Abstract
The contribution of elevated glucagon-like peptide 1 (GLP-1) to postprandial glucose metabolism after Roux-en-Y gastric bypass (RYGB) has been the subject of uncertainty. We used exendin-9,39, a competitive antagonist of GLP-1, to examine glucose metabolism, islet hormone secretion, and gastrointestinal transit in subjects after RYGB and in matched control subjects. Subjects were studied in the presence or absence of exendin-9,39 infused at 300 pmol/kg/min. Exendin-9,39 resulted in an increase in integrated postprandial glucose concentrations post-RYGB (3.6 ± 0.5 vs. 2.0 ± 0.4 mol/6 h, P = 0.001). Exendin-9,39 decreased insulin concentrations (12.3 ± 2.2 vs. 18.1 ± 3.1 nmol/6 h, P = 0.002) and the β-cell response to glucose (ϕTotal, 13 ± 1 vs. 11 ± 1 × 10−9 min−1, P = 0.01) but did not alter the disposition index (DI). In control subjects, exendin-9,39 also increased glucose (2.2 ± 0.4 vs. 1.7 ± 0.3 mol/6 h, P = 0.03) without accompanying changes in insulin concentrations, resulting in an impaired DI. Post-RYGB, acceleration of stomach emptying during the first 30 min by exendin-9,39 did not alter meal appearance, and similarly, suppression of glucose production and stimulation of glucose disappearance were unaltered in RYGB subjects. These data indicate that endogenous GLP-1 has effects on glucose metabolism and on gastrointestinal motility years after RYGB. However, it remains uncertain whether this explains all of the changes after RYGB.
Introduction
Type 2 diabetes is a common metabolic disorder characterized by hyperglycemia arising from defects in insulin secretion and action. The increase in incidence and prevalence of type 2 diabetes is strongly associated with an increase in obesity in the general population (1). Prior observational studies have suggested that bariatric surgery is the most effective long-term intervention for weight loss, leading to an increase in the number of procedures performed annually (2,3).
Bariatric surgery has been associated with remission of type 2 diabetes. Intriguingly, there appears to be some heterogeneity within procedures with regard to their efficacy in improving type 2 diabetes, with Roux-en-Y gastric bypass (RYGB) being superior to adjustable gastric banding (4) or to medical therapy alone in achieving glycemic control (5,6). However, while sleeve gastrectomy was associated with rates of resolution of type 2 diabetes similar to those of RYGB 1 year after intervention, at 24 months subjects with RYGB exhibited greater reduction in truncal fat and improvement in β-cell function compared with sleeve gastrectomy or intensive medical therapy (7). This would suggest that RYGB differs from purely restrictive procedures in terms of direct benefit to islet function. One of the changes known to occur after RYGB is an increase in postprandial glucagon-like peptide 1 (GLP-1) concentrations compared with control subjects (8–10). GLP-1 is an insulin secretagogue released by enteroendocrine L cells (11). In addition, it also suppresses glucagon secretion (12). These and numerous other observations have led to the development of GLP-1–based therapy for type 2 diabetes (13).
We therefore set out to examine the effect of endogenous GLP-1 secretion on glucose metabolism after RYGB using exendin-9,39, a competitive antagonist of GLP-1 at its cognate receptor (GLP-1R) (14,15). To control for the potential effects of this compound on β-cell function (measured as disposition index [DI] [16]), we also studied age- and weight-matched subjects. Fasting and postprandial glucose metabolism was measured using the isotope dilution method (17), while indices of insulin secretion and action were measured using the oral minimal model (18). To avoid potential confounders such as the effect of diabetes on endogenous insulin secretion (and therefore the ability to respond to an endogenous secretagogue such as GLP-1) we studied nondiabetic subjects >1 year after surgery when subjects were at a stable weight. Subjects were studied on two occasions in random order, receiving a saline or exendin-9,39 infusion (at a rate of 300 pmol/kg/min) during the study. We report that while exendin-9,39 increased integrated postprandial glucose concentrations in both post-RYGB subjects and control groups, it decreased peak and integrated postprandial insulin and C-peptide in post-RYGB subjects but not in control subjects. On the other hand, exendin-9,39 did not alter insulin action or total DI in the post-RYGB subjects. While exendin-9,39 accelerated gastric emptying after RYGB, it did not alter the Ra of the ingested glucose, postprandial suppression of endogenous glucose production (EGP), or postprandial stimulation of glucose disappearance. We conclude that the marked elevation of endogenous GLP-1 concentrations after RYGB has effects on insulin secretion and gastrointestinal motility in nondiabetic subjects.
Research Design and Methods
After approval from the Mayo Institutional Review Board, local advertisement was used to recruit 12 subjects without type 2 diabetes and not taking any glucose-lowering medication after uncomplicated RYGB. Subjects gave written, informed consent to participate in the study. Concurrently, eight age-, sex-, and weight-matched subjects with no history of type 2 diabetes who were recruited by the same process gave written informed consent to participate. All subjects were in good health, at a stable weight, and did not engage in regular exercise. All subjects were instructed to follow a weight-maintenance diet (~55% carbohydrate, 30% fat, and 15% protein) for the period of study. Body composition was measured using dual-energy X-ray absorptiometry (DPX scanner; Lunar, Madison, WI).
Experimental Design
Subjects were studied on two occasions in random order 14 days apart. On one occasion, subjects received an infusion of exendin-9,39 (1,200 pmol/kg bolus followed by infusion at 300 pmol/kg/min as previously described [16]), while on the other occasion they received saline. They were admitted to the clinical research unit at 1700 h on the evening prior to all of the meal studies. Subsequently, they consumed a standardized low-calorie meal (10 kcal/kg body wt: 40% carbohydrate, 30% fat, and 30% protein) tolerable to patients who have undergone restrictive upper gastrointestinal procedures and then fasted overnight. At 0630 h on the following morning (−180 min), a forearm vein was cannulated with an 18-g needle to allow infusions to be performed. An 18-g cannula was inserted retrogradely into a vein of the dorsum of the contralateral hand. This was placed in a heated Plexiglas box maintained at 55°C to allow sampling of arterialized venous blood (Supplementary Table 1). A primed (12 mg/kg) continuous (0.12 mg/kg/min) infusion of [6,6-2H2]glucose was initiated. At time 0 (0930 h), subjects consumed a meal (220 kcal, 56% carbohydrate, 25% fat, and 19% protein) consisting of one scrambled egg, 15 g Canadian bacon, 100 mL water, and Jell-O containing 35 g glucose labeled with [1-13C]glucose (4% enrichment). To enable measurement of solid-phase gastric emptying, we labeled the egg with 0.1 mCi 111In-DTPA to allow for measurement of gastric emptying and orocecal transit. Simultaneously, an infusion of saline or exendin-9,39 was initiated. An infusion of [6-3H]glucose was also started at this time, and the infusion rate varied to mimic the anticipated appearance of meal [1-13C]glucose. The rate of infusion of [6,6-2H2]glucose was altered to approximate the anticipated fall in EGP, thereby minimizing changes in specific activity (19,20).
The consumption of the test meal was standardized; subjects fed themselves by repetitively consuming a spoonful of Jell-O followed by a spoonful of bacon and egg. Subjects were instructed to consume all of their meal within 15 min from the study start. At the end of the meal, subjects drank 30 mL water. The meal was consumed in the sitting position.
Measurement of Gastrointestinal Transit
Gastrointestinal and colonic transit was measured by anterior and posterior γ camera images obtained in the supine position immediately after meal ingestion, then every 15 min for the first 2 h, and then every 30 min for the next 2 h (total 4 h after the meal) (21). A region of interest analysis around the stomach or, in the case of RYGB subjects, around the gastric pouch was created using ANALYZE PC 2.5 (Biomedical Imaging Resource; Mayo Foundation, Rochester, MN), and the counts in the stomach and their change over time were quantitated. The counts were corrected for radionuclide decay and tissue attenuation.
Analytical Techniques
Plasma samples were placed on ice, centrifuged at 4°C, separated, and stored at −20°C until assayed. Glucose concentrations were measured using a glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured using a chemiluminescence assay (Access Assay; Beckman, Chaska, MN). Plasma glucagon and C-peptide were measured by Radio-Immunoassay (Linco Research, St. Louis, MO). Collection tubes for GLP-1 had 100 μmol/L dipeptidyl peptidase-4 inhibitor (Linco Research, St. Louis, MO) added. Total GLP-1 concentrations were measured using a COOH-terminal assay (Linco Research). Plasma [6,6-2H2]glucose and [1-13C]glucose enrichments were measured using gas chromatographic mass spectrometry (Thermoquest, San Jose, CA) to simultaneously monitor the C-1 and C-2 and C-3 to C-6 fragments, as described by Beylot et al. (22). In addition, [6-3H]glucose specific activity was measured by liquid scintillation counting after deproteinization and passage over anion- and cation-exchange columns (20).
Calculations
The systemic rates of meal appearance (Ra meal), EGP, and Rd were calculated using Steele’s model (23). Ra meal was calculated by multiplying Ra of [1-13C]glucose (obtained from the infusion rate of [6-3H]glucose and the clamped plasma ratio of [6-3H]glucose and [1-13C]glucose) by the meal enrichment. EGP was calculated from the infusion rate of [6,62H2]glucose and the ratio of [6,62H2]glucose to endogenous glucose concentration. Rd was calculated by subtracting the change in glucose mass from the overall rate of glucose appearance (i.e., Ra meal + EGP). Values from –30 to 0 min were averaged and considered as basal. Area above basal (AAB) was calculated using the trapezoidal rule.
Net insulin action (insulin sensitivity index [Si]) was measured using the oral minimal model (24). β-Cell responsivity indices were estimated using the oral C-peptide minimal model (25), incorporating age-associated changes in C-peptide kinetics (26). The model assumes that insulin secretion comprises a static and a dynamic component with an index of total β-cell responsivity to glucose (ϕtotal) derived from these two components (18). DIs were subsequently calculated by multiplying ϕtotal by Si.
Statistical Analysis
Data in the text are presented as (observed) means ± SEM. The primary analyses compared changes in fasting, peak, and integrated hormone concentrations or glucose flux (saline vs. exendin-9,39 treatment) was assessed separately for each group using a paired, two-tailed t test. Between-group differences were assessed using an unpaired, two-tailed t test. A P value <0.05 was considered statistically significant. The SD of the change in a given parameter between saline and exendin-9,39 studies in the RYGB subjects and separately in the control subjects was used to calculate the detectable difference between study days with 80% power using a paired t test with a two-sided α-level of 0.05 (Supplementary Table 2).
Results
Volunteer Characteristics
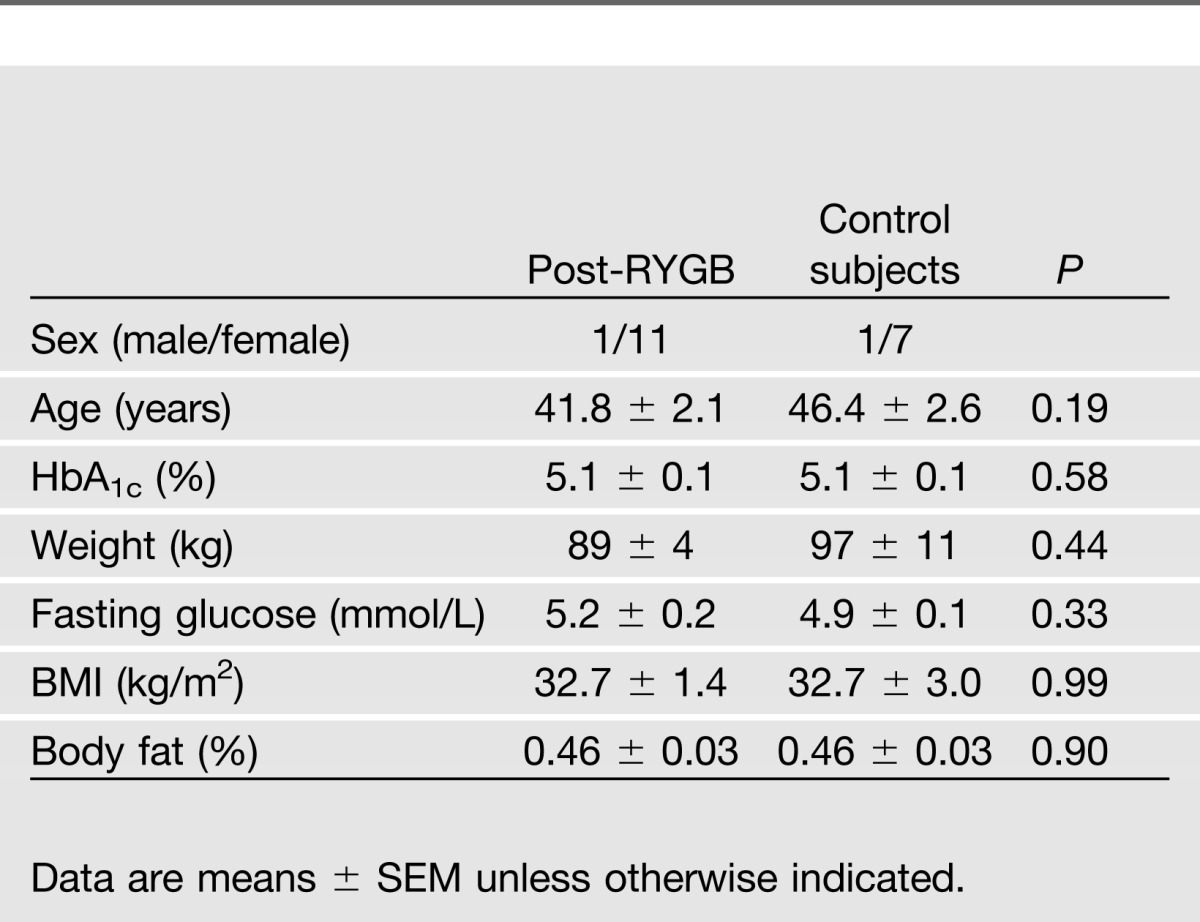
There were 12 subjects studied 5.0 ± 0.9 years after RYGB and 8 age-, weight-, and sex-matched control subjects. Average weight prior to surgery was 117 ± 3 kg, and the average change in weight was −25 ± 3%). No important differences were observed in other baseline characteristics between the groups (Table 1).
Table 1.
Baseline characteristics of the participants in each group
Total GLP-1 Concentrations
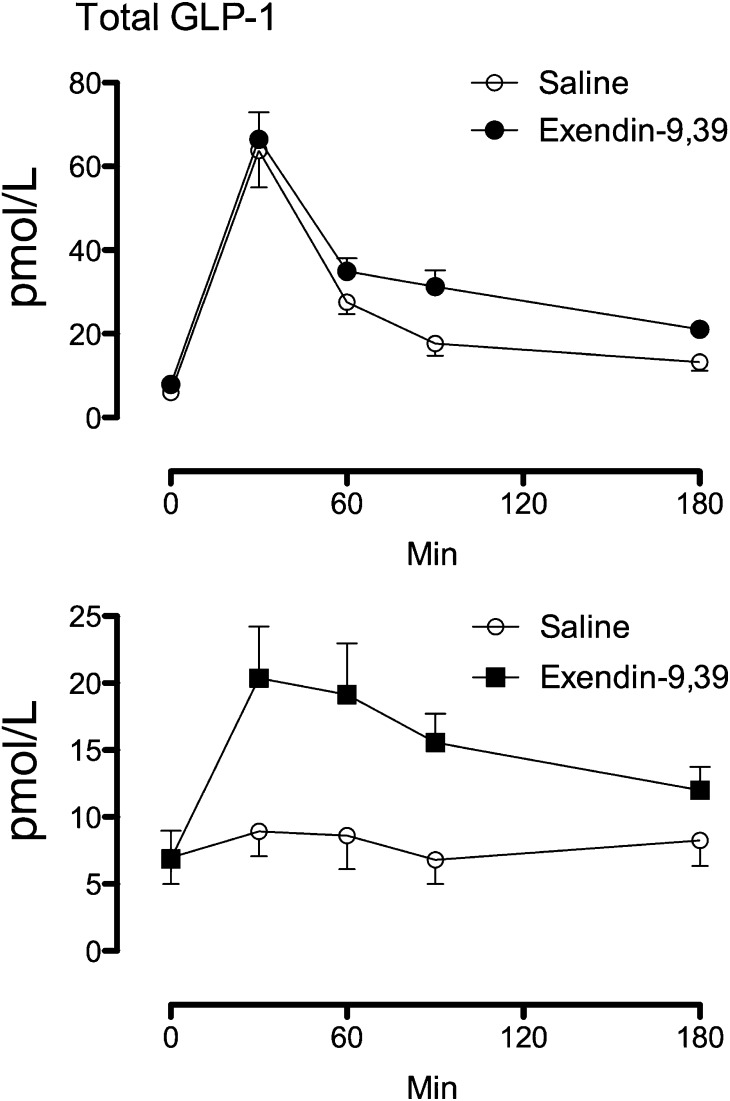
Fasting, total GLP-1 concentrations did not differ between the post-RYGB and control groups. In contrast, peak GLP-1 concentrations after meal ingestion during saline infusion were higher (P < 0.001) in the post-RYGB group compared with the control group, respectively (64 ± 9 vs. 11 ± 2 pmol/L). Exendin-9,39 infusion did not alter peak GLP-1 concentrations in the post-RYGB group. However, in the control group exendin-9,39 increased (P = 0.003) peak GLP-1 concentrations compared with saline infusion (11 ± 2 vs. 25 ± 4 pmol/L). Integrated AAB GLP-1 concentrations were increased by exendin-9,39 in both the post-RYGB (3,519 ± 499 vs. 4,625 ± 458 pmol/3 h, P = 0.04) and control (158 ± 269 vs. 1,525 ± 202 pmol/3 h, P < 0.001) groups, respectively (Fig. 1).
Figure 1.
Total GLP-1 concentrations during saline infusion and during exendin-9,39 infusion in the post-RYGB (upper panel) or control (lower panel) groups.
Plasma Glucose, Insulin, C-peptide, and Glucagon Concentrations
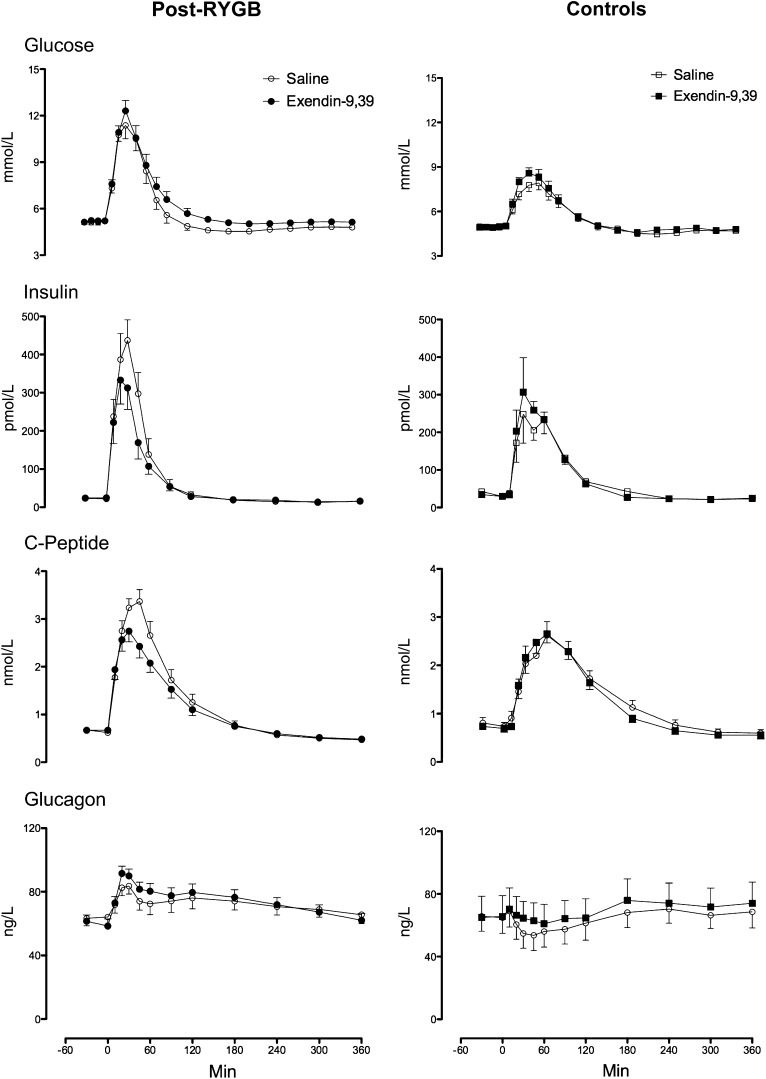
Fasting glucose (Fig. 2 [top panel]) did not differ between the post-RYGB and control groups (5.2 ± 0.2 vs. 4.9 ± 0.1 mmol/L, P = 0.33), respectively. In contrast, peak glucose concentrations after meal ingestion during saline infusion were higher (P < 0.001) in the RYGB group (12.3 ± 0.7 vs. 8.4 ± 0.3 mmol/L), likely reflecting different upper gastrointestinal anatomy. Exendin-9,39 did not alter peak glucose concentrations after meal ingestion in either the post-RYGB (12.3 ± 0.7 vs. 12.3 ± 0.7 mmol/L, P = 0.91) or control (8.4 ± 0.3 vs. 9.0 ± 0.3 mmol/L, P = 0.14) group. Exendin-9,39 increased integrated (AAB) glucose concentrations in both the post-RYGB (198 ± 31 vs. 364 ± 46 mmol/6 h, P = 0.001) and control (170 ± 34 vs. 224 ± 37 mmol/6 h, P = 0.03) groups.
Figure 2.
Glucose (upper panel), insulin (upper middle panel), C-peptide (lower middle panel), and glucagon concentrations (lower panel) during saline infusion and during exendin-9,39 infusion in the post-RYGB (left panel) and control (right panel) groups.
Fasting insulin concentrations (upper middle panels) were higher (P = 0.04) in the control groups (23 ± 3 vs. 36 ± 6 pmol/L). However, peak insulin concentrations after meal ingestion during saline infusion did not differ (491 ± 69 vs. 309 ± 68 pmol/L, P = 0.09). Exendin-9,39 decreased (P < 0.001) peak (491 ± 69 vs. 389 ± 56 pmol/L) and integrated (AAB) insulin concentrations (18.1 ± 3.1 vs. 12.3 ± 2.2 nmol/6 h, P = 0.002) after meal ingestion in the post-RYGB group. On the other hand, exendin-9,39 did not alter peak or integrated insulin concentrations in the control group.
Fasting C-peptide concentrations (lower middle panels) did not differ between the post-RYGB and control groups. However, peak C-peptide concentrations after meal ingestion during saline infusion were higher in the post-RYGB group (3.5 ± 0.2 vs. 2.8 ± 0.3 nmol/L, P = 0.05). Postprandial C-peptide concentrations mirrored insulin concentrations in exendin-9,39 decreased peak (3.5 ± 0.2 vs. 2.9 ± 0.2 nmol/L, P = 0.001) and integrated (AAB) C-peptide concentrations (204 ± 25 vs. 133 ± 21 nmol/6 h, P = 0.001) after meal ingestion in the post-RYGB group but did not affect these parameters in the control group (Supplementary Table 3).
Fasting and nadir postprandial glucagon concentrations (bottom panels) did not differ between the post-RYGB and control groups and were not altered by exendin-9,39 in either group. However, post hoc analysis revealed an effect of exendin-9,39, which increased glucagon concentrations at 20 and 30 min in the control subjects and at 20, 45, and 60 min in the post-RYGB subjects (Supplementary Table 6).
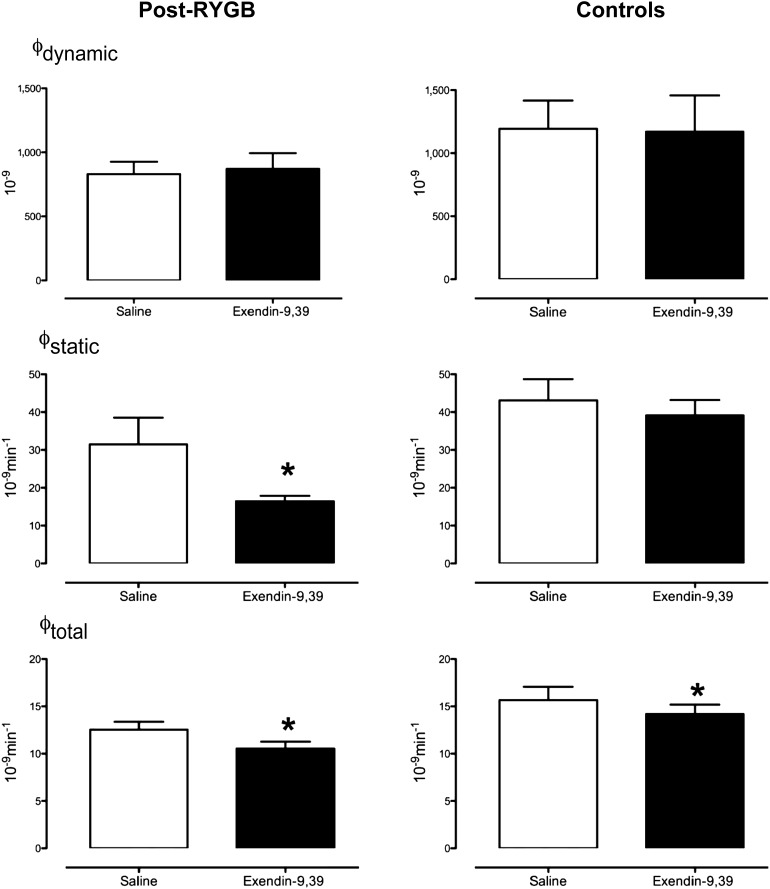
Dynamic β-Cell Responsivity (ϕDynamic), Static β-Cell Responsivity (ϕStatic), and Total β-Cell Responsivity (ϕTotal)
The dynamic component of insulin secretion (ϕDynamic) was unchanged in either group by exendin-9,39 infusion (Fig. 3 [top panel]). In subjects after RYGB, exendin-9,39 significantly decreased (P = 0.03) the static component of insulin secretion (ϕStatic) (31 ± 7 vs. 16 ± 1 10−9 ⋅ min−1), while there was no effect observed in the control group (middle panel). Total β-cell responsivity (ϕTotal) was decreased in both RYGB (13 ± 1 vs. 11 ± 1 10 × −9 ⋅ min−1, P = 0.01) and control groups (16 ± 7 vs. 14 ± 1 × 10−9 ⋅ min−1, P = 0.04) (lower panel).
Figure 3.
ϕDynamic (top panel), ϕStatic (middle panel), and ϕTotal (bottom panel) during saline infusion and during exendin-9,39 infusion in the post-RYGB subjects and in control subjects. *P < 0.05.
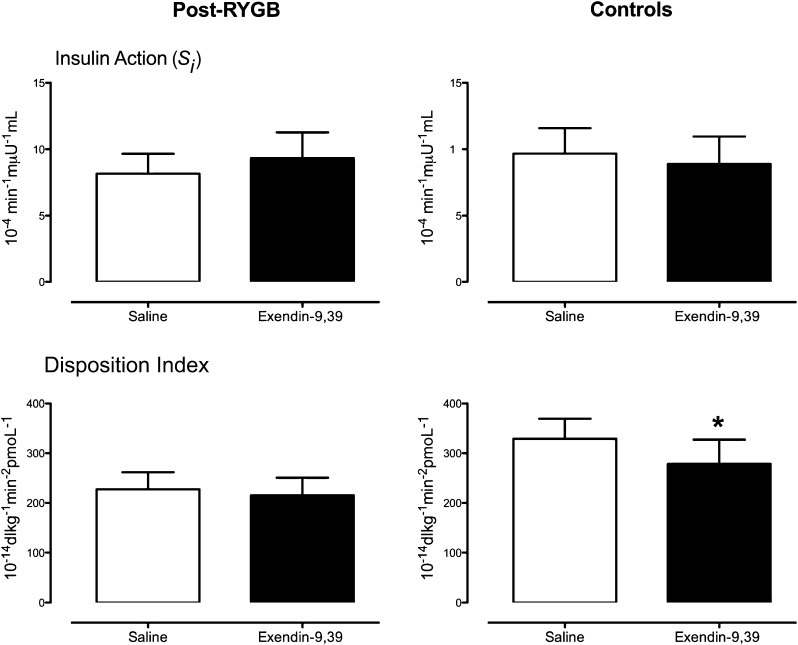
Insulin Action and DITotal
Exendin-9,39 infusion did not significantly alter insulin action (Si) in either group (Fig. 4 [top panel]). Expressing ϕTotal as a function of the prevailing insulin action as the DI (DITotal [bottom panel]) revealed no net effect of exendin-9,39 in the post-RYGB group (227 ± 34 vs. 215 ± 36 × 10−14 dL ⋅ kg−1 ⋅ min−2 ⋅ pmol−1, P = 0.59) but an effect (P = 0.03) in the control group (329 ± 40 vs. 279 ± 49 × 10−14 dL ⋅ kg−1 ⋅ min−2 ⋅ pmol−1).
Figure 4.
Si (top panel) and DITotal (bottom panel) during saline infusion and during exendin-9,39 infusion in the post-RYGB subjects (left panel) and in control subjects (right panel). *P < 0.05.
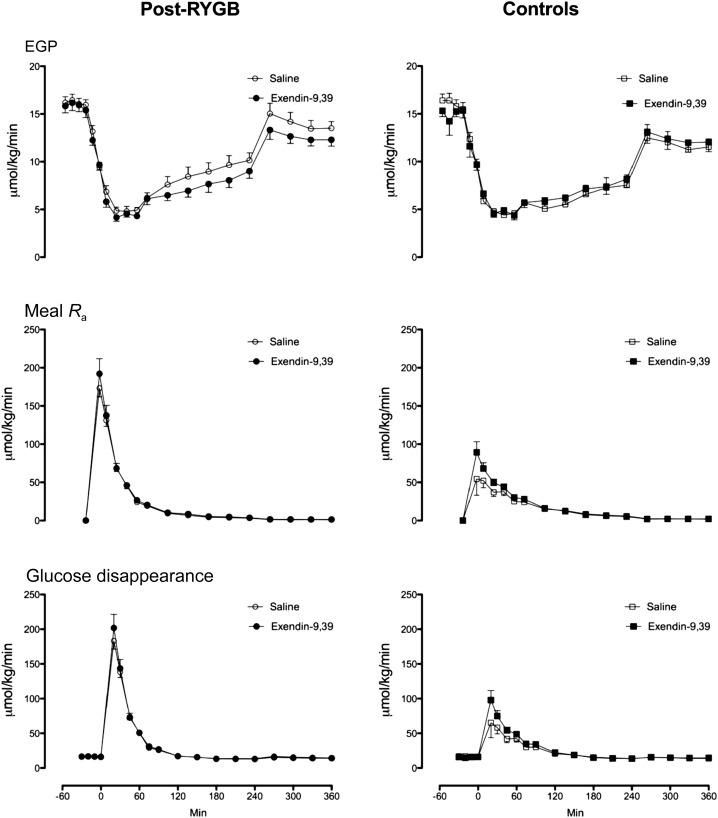
EGP, Meal Appearance, and Glucose Disappearance
Fasting EGP as well as postprandial suppression of EGP did not differ between the post-RYGB and control groups (Fig. 5 [top panel]) and was unchanged by exendin-9,39 in either group. Peak meal Ra (middle panels) was higher (P < 0.001) in the post-RYGB group compared with control subjects (175 ± 11 vs. 57 ± 10 μmol/kg/min). Integrated AAB meal Ra also was also higher (P < 0.001) in the post-RYGB group compared with control subjects (8.6 ± 0.6 vs. 5.0 ± 0.5 mmol/6 h). Exendin-9,39 infusion did not alter peak or integrated meal Ra in either group, although there was a trend to higher meal Ra in the control group during exendin-9,39 infusion (5.0 ± 0.5 vs. 5.8 ± 0.4 mmol/6 h, P = 0.09). Exendin-9,39 infusion did not alter peak or integrated Rd (bottom panel) in either group.
Figure 5.
EGP (upper panel), meal Ra (middle panel), and glucose disappearance (lower panel) during saline infusion and during exendin-9,39 infusion in the post-RYGB (left panel) and control (right panel) groups.
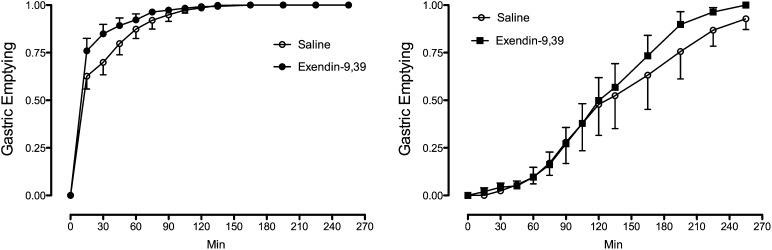
Gastrointestinal Transit
Exendin-9,39 significantly increased (P < 0.01) the rate of gastric emptying of the radiolabeled solid meal during the first 45 min after food ingestion in the post-RYGB group but did not alter the rate of gastric emptying in the control group (Fig. 6, Supplementary Table 3, and Supplementary Fig. 2).
Figure 6.
Gastric emptying during saline infusion and during exendin-9,39 infusion in the post-RYGB (left panel) and control (right panel) groups.
Discussion
RYGB results in increased postprandial concentrations of GLP-1. To determine the contribution of endogenous GLP-1 to postprandial glucose metabolism after RYGB, we used exendin-9,39, a competitive antagonist of GLP-1 at its receptor. Exendin-9,39 decreased peak and integrated insulin concentrations resulting in a significant increase in integrated glucose concentrations and also an acceleration of gastric emptying. However, it did not alter the postprandial suppression of EGP, the systemic Ra of ingested glucose, or the postprandial stimulation of glucose disappearance. We conclude that the elevated GLP-1 concentrations post-RYGB have effects on glucose metabolism in nondiabetic subjects >1 year after RYGB.
The active forms of GLP-1 infused in pharmacologic concentrations increase insulin secretion, delay gastric emptying, and suppress glucagon secretion (27,28). However, given the short half-life of active GLP-1 in the circulation, the contribution of elevated GLP-1 concentrations to glucose metabolism after RYGB has been the subject of uncertainty. We used exendin-9,39 to examine the contribution of endogenous GLP-1 to postprandial glucose metabolism after RYGB. Given the recently observed effects of exendin-9,39 on DI in the absence of endogenous incretin hormone secretion (16), we also studied age- and weight-matched control subjects to ensure that our observations could not be explained by direct effects of exendin-9,39 per se.
We infused exendin-9,39 at a rate of 300 pmol/kg/min, as this has previously been shown to block the effects of GLP-1 infused at supraphysiologic doses and the effects of endogenous GLP-1 on gastrointestinal motility and insulin secretion (14,15,29). Although it is conceivable that the effects of GLP-1 after RYGB were not completely blocked by exendin-9,39 in this experiment, our data are qualitatively similar to those in two recent publications using infusion rates 2.5- to 3.0-fold higher than those used in our current study (30,31). Ionut et al. (32) previously reported portal GLP-1 levels twofold higher than in the systemic circulation. Assuming this is the case, the infusion rate of exendin-9,39 should be sufficient to inhibit GLP-1 actions at its receptor (33). While it is possible that endogenous GLP-1 still contributes to insulin secretion in the presence of exendin-9,39, the residual effect is likely to be small (30). For example, Jørgensen et al. (31) used an infusion of 900 pmol/kg/min and experienced a ∼25% increase in integrated glucose concentrations, which compares with the change in glucose concentrations that we observed in our study (Supplementary Table 3) in glucose concentrations. On the other hand, despite the higher infusion rate of exendin-9,39, Jørgensen et al. did not observe significant effects on gastrointestinal motility, which was evaluated with acetaminophen-based methodology that assesses emptying of the liquid phase of the meal. This may be insufficiently sensitive to detect subtle changes in motility, especially in the presence of RYGB, which normally causes rapid emptying of the meal’s liquid phase.
Exendin-9,39 infusion resulted in increased total GLP-1 secretion, an effect that has been observed in the absence of an oral stimulus (16), after RYGB, and, albeit to a lesser extent, in matched control subjects. However, while both peak and integrated GLP-1 concentrations were increased by exendin-9,39 in the control subjects, peak concentrations were unaffected post-RYGB. One possible explanation is that peak GLP-1 secretion after RYGB is maximized and cannot be increased further by blockade of the GLP-1R.
In subjects post-RYGB, exendin-9,39 decreased peak and integrated insulin concentrations. While insulin concentrations reflect the net sum of insulin secretion and hepatic clearance, a parallel decrease in C-peptide concentrations during GLP-1R inhibition by exendin-9,39 implies that this was due not to an increase in hepatic insulin extraction but to decreased insulin secretion. Indeed, the minimal model indices demonstrate decreased β-cell responsivity to glucose in the presence of exendin-9,39 post-RYGB. This effect was manifest on the component of β-cell responsivity (ϕstatic) that represents the provision of new insulin to the releasable pool, whereas the component representing promptly releasable insulin (ϕdynamic) was unchanged (25). This observation mirrors the effect of GLP-1–based pharmacotherapy on β-cell responsivity (20), implying that activation of GLP-1R modulates insulin synthesis and release in response to a mixed-meal challenge. Blockade of GLP-1R after RYGB has a converse effect, suggesting that postprandial elevation of GLP-1 after RYGB also modulates insulin synthesis and secretion. On the other hand, when total β-cell responsivity (ϕTotal) is expressed as a function of the prevailing insulin action (Si) to calculate DI—a measure of net β-cell function—this was unchanged by exendin-9,39, emphasizing the relatively minor contribution of endogenous GLP-1 secretion to overall β-cell function after RYGB in this experiment.
In the control subjects, exendin-9,39 infusion also decreased total DI indicating a decrease in β-cell function when considered in light of the prevailing insulin action. This in turn resulted in a slight, but significant, increase in glucose concentrations. Exendin-9,39 decreased DI in otherwise healthy subjects in the absence of endogenous incretin hormone secretion (16). However, in the present experiment, potential effects of exendin-9,39 per se would represent a conservative error in terms of quantifying the effects of GLP-1 post-RYGB. Despite reports that GLP-1 increases insulin action (rev. in 34), insulin action did not differ in the presence or absence of exendin-9,39.
In experiments that used sustained hyperglycemia as a β-cell stimulus, regardless of whether intravenous glucose was the sole stimulus (35) or combined with a nutrient challenge delivered orally (36) or by nasoduodenal tube (14,37), exendin-9,39 decreased insulin secretion in healthy subjects (14), in subjects with type 2 diabetes (36,37), and after RYGB (35). These experiments differ from the current study that used a less sustained challenge that more closely resembles normal physiology.
Despite decreased insulin secretion after meal ingestion, postprandial suppression of EGP was unaffected by exendin-9,39 after RYGB. Since hyperglycemia per se potentially suppresses glucose production and stimulates glucose uptake, the high postprandial glucose concentrations due to the rapid absorption of ingested glucose, along with the rapid increase in insulin concentrations, albeit to a slightly lower peak, are sufficient to result in comparable glucose fluxes in the presence or absence of exendin-9,39 (38). Whether these differences would be more apparent in individuals with impaired glucose effectiveness (39), such as those with type 2 diabetes, is uncertain. The other noteworthy observation is that in the presence of GLP-1R inhibition post-RYGB, despite the modest acceleration of gastrointestinal transit, the systemic Ra of ingested glucose was also unchanged. A possible explanation is that the rate of emptying from the gastric pouch of the portion of the meal labeled with [1-13C]glucose emptied at a rate different from that associated with the (solid) portion labeled with indium. In the control subjects, the changes in meal appearance and glucose disappearance also did not differ significantly between study days.
It has been presumed that the increase in GLP-1 after meal ingestion post-RYGB is related to rapid delivery of food to the incretin-secreting portions of the intestine (9,40,41). As expected in our experiment given the upper gastrointestinal anatomy after RYGB, gastric emptying of the solid portion of the meal differed dramatically between post-RYGB subjects and control subjects. However, exendin-9,39 accelerated gastric emptying in the post-RYGB subjects, suggesting that postprandial GLP-1 concentrations in this situation delay transit of ingested solids. Given the nature of the surgical reconstruction that leads to rapid emptying of solids, it is not surprising that this effect was only observed early after meal ingestion (42).
In our experiment, we failed to observe an effect of exendin-9,39 on gastrointestinal transit in the control group. This may have been in part because we were only powered to detect a relatively large change (23 min) in the time taken to empty half the stomach contents (gastric emptying T1/2) (Supplementary Table 2). Previously, Schirra et al. (14) measured gastroduodenal motility using perfusion manometry and concluded that in the presence of intraduodenal glucose infusion, exendin-9,39 increased motility. Subsequently, a surrogate measurement of gastric emptying, i.e., the rate of systemic appearance of d-xylose ingested together with glucose in healthy subjects (43) or with a mixed meal in people with type 2 diabetes (36), failed to show an effect of exendin-9,39. Measurement of gastric emptying using scintigraphy has demonstrated either no effect (44) or accelerated emptying (45) in otherwise healthy humans. However, Deane et al. (45) infused exendin-9,39 for 30 min prior to meal ingestion and, given the effect of GLP-1 on gastric volume and accommodation (46), it is possible that under these experimental conditions the compound accelerated gastric emptying because of decreased gastric volume prior to meal ingestion (45).
In summary, exendin-9,39 results in an increase in postprandial glucose concentrations and a decrease in insulin secretion in subjects post-RYGB. This demonstrates that even several years after RYGB in nondiabetic subjects, the elevated postprandial GLP-1 concentrations affect insulin secretion and gastrointestinal motility. Whether such GLP-1–mediated effects explain the preservation or improvement in β-cell function in RYGB compared with other bariatric procedures (7) remains to be ascertained.
Supplementary Material
Article Information
Funding. The authors acknowledge the support of the Mayo Clinic General Clinical Research Center (NIH DK TR000135). C.C. and A.V. are supported by NIH R01 DK-78646 and by NIH R01 DK-82396.
Duality of Interest. A.V. has received research grants from Merck and Daiichi-Sankyo; is an investigator in multicenter studies sponsored by Novartis and GI dynamics, respectively; and has consulted for XOMA, Sanofi, Novartis, and Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.Sh. and J.H.L researched data and ran the studies. F.M. undertook mathematical modeling of insulin secretion and action. M.Sa. conducted some of the meal studies and researched data. C.D.M. undertook mathematical modeling of insulin secretion and action. C.C. reviewed and edited the manuscript. R.A.R. contributed to discussion and reviewed and edited manuscript. M.C. supervised the gastric emptying studies and reviewed and edited the manuscript. A.R.Z. performed statistical analysis. A.V. researched data and wrote the manuscript. A.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01843881, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0954/-/DC1.
See accompanying commentary, p. 387.
References
- 1.Nguyen NT, Nguyen X-MT, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999-2006. Obes Surg 2011;21:351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med 2005;142:547–559 [DOI] [PubMed] [Google Scholar]
- 3.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA 2005;294:1909–1917 [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 7.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 2013;36:2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellum JM, Kuemmerle JF, O'Dorisio TM, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 1990;211:763–770 [DOI] [PMC free article] [PubMed]
- 9.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006;243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieffer TJ. Gastro-intestinal hormones GIP and GLP-1. Ann Endocrinol (Paris) 2004;65:13–21 [DOI] [PubMed] [Google Scholar]
- 12.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev 1999;20:876–913 [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 14.Schirra J, Nicolaus M, Roggel R, et al. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 2006;55:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schirra J, Nicolaus M, Woerle HJ, et al. GLP-1 regulates gastroduodenal motility involving cholinergic pathways. Neurogastroenterol Motil 2009;21:609–618 [DOI] [PubMed]
- 16.Sathananthan M, Farrugia LP, Miles JM, et al. Direct effects of exendin-(9,39) and GLP-1-(9,36)amide on insulin action, β-cell function, and glucose metabolism in nondiabetic subjects. Diabetes 2013;62:2752–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vella A, Rizza RA. Application of isotopic techniques using constant specific activity or enrichment to the study of carbohydrate metabolism. Diabetes 2009;58:2168–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293:E1–E15 [DOI] [PubMed] [Google Scholar]
- 19.Bock G, Dalla Man C, Micheletto F, et al. The effect of DPP-4 inhibition with sitagliptin on incretin secretion and on fasting and postprandial glucose turnover in subjects with impaired fasting glucose. Clin Endocrinol (Oxf) 2010;73:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalla Man C, Bock G, Giesler PD, et al. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care 2009;32:14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology 1997;112:1155–1162 [DOI] [PubMed] [Google Scholar]
- 22.Beylot M, Previs SF, David F, Brunengraber H. Determination of the 13C-labeling pattern of glucose by gas chromatography-mass spectrometry. Anal Biochem 1993;212:526–531 [DOI] [PubMed] [Google Scholar]
- 23.Steele R, Bjerknes C, Rathgeb I, Altszuler N. Glucose uptake and production during the oral glucose tolerance test. Diabetes 1968;17:415–421 [DOI] [PubMed] [Google Scholar]
- 24.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643 [DOI] [PubMed] [Google Scholar]
- 25.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–158 [DOI] [PubMed] [Google Scholar]
- 26.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 27.Ahrén B. GLP-1 for type 2 diabetes. Exp Cell Res 2011;317:1239–1245 [DOI] [PubMed] [Google Scholar]
- 28.Marathe CS, Rayner CK, Jones KL, Horowitz M. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Exp Diabetes Res 2011;2011:279530 [DOI] [PMC free article] [PubMed]
- 29.Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 1998;101:1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiménez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013;36:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013;62:3044–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ionut V, Liberty IF, Hucking K, et al. Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab 2006;291:E779–E785 [DOI] [PubMed] [Google Scholar]
- 33.Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 1999;48:86–93 [DOI] [PubMed] [Google Scholar]
- 34.Vella A, Rizza RA. Extrapancreatic effects of GIP and GLP-1. Horm Metab Res 2004;36:830–836 [DOI] [PubMed] [Google Scholar]
- 35.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salehi M, Aulinger B, Prigeon RL, D’Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 2010;59:1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woerle HJ, Carneiro L, Derani A, Göke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 2012;61:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu A, Alzaid A, Dinneen S, Caumo A, Cobelli C, Rizza RA. Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J Clin Invest 1996;97:2351–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu A, Caumo A, Bettini F, et al. Impaired basal glucose effectiveness in NIDDM: contribution of defects in glucose disappearance and production, measured using an optimized minimal model independent protocol. Diabetes 1997;46:421–432 [DOI] [PubMed] [Google Scholar]
- 40.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 2003;52:1147–1154 [DOI] [PubMed] [Google Scholar]
- 41.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012;61:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fich A, Neri M, Camilleri M, Kelly KA, Phillips SF. Stasis syndromes following gastric surgery: clinical and motility features of 60 symptomatic patients. J Clin Gastroenterol 1990;12:505–512 [PubMed] [Google Scholar]
- 43.Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab 2008;93:4909–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicolaus M, Brödl J, Linke R, Woerle H-J, Göke B, Schirra J. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab 2011;96:229–236 [DOI] [PubMed] [Google Scholar]
- 45.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 2010;95:215–221 [DOI] [PubMed] [Google Scholar]
- 46.Delgado-Aros S, Kim DY, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol 2002;282:G424–G431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.