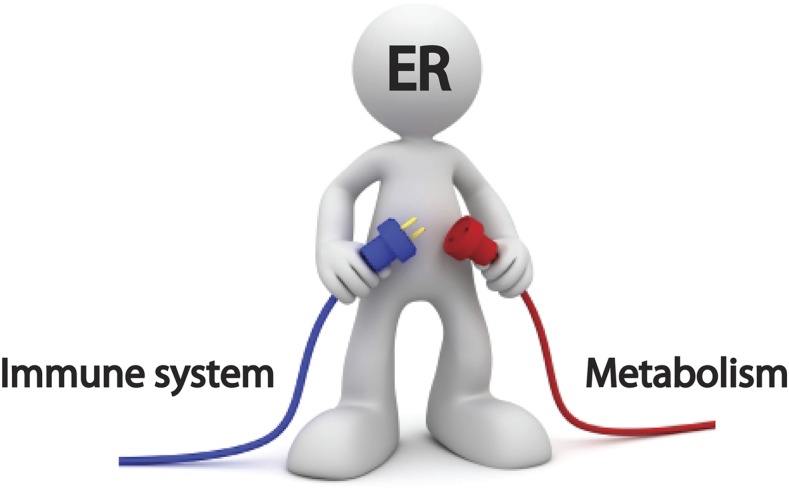
Endoplasmic reticulum (ER) is a busy cell organelle that participates in many important cellular tasks. It has been established that ER is involved in protein and lipid biosynthesis, calcium regulation, redox regulation, cell signaling, and cell death. Given the many vital and complex functions of ER, there is little wonder that its failure can trigger a range of diseases. Recent genetic and clinical evidence indicates that inherited or acquired dysregulation of ER homeostasis can give rise to genetic diseases, including Wolfram syndrome (which is characterized by juvenile-onset diabetes and neurodegeneration) and a number of common metabolic diseases including diabetes and atherosclerosis. Accelerating interest in the role of ER in metabolic disease has been fueled by recent reports showing pathways that link ER to inflammation. The role of ER as an interface between the immune system and metabolism is an emerging concept (Fig. 1). However, currently there is no treatment targeting ER for combating immunometabolic diseases. To fulfill this unmet medical need, we need to identify pathways and molecules that link the immune system to metabolism at ER.
Figure 1.
ER is an interface between the immune system and metabolism. ER is an intersection between inflammation and metabolism and an attractive target for immunometabolic diseases, including type 1 and type 2 diabetes, atherosclerosis, and Wolfram syndrome.
ER dysfunction has been a suspect as a major pathogenic component of human chronic diseases, such as diabetes, atherosclerosis, and Wolfram syndrome (1–10). However, the precise role of ER in the etiology of these diseases is not clear. It has been recognized that inflammation plays a central role in chronic metabolic diseases, raising the possibility that ER is at the intersection of inflammation and metabolism (7,11–14). Over the past several years, this concept has been supported by genetic, experimental, and clinical evidence (10,15). One of the unmet scientific needs in this emerging field is to identify the pathways linking ER to production of inflammatory cytokines. Two molecular pathways linking ER to production of interleukin (IL)-1β, a major player in inflammation, have been recently identified. These are activating transcription factor (ATF) 5 and miR-17 (16,17). Both molecules are regulated by key regulators of the ER stress response, Perk and Ire1α.
In this issue, Iwasaki et al. (18) describe the molecular pathway linking ER to IL-6 production. Using DNA microarray and network analyses of macrophages, they show compelling evidence that ATF4, which is involved in the ER stress response, plays an essential role in IL-6 expression induced by various metabolic stresses, including ER stress. Furthermore, they reveal that the ATF4 pathway has a synergistic effect on the Toll-like receptor-4 signaling pathway, enhancing IL-6 expression. IL-6 has been shown to play crucial roles in insulin resistance and type 2 diabetes (19), raising the possibility that ATF4 signaling is a novel target for the treatment of metabolic diseases. The new findings from Iwasaki et al. (18) also suggest that ER-stressed macrophages may trigger autoimmune diseases through IL-6 production.
Compelling evidence indicates that ER is at the intersection of inflammation and metabolism and is therefore an attractive target for immunometabolic diseases. For example, recent evidence strongly suggests that ER dysfunction in antigen-presenting macrophages and β-cells triggers autoimmunity during the onset and progression of type 1 diabetes (20,21). Despite the underlying importance of ER dysfunction in these diseases, no current therapies target ER. The unmet scientific and medical need in the field of ER immunometabolism is to target the common molecular processes that are altered in ER diseases as a novel therapeutic discovery strategy. The strategy of performing clinical studies using drugs previously known to target ER, such as glucagon-like peptide 1 agonists and vitamin D, on patients with immunometabolic diseases should be explored (22,23).
Article Information
Funding. F.U. is supported by grants from the National Institutes of Health (DK-067493, DK-020579, and UL1 TR000448), JDRF (47-2012-760, 17-2013-512), American Diabetes Association (1-12-CT-61), the Ellie White Foundation for Rare Genetic Disorders, and the Jack and J.T. Snow Scientific Research Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 152.
References
- 1.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011;334:1081–1086 [DOI] [PubMed] [Google Scholar]
- 2.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev 2008;29:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oslowski CM, Urano F. The binary switch that controls the life and death decisions of ER stressed β cells. Curr Opin Cell Biol 2011;23:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca SG, Fukuma M, Lipson KL, et al. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem 2005;280:39609–39615 [DOI] [PubMed] [Google Scholar]
- 5.Fonseca SG, Ishigaki S, Oslowski CM, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 2010;120:744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil GS. Endoplasmic reticulum stress and atherosclerosis. Nat Med 2010;16:396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med 2012;63:317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman RJ. Beta-cell failure, stress, and type 2 diabetes. N Engl J Med 2011;365:1931–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17:314–321 [DOI] [PubMed] [Google Scholar]
- 12.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 13.Donath MY, Mandrup-Poulsen T. The use of interleukin-1-receptor antagonists in the treatment of diabetes mellitus. Nat Clin Pract Endocrinol Metab 2008;4:240–241 [DOI] [PubMed] [Google Scholar]
- 14.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008;29:42–61 [DOI] [PubMed] [Google Scholar]
- 15.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 16.Oslowski CM, Hara T, O’Sullivan-Murphy B, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab 2012;16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerner AG, Upton JP, Praveen PV, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 2012;16:250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki Y, Suganami T, Hachiya R, et al. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes 2014;63:152–161 [DOI] [PubMed] [Google Scholar]
- 19.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 2005;54(Suppl. 2):S114–S124 [DOI] [PubMed] [Google Scholar]
- 20.Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 2012;61:818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol 2012;90:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 2006;4:391–406 [DOI] [PubMed] [Google Scholar]
- 23.Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol 2013;136:309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]