Abstract
OBJECTIVE
Glucagon-like peptide 1 (GLP-1) is an incretin hormone that is released from the gastrointestinal tract. Treatment with GLP-1 analogs has proven to be of clinical use for patients with type 2 diabetes. Patients with type 1 diabetes, particularly those with residual β-cell function, may also respond to treatment, but the acute metabolic effects of GLP-1 analogs on these patients in reaction to both oral and intravenous glucose challenges are not well understood.
RESEARCH DESIGN AND METHODS
Seventeen patients with type 1 diabetes, half of whom had residual insulin production, underwent two mixed-meal tolerance tests (MMTTs) and two intravenous glucose tolerance tests (IVGTTs), with and without pretreatment with exenatide. No exogenous bolus insulin was administered for the studies. Glucose excursions, insulin secretion rates (ISRs), and levels of glucagon, endogenous GLP-1, and gastric inhibitory polypeptide were measured after the meal or glucose loads.
RESULTS
During the MMTT, glucose levels were suppressed with exenatide in patients with or without residual insulin production (P = 0.0003). Exenatide treatment did not change the absolute ISR, but the ISR to glucose levels were increased (P = 0.0078). Gastric emptying was delayed (P = 0.0017), and glucagon was suppressed (P = 0.0015). None of these hormonal or glucose changes were detected during the IVGTT with exenatide administration.
CONCLUSIONS
Exenatide showed a significant antidiabetogenic effect prior to an oral meal in patients with type 1 diabetes involving glucagon suppression and gastric emptying, while preserving increased insulin secretion. GLP-1 analogs may be useful as an adjunctive treatment in type 1 diabetes.
Introduction
Glucagon-like peptide 1 (GLP-1) is an incretin secreted from the L cells of the gastrointestinal tract in response to nutrient ingestion. In healthy control subjects, its physiologic effects control glucose levels by stimulating glucose-dependent insulin secretion, inhibiting glucagon secretion, and delaying gastric emptying (1). GLP-1 analogs have been developed to mimic the incretin response (2,3). Extensive studies of the mechanisms of GLP-1 analogs in patients with type 2 diabetes have confirmed their physiologic actions (4,5). These drugs as well as dipeptidyl-peptidase IV (DPPIV) inhibitors, such as sitagliptin, saxagliptin, linagliptin, alogliptin, and vildagliptin, which inhibit the degradation of GLP-1, are routinely used for treatment of this form of diabetes (6,7).
GLP-1 analogs may also have a role in the treatment of type 1 diabetes. Brown et al. (8) have demonstrated a progressive rise in meal-stimulated glucagon response associated with declining endogenous insulin production. GLP-1 analogs have been shown to inhibit glucagon levels, and therefore insulin-deficient individuals with type 1 diabetes may show a beneficial response on the basis of reduced glucagon secretion. Moreover, animal studies have suggested that GLP-1 therapy may promote the proliferation of β-cells, enhance β-cell recovery, and suppress β-cell apoptosis (9,10), suggesting that there may be long-term primary benefit to its use.
In patients with type 2 diabetes, GLP-1 analogs have been shown to augment glucose-dependent insulin secretion (11,12), but the significance of this action is not clear inasmuch as detailed analyses of GLP-1 receptor agonists in patients with residual insulin production are limited. Although older studies have highlighted the progression of type 1 diabetes to complete insulin deficiency, more recent studies have identified subjects with long-standing type 1 diabetes with residual insulin production (13,14). In these subjects, GLP-1 analogs may improve glucose control and reduce the need for exogenous insulin (15), because there may be a significant functional component to the loss of insulin secretion, possibly due to β-cell exhaustion from hyperglycemia (16). To assess whether combination therapies aimed at promoting β-cell growth in addition to agents that decrease the autoimmune destruction of β-cells, 20 subjects with long-standing type 1 diabetes were enrolled in a trial and randomized to exenatide with or without daclizumab (17). While C-peptide secretion increased with exenatide treatment, the difference failed to reach statistical significance. Other studies have suggested that residual insulin production is not a significant contributor to the effects of GLP-1 receptor agonists. Infusion of GLP-1 has been shown to reduce postprandial glycemic excursions in half in subjects with type 1 diabetes regardless of residual endogenous insulin production (18). Furthermore, GLP-1 infusion was found to delay gastric emptying. However, this study was conducted with infusions of GLP-1, and assessment was performed solely with orally administered glucose, which may not reflect physiologic stimuli.
To determine the metabolic effects of GLP-1 in patients with type 1 diabetes, we studied the acute effects of exenatide, a short-acting GLP-1 analog, on glucose tolerance to a mixed-meal tolerance test (MMTT) and an intravenous glucose tolerance test (IVGTT). We analyzed insulin secretion rates (ISRs), gastric emptying, and hormonal responses including glucagon, gastric inhibitory polypeptide (GIP), and endogenous GLP-1 release. We studied individuals with and without residual insulin production to determine the importance of insulin secretion on mediating the metabolic effects of the analog.
Research Design and Methods
Human Subjects
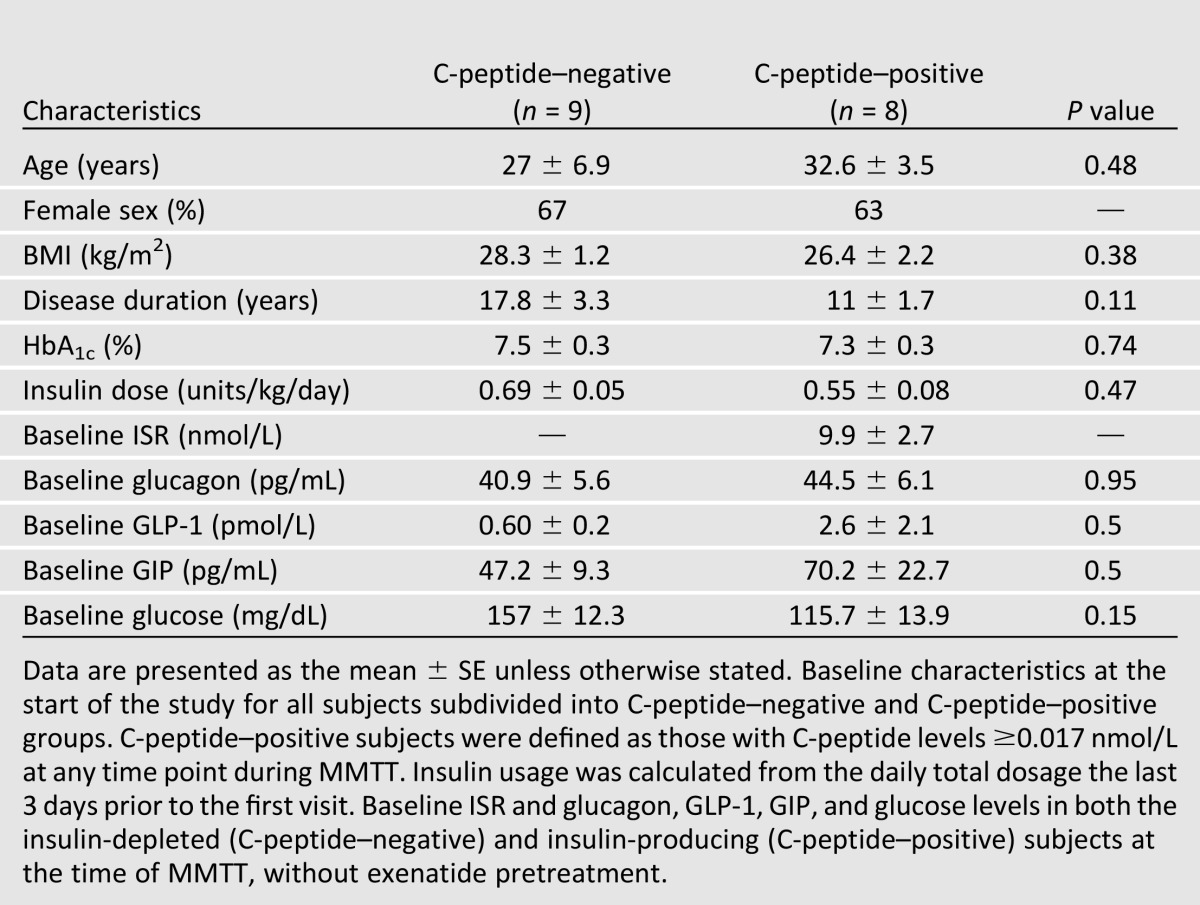
We studied 17 patients with type 1 diabetes with (C-peptide–positive) (n = 8) and without (C-peptide–negative) (n = 9) residual insulin production who were recruited from the Yale Diabetes Center. The presence of residual insulin production was identified post hoc after completion of the metabolic studies, and this information was used for the comparative data analysis. Patients were considered C-peptide–positive if they had a C-peptide value of ≥0.017 nmol/L at any time point during the MMTT. To enhance our detection of individuals with residual insulin production, we recruited individuals between the ages of 18 and 56 years, with a diabetes duration of at least 2 years, HbA1c level of <9%, and insulin usage of <0.9 units/kg/day. Table 1 shows the baseline characteristics of the study participants. The study protocol was approved by Yale University Institutional Review Board. Written informed consent was obtained from all patients.
Table 1.
Patient baseline characteristics
Study Procedures
Subjects underwent two MMTTs and two IVGTTs. During all studies, subjects received basal insulin (either as basal insulin through an insulin pump or as long-acting insulin); however, no short-acting insulin was administered for either the MMTT or IVGTT. The tests were performed in a randomized manner under two study conditions, with and without pretreatment with exenatide, 5 μg s.c., 15 min prior to the start of the test.
A standard 4-h MMTT was extended to 5 h because previous studies had shown delayed gastric emptying with GLP-1 use. For the MMTTs, patients drank a liquid meal (Boost High Protein, 6 mL/kg), and blood samples were collected at 13 time points (−10, 0, 15, 30, 60, 90, 120, 150, 180, 210, 240, 270, and 300 min) for the measurement of glucose, C-peptide, and glucagon, and, on a subset of subjects (n = 8), GLP-1 and GIP. Gastric emptying was evaluated by measuring the plasma levels of acetaminophen after a dose of 20 mg/kg to a maximum of 1,300 mg at the start of the meal. IVGTTs were performed by infusing a 20% dextrose solution at a dose of 0.5 g/kg up to a maximum of 35 g. Blood samples for the measurement of glucose and C-peptide were collected at −10, −4, 1, 3, 5, 7, and 10 min. GLP-1 (n = 6) and GIP (n = 4) samples were collected at the start and the end of the test. The IV glucose bolus was given at −3 min over 3 min.
Laboratory Measurements
Plasma C-peptide levels were measured at Northwest Lipid Metabolism and Diabetes Research Laboratories (Seattle, WA) using the Tosoh AIA 1800 assay. The lower limit of detection was 0.017 nmol/L with intra-assay and interassay coefficients of variation (CVs) of 1.71 and 4.68%, respectively. Glucagon samples were collected in EDTA tubes containing aprotinin but not DPPIV inhibitor. Glucagon levels were measured by radioimmunoassay (Millipore, St. Charles, MO). The lower limit of detection was 19 pg/mL, with intra-assay and interassay CVs of 6.58 and 6.64%. Total GLP-1 and GIP levels were collected in tubes containing EDTA and DPPIV inhibitor, and measured by ELISA (Alpco Diagnostics and Millipore). The GLP-1 assay did not detect exenatide and therefore measured endogenous GLP-1 production. The interassay CVs for low and high GLP-1 level controls are 8.8 and 7.0%, respectively, and for the low and high GIP level controls were 9.2 and 8.1%, respectively. Acetaminophen was measured by Roche Modular P analyzer using Syva Emit reagents (Siemens Healthcare Diagnostics Ltd, Newark, DE). HbA1c was measured using a Siemens DCA vantage analyzer machine. Plasma glucose levels were measured at the bedside using a biochemistry analyzer (Model 2700 Select; YSI and Xylem).
ISRs
To determine ISRs, the C-peptide levels obtained during MMTTs and IVGTTs were deconvoluted using a two-compartment model for hormone clearance with the Chronobiological Series Analyzer (CSA) software (19,20). Standard kinetic parameters for C-peptide were used based on the findings of Van Cauter et al. (21), who estimated rate constants based on extrapolations from C-peptide decay curves of 200 subjects. Parameters used for ISR calculation accounted for the patient’s age, sex, height, weight, and C-peptide values (in nanomoles per liter). Undetectable levels of C-peptide were assigned a value of 0.017 nmol/L, the lower limit of detection in the assay.
Statistical Analyses and Calculations
The baseline insulin dose was calculated as the average number of units used per day for 3 days prior to the first visit. Subjects without detectable fasting C-peptide levels were classified as not having residual insulin production, and we did not observe detectable levels after the MMTT or IVGTT. Total area under the curve (AUC) was calculated for ISR, and glucose, glucagon, GLP-1, GIP, and acetaminophen levels using the trapezoidal rule. The ISR/glucose ratio was calculated using the ISR calculated from the time interval initiated with the glucose level. Statistical analyses were performed with GraphPad (San Diego, CA) Prism version 5.04. Data are presented as means ± SE, unless indicated otherwise. For comparison between the two groups, a Wilcoxon matched-pairs signed rank test was used. Differences between groups resulting in two-tailed P values <0.05 were considered statistically significant.
Results
Metabolic Profiles of Subjects With Long-Standing Type 1 Diabetes
We studied 17 subjects with type 1 diabetes with (n = 8) and without (n = 9) endogenous C-peptide secretion. Their baseline characteristics are presented in Table 1. Although all of the subjects had type 1 diabetes for at least 2 years, those with residual insulin production tended to have a shorter duration of diabetes (P = 0.11). The HbA1c and total daily insulin doses were not significantly different between the groups.
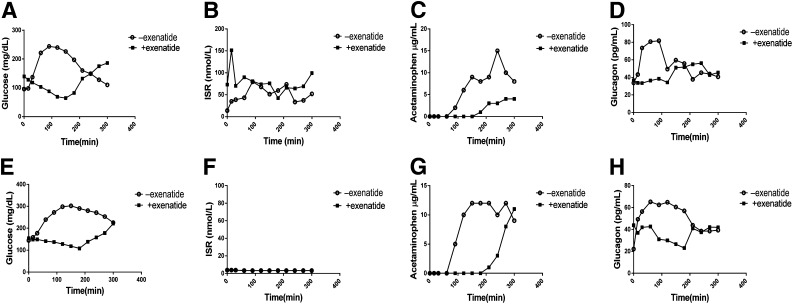
The insulin production that was detectable in C-peptide–positive subjects was responsive to the metabolic stimuli. ISRs increased approximately fourfold in those with residual insulin production during the MMTT without exenatide pretreatment, but only 1.3-fold in response to IV glucose (Figs. 1 and 2C and D).
Figure 1.
Metabolic effects of exenatide during MMTT: the kinetics of ISRs and glucose, acetaminophen, and glucagon levels are shown in a representative patient with (A–D) or without (E–H) detectable C-peptide levels. +, with; −, without.
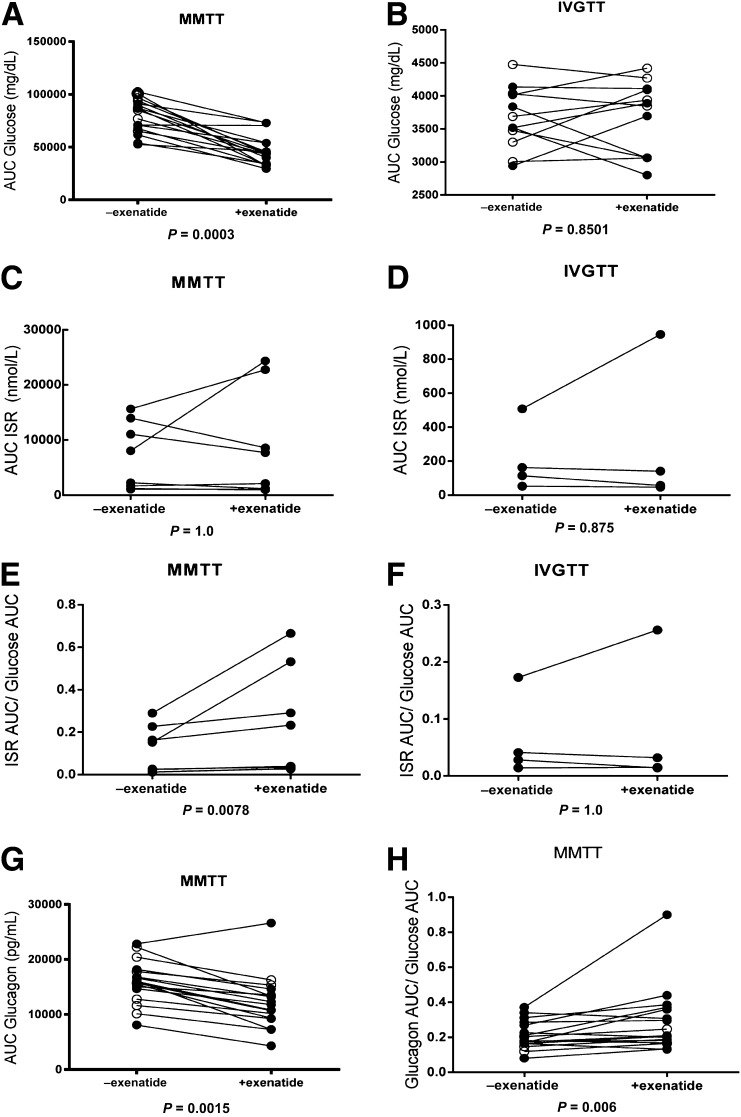
Figure 2.
Changes in hormonal responses during MMTT and IVGTT with exenatide.A: AUC for glucose (in milligrams per deciliter) during an MMTT without and with administration of exenatide, 5 µg s.c. (P = 0.0003). B: AUC for glucose (in milligrams per deciliter) during IVGTT without and with exenatide (P = 0.8501). C–F: Only patients with detectable C-peptide levels are shown. C: AUC for ISR (in nanomoles per liter) during MMTT without and with subcutaneous injection of exenatide (P = 1.0). D: AUC for ISR during an IVGTT (P = 0.875). E: AUC ISR/AUC glucose during MMTT with and without exenatide (P = 0.0078). F: ISR AUC/glucose AUC at the time of IVGTT with and without pretreatment with exenatide (P = 1.0). G: AUC for glucagon (in picograms per milliliter) during MMTT with and without administration of exenatide (P = 0.0015) in all subjects. H: AUC of the glucagon/glucose ratio in all patients during MMTT, which shows a significant increase with exenatide (P = 0.006). Open circles (○) indicate C-peptide–negative patients, and closed circles (●) indicate C-peptide–positive patients. +, with; −, without.
Effects of Exenatide on the Metabolic Responses in MMTTs and IVGTTs
The glucose, ISR, glucagon, and acetaminophen responses of representative subjects with and without endogenous C-peptide secretion are shown in Fig. 1. When all subjects with and without residual insulin production were considered together, the glucose excursions after the liquid meal were reduced by 33% with exenatide pretreatment (without residual insulin production 79,626 ± 3,869 mg/dL; with residual insulin production 4,665 ± 3,427 mg/dL; P < 0.001) (Fig. 2A). We did not find a difference in the exenatide effects on glucose excursions in subjects with versus those without residual insulin production. In contrast, we did not detect an effect of exenatide on glucose responses to IVGTT (without residual insulin production 3,661 ± 133.2 mg/dL; with residual insulin production 3,686 ± 158.2 mg/dL; P = 0.8501) (Fig. 2B).
Exenatide is known to delay gastric emptying. To assess this effect in patients with type 1 diabetes, we measured the absorption of acetaminophen during the MMTT performed with or without exenatide pretreatment. Gastric emptying was delayed, and the total acetaminophen absorption was reduced from 2,058 ± 196 to 686 ± 138 µg/mL (P = 0.0017). The effects on gastric emptying were also similar in subjects with and without residual insulin production.
Effects of Exenatide on Hormonal Responses
We analyzed the ISR in the eight subjects with detectable levels of C-peptide. Of these, five subjects showed a reduction in the absolute ISR AUC when exenatide was given, and the remainder showed an increase. Thus, overall the absolute levels of ISR were not changed by the exenatide (Fig. 2C) (P = 1.0). Likewise, we did not see an effect of exenatide treatment on the insulin secretory response to IVGTT (Fig. 2D). Interestingly, when we evaluated the relationship of ISR to the glucose levels (AUC ISR/AUC glucose) in C-peptide–positive patients, we found that the levels were significantly higher in the exenatide-treated group (without treatment 0.1134 ± 0.0388; with treatment 0.2318 ± 0.08855; P = 0.0078) (Fig. 2E). We did not see an effect of exenatide treatment on the insulin secretory response to IVGTT (without treatment 209.5 ± 102.1 nmol/L; with treatment 297 ± 217.1 nmol/L; P = 0.875) (Fig. 2D) or an effect on the relationship between ISR and glucose in C-peptide–positive patients during the IVGTTs (without treatment 0.0640 ± 0.03675; with treatment 0.07925 ± 0.05906; P = 1.0) (Fig. 2F).
Glucagon levels were significantly suppressed in the presence of exenatide (without treatment 15,909 ± 945.8 pg/mL; with treatment 12,124 ± 1,182 pg/mL; P = 0.0015) (Fig. 2G). However, the glucagon/glucose ratio was significantly increased after exenatide treatment (without treatment 0.2116 ± 0.01925; with treatment 0.2875 ± 0.0446; P = 0.006), suggesting that the decrease in glucose excursion involved factors in addition to the effects on glucagon itself (Fig. 2H). The responses in patients with and without residual insulin were similar.
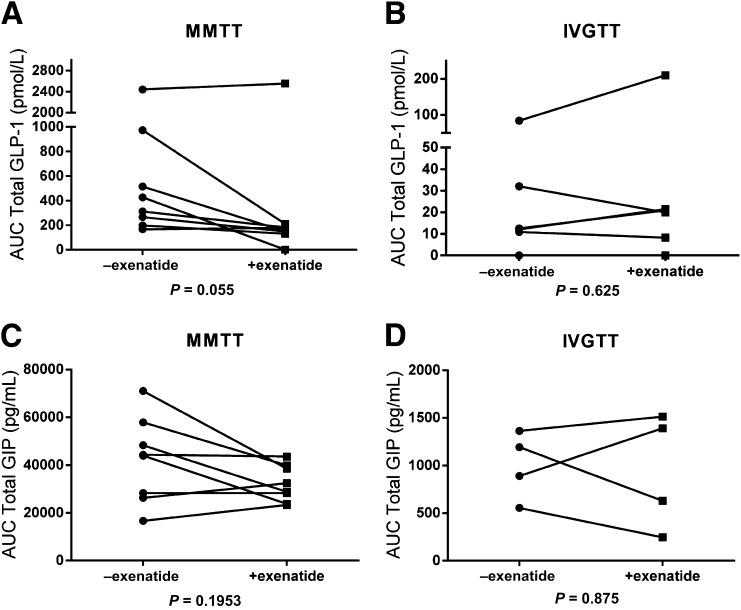
Using an assay that was specific for endogenous GLP-1 and did not cross-react with the exenatide that was administered, we found that there were reduced GLP-1 levels in six of the eight subjects, while the levels increased in two of eight subjects when they received exenatide, although the differences in the hormone levels did not reach statistical significance (without exenatide 662.6 ± 270 pmol/L; with exenatide 444.2 ± 302.1 pmol/L; P = 0.055) (Fig. 3A). We did not find a significant change in the levels of GIP when exenatide was administered (without exenatide 1,000 ± 177.8 pg/mL; with exenatide 944.5 ± 304.2 pg/mL; P = 0.875) (Fig. 3B–D).
Figure 3.
Changes in incretins with exenatide. A: AUC for GLP-1 during MMTT without exenatide 662.6 ± 270 pmol/L vs. with exenatide 444.2 ± 302.1 pmol/L in eight subjects (P = 0.055). B: AUC for GLP-1 during IVGTT (without exenatide 25.28 ± 12.48 pmol/L vs. with exenatide 46.76 ± 32.83 pmol/L) in six subjects (P = 0.625). C: AUC for GIP during MMTT without and with exenatide in eight subjects. D: AUC for GIP (in picograms per milliliter) during IVGTT (P = 0.875). +, with; −, without.
Conclusions
We studied the acute effects of exenatide, a short-acting GLP-1 receptor agonist, in individuals with type 1 diabetes with and without residual insulin production. The primary objective of our study was to determine whether exenatide would affect the acute metabolic responses to an MMTT or an IVGTT in subjects with established type 1diabetes, and to determine the significance of residual insulin production on those responses. We calculated the ISR rather than using raw C-peptide values, which gives a more accurate assessment of β-cell function, inasmuch as the C-peptide levels may not reflect the true levels of insulin secretion because of the relatively long half-life of C-peptide. Moreover, we were able to study the effects of GLP-1 analogs on endogenous GLP-1 and GIP in a subset of subjects. We found that administration of exenatide reduced glucose excursion and absolute glucagon secretion during an MMTT and delayed gastric emptying, similar to previous reports (22–25). The total absorption of acetaminophen was reduced, and gastric emptying was delayed with exenatide administration. On average, we did not find an absolute increase in insulin secretion in subjects who were able to secrete insulin, but the relative amount of insulin secreted for the glucose was increased.
Because we did not find an increase in the absolute amount of insulin that was secreted, the proportional increase in insulin for the glucose most likely is a reflection of the reduced glucose levels in subjects who were already maximally secreting insulin. The changes that we found in the glucagon/glucose ratio are consistent with the major effect on reducing glucose levels. Therefore, our studies indicate that the metabolic effects of exenatide involve two mechanisms, including its effects on the absorption of nutrients and glucagon inhibition, but based on our study design we cannot exclude an effect on augmenting insulin production as well.
The metabolic effects require oral absorption of nutrients because we found no glycemic or hormonal effects of exenatide on the responses to intravenous glucose. The stimuli of a mixed meal and intravenous glucose are different—the former includes protein and fat. In addition, the route of administration may be important because DPPIV inhibitors have been shown to regulate glycemia by local inhibition of intestinal DPPIV activity, activation of incretin receptors, and activation of the gut-to-pancreas neural axis (26).
Endogenous GLP-1 levels were decreased with exenatide administration in six of eight patients, which may have been due to the effects of the drug on gastric emptying or a feedback inhibition of GLP-1 secretion (27). Kielgast et al. (15) studied the effects of exenatide prior to an MMTT in eight subjects with and eight subjects without residual insulin production. In these subjects, one-half of the usual dose of fast-acting insulin was administered together with exenatide. They reported that the incretin responses were similar in patients compared with healthy control subjects and found that the responses were also similar between those with and without residual insulin production. Similarly, Gutniak et al. (28) studied hormonal responses in insulin-deficient patients with type 1 diabetes and found reduced insulin requirements and glucagon responses when GLP-1 was infused. In addition to a similar reduction in glucagon release in response to a mixed meal that was seen by these previous authors, our data suggest a reduced level of GLP-1 in most patients, but the number of subjects that we studied was small.
Despite the profound effect of the GLP-1 receptor agonist on glycemic excursion in our short-term studies, the effects of therapy with GLP-1 receptor agonist on the clinical management of patients with type 1 diabetes have been relatively modest (29). The most significant metabolic consequence of exenatide administration appears to involve the delay in absorption of nutrients and the reduced rise in glucose as a consequence, but testing for additional benefits to individuals with residual insulin production may require further experience with larger numbers of subjects and longer use of an agonist. In addition to consideration of GLP-1 receptor agonists in the chronic metabolic management, these agents might be considered in combination with other agents, including immune modulators in the new-onset period (30).
In summary, in patients with long-standing type 1 diabetes, with and without residual insulin production, we observed a marked reduction in glycemic excursion during an MMTT with exenatide pretreatment, but no changes were observed in glucose excursion in response to an intravenous glucose challenge with exenatide pretreatment. In patients with residual insulin production, the insulin secretion was preserved even with reduced glucose levels. The value of GLP-1 receptor agonists in long-term management of type 1 diabetes, particularly those with residual insulin production, will require long-term studies with larger numbers of subjects.
Funding and Duality of Interest.
This research was supported by JDRF grant 17-2011-559; by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1 RR024986; by an American Diabetes Association and Merck Clinical/Translational Science Postdoctoral Fellowship Award, and a supplement to the University of Michigan Clinical and Translational Science Award; and by grants from Amylin Inc. and the Brehm Foundation. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.G. researched data, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. L.R. researched data. J.L.S. researched data and reviewed and edited the manuscript. K.C.H. researched data, contributed to the discussion, and reviewed and edited the manuscript. T.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
References
- 1.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987;2:1300–1304 [DOI] [PubMed] [Google Scholar]
- 2.Ahrén B, Larsson H, Holst JJ. Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997;82:473–478 [DOI] [PubMed] [Google Scholar]
- 3.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 1996;81:327–332 [DOI] [PubMed] [Google Scholar]
- 4.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 5.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, NN2211-1310 International Study Group Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care 2004;27:1335–1342 [DOI] [PubMed] [Google Scholar]
- 6.Solis-Herrera C, Triplitt C, Garduno-Garcia JJ, Adams J, DeFronzo RA, Cersosimo E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care 2013;36:2756–2762 [DOI] [PMC free article] [PubMed]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 8.Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care 2008;31:1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farilla L, Hui H, Bertolotto C, et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 2002;143:4397–4408 [DOI] [PubMed] [Google Scholar]
- 10.Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003;144:5149–5158 [DOI] [PubMed] [Google Scholar]
- 11.Chang AM, Jakobsen G, Sturis J, et al. The GLP-1 derivative NN2211 restores β-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes 2003;52:1786–1791 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004;117:77–88 [DOI] [PubMed] [Google Scholar]
- 13.Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes 2004;53:426–433 [DOI] [PubMed] [Google Scholar]
- 14.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta-cell function. Diabetes Care 2011;34:1463–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 2006;55:3238–3245 [DOI] [PubMed] [Google Scholar]
- 17.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on β-cell function in long-standing type 1 diabetes. Diabetes Care 2009;32:2251–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes 2011;60:1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ibridge. Chronobiological Series Analyzer (CSA) [article online], 2013. Available from http://www.ibridgenetwork.org/uctech/chronobiological-series-analyzer-csa Accessed 14 August 2013
- 20.Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B. Use of biosynthetic human c-peptide in the measurement of insulin secretion rates in normal volunteers and type 1 diabetic patients. J Clin Invest 1986;77:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 22.Varanasi A, Bellini N, Rawal D, et al. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol 2011;165:77–84 [DOI] [PubMed] [Google Scholar]
- 23.Raman VS, Mason KJ, Rodriguez LM, et al. The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care 2010;33:1294–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care 1996;19:580–586 [DOI] [PubMed] [Google Scholar]
- 25.Dupré J, Behme MT, McDonald TJ. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab 2004;89:3469–3473 [DOI] [PubMed] [Google Scholar]
- 26.Waget A, Cabou C, Masseboeuf M, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology 2011;152:3018–3029 [DOI] [PubMed] [Google Scholar]
- 27.Vilsbøll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 2003;88:2706–2713 [DOI] [PubMed] [Google Scholar]
- 28.Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendić S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992;326:1316–1322 [DOI] [PubMed] [Google Scholar]
- 29.Harrison LB, Mora PF, Clark GO, Lingvay I. Type 1 diabetes treatment beyond insulin: role of GLP-1 analogs. J Investig Med 2013;61:40–44 [DOI] [PubMed] [Google Scholar]
- 30.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 2007;148:5136–5144 [DOI] [PubMed] [Google Scholar]