenitors and hormone-positive islet cells in part by controlling cell cycle exit. It is, however, important to keep in mind that although only Pak3 is endocrine specific, functional redundancy with Pak1 and Pak2 as well as with other cell cycle inhibitors, such as Cdkn1a, might explain the relatively mild phenotype observed in Pak3-deficient mice. Interestingly, inhibition of XPak3 in Xenopus results in increased cell proliferation and inhibition of neuronal differentiation (19), providing additional evidence that islet and neural cells share similar developmental programs.
In the adult mice, we demonstrate that Pak3 is necessary for normal glucose homeostasis. Under normal diet, Pak3 KO mice show very mild differences in the control of glucose homeostasis compared with WT. This phenotype could be explained by the redundant function of the remaining group A members that we also found to be expressed in adult islets. Particularly, it has been demonstrated recently that the Cdc42-Pak1 signaling pathway is essential for glucose-induced second-phase insulin secretion in mouse and human islets (21). However, potential redundancy of Pak1 or Pak2 is not sufficient under challenging conditions as Pak3 KO mice are glucose intolerant under HFD. Further studies are needed to understand the origin of this intolerance, as β- and α-cell proliferation and mass and insulin secretion and sensitivity seem normal. Of note, we cannot exclude that a mild effect in the first or second phase of insulin secretion may not have been detected. Although both mutants are glucose intolerant, Pak1 KO and Pak3 KO mouse phenotypes are, however, fairly different, especially since we show that Pak3 KO mice do not develop peripheral insulin resistance even under HFD (Fig. 7I). During the OGTT, the defects in glucose regulation appear very early in Pak3-deficient mice, suggesting that intestinal incretin (GLP-1 and GIP) hormones are involved. However, we showed that enteroendocrine cells differentiated properly in Pak3-deficient mice, implying that the metabolic phenotype is independent of any incretin effect. PAKs have been shown to signal through different pathways; among them, MAPK and LIMK-cofilin have been reported to impact insulin secretion or action (21,41,42). For example, Wang et al. (21) showed that Pak1 signaling in β-cells relies primarily on extracellular signal–related kinase 1/2 (ERK 1/2) activation. Whether Pak3 signals through similar pathways remains an open question.
In summary, this study is the first evidence that the kinase Pak3 regulates the proliferation and differentiation of endocrine progenitors and β-cells in the mouse embryonic pancreas. Importantly, this work also reveals that Pak3 is necessary to maintain glucose homeostasis in adult mice, suggesting that a careful metabolic survey of patients with mental retardation linked to mutations in Pak3 might be of interest.
Supplementary Material
Article Information
Acknowledgments. The authors thank laboratory members A. Beucher and Y. Felsen for sharing their data on Pak3 expression in the intestine, M. Poulet for technical assistance, and M. Ejarque for careful reading of the manuscript and help with FACS sorting (Institut de Génétique et de Biologie Moléculaire et Cellulaire [IGBMC]). The authors are grateful to C. Ebel (IGBMC) for assistance with cell sorting and to H. Gehart and E. Erbs (IGBMC) for their help with islet purification and I. Sumara (IGBMC) for useful discussions. The authors thank L. Pouilly, M.F. Champy, Y. Herault, and all members of the Mouse Clinical Institute for assistance with the metabolic analyses and J. Hecksher-Sørensen (Novo Nordisk, Copenhagen, Denmark) for training in β-cell mass determination. The authors thank C. Thibault and all members of the Microarray and Deep Sequencing Platform (IGBMC) for RNA quality control and amplification.
Funding. This work was supported by grants from the Fondation pour la Recherche Médicale, the Association Francaise des Diabetiques, and the Beta Cell Biology consortium (Grant U19-DK-072495). J.P. is a recipient of a fellowship from the French Ministère de la Recherche.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.P. designed the experiments, acquired and analyzed the data, and wrote the manuscript. A.M. participated in the acquisition and analysis of the data. C.M. designed the mouse that was used for the sorting of β-cells. Z.J. and J.-V.B. shared critical material and participated in the interpretation of data and editing of the manuscript. G.G. designed the experiments, interpreted the data, and wrote the manuscript. G.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0384/-/DC1.
References
- 1.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 2011;240:530–565 [DOI] [PubMed] [Google Scholar]
- 2.Seymour PA, Sander M. Historical perspective: beginnings of the beta-cell: current perspectives in beta-cell development. Diabetes 2011;60:364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res (Amst) 2012;8:274–284 [DOI] [PubMed] [Google Scholar]
- 4.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 5.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 6.Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 2000;97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson KA, Dursun U, Jordan N, et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007;12:457–465 [DOI] [PubMed] [Google Scholar]
- 8.Collombat P, Mansouri A, Hecksher-Sorensen J, et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003;17:2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 1997;386:399–402 [DOI] [PubMed] [Google Scholar]
- 10.Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol 2000;20:3292–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellitzer G, Bonné S, Luco RF, et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J 2006;25:1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SB, Qu HQ, Taleb N, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature 2010;463:775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soyer J, Flasse L, Raffelsberger W, et al. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development 2010;137:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreis P, Barnier JV. PAK signalling in neuronal physiology. Cell Signal 2009;21:384–393 [DOI] [PubMed] [Google Scholar]
- 15.Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol 2002;34:713–717 [DOI] [PubMed] [Google Scholar]
- 16.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 1994;367:40–46 [DOI] [PubMed] [Google Scholar]
- 17.Allen KM, Gleeson JG, Bagrodia S, et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet 1998;20:25–30 [DOI] [PubMed] [Google Scholar]
- 18.Meng J, Meng Y, Hanna A, Janus C, Jia Z. Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene Pak3. J Neurosci 2005;25:6641–6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souopgui J, Sölter M, Pieler T. XPak3 promotes cell cycle withdrawal during primary neurogenesis in Xenopus laevis. EMBO J 2002;21:6429–6439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu TT, Jensen TE, Sylow L, Richter EA, Klip A. Rac1 signalling towards GLUT4/glucose uptake in skeletal muscle. Cell Signal 2011;23:1546–1554 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem 2011;286:41359–41367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem 2007;282:9536–9546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellitzer G, Martín M, Sidhoum-Jenny M, et al. Pancreatic islet progenitor cells in neurogenin 3-yellow fluorescent protein knock-add-on mice. Mol Endocrinol 2004;18:2765–2776 [DOI] [PubMed] [Google Scholar]
- 24.Miyata T, Maeda T, Lee JE. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev 1999;13:1647–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki J, Araki K, Yamato E, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990;127:126–132 [DOI] [PubMed] [Google Scholar]
- 26.Lilla V, Webb G, Rickenbach K, et al. Differential gene expression in well-regulated and dysregulated pancreatic beta-cell (MIN6) sublines. Endocrinology 2003;144:1368–1379 [DOI] [PubMed] [Google Scholar]
- 27.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online 2009;11:3–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreis P, Rousseau V, Thévenot E, Combeau G, Barnier JV. The four mammalian splice variants encoded by the p21-activated kinase 3 gene have different biological properties. J Neurochem 2008;106:1184–1197 [DOI] [PubMed] [Google Scholar]
- 29.Rousseau V, Goupille O, Morin N, Barnier JV. A new constitutively active brain PAK3 isoform displays modified specificities toward Rac and Cdc42 GTPases. J Biol Chem 2003;278:3912–3920 [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 2007;13:103–114 [DOI] [PubMed] [Google Scholar]
- 31.Dubois CL, Shih HP, Seymour PA, et al. Sox9-haploinsufficiency causes glucose intolerance in mice. PLoS ONE 2011;6:e23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyatsuka T, Kosaka Y, Kim H, German MS. Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc Natl Acad Sci USA 2011;108:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beucher A, Martín M, Spenle C, Poulet M, Collin C, Gradwohl G. Competence of failed endocrine progenitors to give rise to acinar but not ductal cells is restricted to early pancreas development. Dev Biol 2012;361:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol 2010;323:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenny M, Uhl C, Roche C, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 2002;21:6338–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellitzer G, Beucher A, Lobstein V, et al. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest 2010;120:1708–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 2013;140:3079–3093 [DOI] [PubMed] [Google Scholar]
- 38.Lacomme M, Liaubet L, Pituello F, Bel-Vialar S. NEUROG2 drives cell cycle exit of neuronal precursors by specifically repressing a subset of cyclins acting at the G1 and S phases of the cell cycle. Mol Cell Biol 2012;32:2596–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih HP, Kopp JL, Sandhu M, et al. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 2012;139:2488–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev 2005;85:1255–1270 [DOI] [PubMed] [Google Scholar]
- 41.Longuet C, Broca C, Costes S, Hani EH, Bataille D, Dalle S. Extracellularly regulated kinases 1/2 (p44/42 mitogen-activated protein kinases) phosphorylate synapsin I and regulate insulin secretion in the MIN6 beta-cell line and islets of Langerhans. Endocrinology 2005;146:643–654 [DOI] [PubMed] [Google Scholar]
- 42.Rondas D, Tomas A, Soto-Ribeiro M, Wehrle-Haller B, Halban PA. Novel mechanistic link between focal adhesion remodeling and glucose-stimulated insulin secretion. J Biol Chem 2012;287:2423–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.