ABSTRACT
The "catch-up growth" phenomenon in children born small for gestational age (SGA) has been linked to early onset obesity with the subsequent emergence of metabolic syndrome (MetS). The intima media thickness of the common carotid artery (CIMT) is a well-known marker of subclinical atherosclerosis.
Aim: to determine the association between being born SGA and CIMT, a measure of atherogenesis and to investigate metabolic risk factors which impact on CIMT in obese children.
Material and methods: A prospective study was carried out over a 1 year period (July 2012-June 2013). We analyzed 122 obese patients, 96 patients appropriate for gestational age (AGA) and 26 patients SGA. Both groups were matched for age, sex and BMI. Blood pressure, lipids and glucose were determined. Oral glucose tolerance tests (oGTT) were performed. Insulin resistance (IR) was assessed by homeostasis model assessment (HOMA). CIMT was measured in all the patients.
Results: CIMT in obese children born SGA was significantly increased as compared with obese children born AGA similar age, sex and BMI (p=0.0035). We demonstrated a strong correlation between CIMT and all other metabolic factors (r=0.98). In both groups, mean CIMT of was significantly related to diastolic blood pressure, triglycerides and HOMA. CIMT was not significantly related to systolic blood pressure and baseline glucose.
Conclusion: High triglycerides levels and low HDL-cholesterol levels, IR and diastolic blood pressure, which are all components of MetS are strong predictors of increased CIMT in obese children. Being born SGA increases the atherogenic risk.
Keywords: small for gestational age, obesity, intima media thickness of the common carotid artery
INTRODUCTION
The "catch-up growth" phenomenon in children born small for gestational age (SGA) has been linked to early onset obesity with the subsequent emergence of metabolic syndrome (MetS) or its components.
About 3-5% of neonates are born small for gestational age (SGA). 85-90% of them recover weight, up to 2 years of age, majority of which become obese up to 4 years of age, later on developing components of MetS. The rapid "catch up" growth during the cell division period up to 2 years of age leads to hyperplasic obesity (1,2).
These children have a high risk of developing MetS with all its components: obesity, impaired glucose tolerance, insulin resistance with subsequent development of diabetes, arterial hypertension, dyslipidemia.
As indicated in previous studies (3-5), children and adolescents with risk factors such as obesity, dyslipidemia, elevated blood pressure and impaired glucose metabolism are at increased risk of developing atherosclerosis in adulthood. It has been found that obesity results in the early onset of adulthood chronic disease such as cardio-cerebrovascular disease.
The intima media thickness of the common carotid artery (CIMT) is a well-known marker of subclinical atherosclerosis and is a noninvasive and inexpensive method for detecting development of subclinical atherosclerosis. Studies in adults have revealed that CIMT was related to cardiovascular risk factors and could predict the possibility of future cardio-cerebrovascular disease (6,7).
There has been no statistical data about the association between CIMT and SGA or between CIMT and the components of MetS in SGA children. This study aimed to determine the association between being born SGA and CIMT, a measure of atherogenesis, in obese children and to investigate metabolic risk factors which impact on CIMT in obese children. ❑
MATERIAL AND METHODS
A prospective study was conducted over a period of 1 year, between July 2012 and June 2013, on cases of obesity in children diagnosed at "Louis Turcanu" Emergency Hospital for Children Timişoara, in the departments of Diabetes and Nutritional Diseases, Endocrinology and Cardiology.
Children were considered obese on the basis of age specific BMI reference guidelines from Centers for Disease Control and Prevention Child Growth Standards 2000 (above 95th percentile) (8). When defining SGA, growth nomograms and charts proposed by Niklasson (9) are being used; newborns weighing less than 2 standard deviations (SD) from the average for gestational age, we considered as being SGA.
Blood pressure was measured; systolic (SBP) and diastolic (DBP) blood pressure were measured at the right arm twice after a 10 minutes rest in the supine position, by using a calibrated sphygmomanometer and averaged. Hypertension was defined by blood pressure above the 95th percentile for height, age, and gender (10). Blood sampling was performed in the fasting status. Serum triglyceride, HDL-cholesterol, LDL-cholesterol, insulin, and glucose concentrations were measured in all children using commercially available test kits (lipids and plasma glucose levels COBAS INTEGRA-400 Roche Diagnostics), plasma insulin levels DPC-Immulite-One (Siemens Medical solutions). oGTT was performed in all children.
Impaired glucose tolerance was defined by 2 h serum glucose >140 mg/dl in the oGTT according to definition of MetS by Weiss (11). Dyslipidemia was defined by triglycerides >95th percentiles, LDL-cholesterol >95th percentiles or HDL-cholesterol <5th percentile (12).
Basal glucose and insulin levels of the patients were measured and IR index was assessed by homeostasis model assessment (HOMA- fasting glucose in mmol/l multiplied by baseline insulin in microunits per milliliter, divided by 22.5). A cut-off HOMA level above 2.5 in the prepubertal period and of >3.5 for adolescents was used to define an IR status.
CIMT was measured by B-mode ultrasound using a 10-MHz linear transducer (General Electric). The subjects were examined supine with the neck extended and the probe in the antero-lateral position. All measurements of CIMT were made in the longitudinal plane at the point of maximum thickness on the far wall of the common carotid artery along a 1 cm section of the artery proximal to the carotid bulb. The CIMT was defined as the distance between the intima-blood interface and the adventitia-media junction. After freezing the image, the measurements were made using electronic calipers. The maximal thicknesses of the intima-media width were measured to give three readings and the mean value was used for statistical purposes.
Exclusion criteria were evidenced for syndromal, chromosomal, or infectious etiology of low birth weight, endocrine or syndromal disorders, systemic disease or acute illness.
We analyzed 122 patients diagnosed with obesity, including 96 patients AGA and 26 patients SGA. Both groups were matched for age, sex and BMI.
The data are expressed as means ± standard deviation or as frequencies. Statistical analysis was performed with SPSS 17.0. We used the unpaired t test (with a confidence interval of 95 percent) to evaluate the differences between the two groups SGA vs. AGA. Multiple stepwise linear regression analysis was used to examine relationships between mean IMT and all other variables investigated. A p<0.05 was considered statistically significant.
Consent was obtained from the parents and the Ethical Committee of the hospital. ❑
RESULTS
The characteristics of the 2 groups:
The two groups were homogenous regarding BMI, age and sex. There was no statistical difference in the age, sex and BMI among the 2 groups (p = 0.68, 0.78, 0.79), as shown in Table 1.
Table 1.
Anthropometric, metabolic and CIMT characteristics of the study groups.
| Total number |
Obese SGA-group I 26 |
Obese AGA-group II 96 |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | P value | |
| Age (years) | 14.208333 | 3.33595911 | 5-17 | 14.79167 | 2.28457 | 4-20 | 0.68 |
| Birth Weight (grams) | 2550 | 403.51933 | 970-2860 | 3446.25 | 461.371 | 2400-5300 | 0.000285 |
| Gestational age (weeks) | 38 | 2.89827534 | 30-41 | 39.368 | 1.14902 | 34-41 | 0.025 |
| Sex (%) Male Female |
42.3% 57.7% |
36.5% 63.5% |
0.78 |
||||
| Residence Urban/rural |
57%/43% |
60%/40% |
0.79 |
||||
| Antropometric data BMI (kg/m2) | 29.623 | 8.13 | 19-54.48 | 30.604 | 6.302 | 17-47 | 0.5 |
| Haemodinamic data SBP (mmHg) DBP (mmHg |
114 70 |
22.6 12.7 |
70-160 50-100 |
117.270 74.010 |
19.10 12.344 |
70-180 40-120 |
0.474 0.0151 |
| Biological data Baseline glucose (mmol/l) 2 hours glucose (mmol/l) HOMA IR Triglycerides (mmol/l) HDL-cholesterol (mmol/l) |
4.38307 5.6177 3 2 1 |
0.406012 1.183376 1.968 0.714 0.313 |
3.8-5.2 3.55-8.18 0.4-9.2 0.47-3 0.65-2.28 |
4.500 5.930 3.418 1.141 1.105 |
0.9342 1.4362 2.5695 0.5343 0.3756 |
3.8-11.3 4-11.8 0.35-28.2 0.33-2.94 0.26-2.91 |
0.346 0.228 0.035 0.000986 0.523 |
| CIMT (mm) | 0.057385 | 0.008537 | 0.4-0.9 | 0.043 | 0.008 | 0.3-0.7 | 0.0035 |
There were significant differences between the two groups regarding birth weight and gestational age.
HOMA-IR was significant different between the two groups. Lipids profile shown differences in triglycerides value more then in HDL-cholesterol values.
We did not find significant differences between the two groups AGA vs SGA regarding systolic blood pressure, baseline glucose and 2 hours serum glucose.
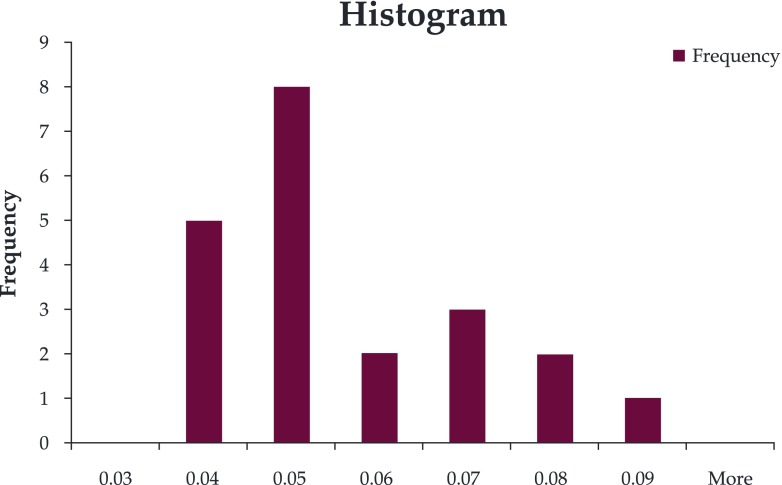
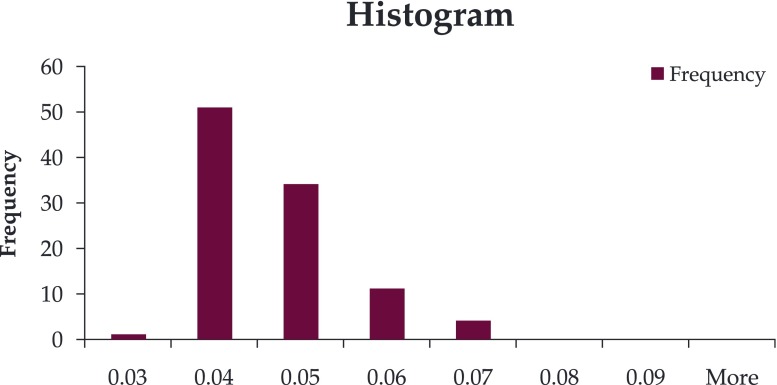
CIMT was increased in the SGA group (mean 0.057 vs 0.043) (Figure 1, Figure 2); there was a significant differences between the two groups (p=0.0035).
Figure 1. CIMT histogram in group I.
Figure 2. CIMT histogram in group II.
The relationship between CIMT and all other variables investigated.
Multiple R is closed to r, meaning a strong correlation between CIMT and all other factors. R square shows that 97.12% from CIMT variation in group I and 99.3% in group II is due to the cumulative influence factors (Table 2, Table 3).
Table 2.
Regression statistic group I.
| Regression Statistics | |
|---|---|
| Multiple R | 0.98554079 |
| R Square | 0.97129066 |
| Adjusted R Square | 0.95562634 |
| Standard Error | 0.00801278 |
| Observations | 96 |
Table 3.
Regression statistic group II.
| Regression Statistics | |
|---|---|
| Multiple R | 0.996631 |
| R Square | 0.993274 |
| Adjusted R Square | 0.902654 |
| Standard Error | 0.007001 |
| Observations | 26 |
In both groups, mean CIMT of was significantly related to gestational age, triglycerides, diastolic blood pressure and HOMA. There were some differences between the 2 groups: group I CIMT was more related to gestational age, triglycerides and HDL-cholesterol and group II was more related to cholesterol, gestational age and 2 hours glucose. CIMT was not significantly related to systolic blood pressure and baseline glucose (Table 4, Table 5). ❑
Table 4.
Correlation between CIMT and other variables group I.
| t Stat | P-value | Lower 95% | Upper 95% | r | |
|---|---|---|---|---|---|
| Gestational age (weeks) | 4.553028 | 1.75E-05 | 0.000498 | 0.001271 | 0.5798969 |
| HDL cholesterol | 1.598727 | 0.011359 | -0.00158 | 0.005068 | 0.464708 |
| HOMA-IR | 1.517442 | 0.013287 | -0.00026 | 0.001929 | 0.414227 |
| Triglycerides | 1.83301 | 0.050302 | -0.00033 | 0.008051 | 0.498442 |
| DBP | 0.487792 | 0.0536953 | -0.00016 | 0.000268 | 0.3924825 |
| Cholesterol | 1.044379 | 0.299271 | -0.00158 | 0.005068 | 0.2699257 |
| SBP | 0.74256 | 0.459797 | -0.00019 | 8.59E-05 | 0.3347851 |
| baseline glucose | 0.28675 | 0.775001 | -0.00261 | 0.001951 | 0.170452 |
| 2 hour glucose | 0.14665 | 0.883752 | -0.0018 | 0.001557 | 0.189122 |
DBP-diastolic blood pressure, SBP-systolic blood pressure
Table 5.
Correlation between CIMT and other variables group II.
| t Stat | P-value | Lower 95.0% | Upper 95.0% | r | |
|---|---|---|---|---|---|
| Cholesterol | 2.865656 | 0.012459 | 0.002695 | 0.018731 | 0.2699257 |
| 2hours glucose | 0.949927 | 0.035826 | -0.00182 | 0.00471 | 0.589122 |
| DBP | 0.929466 | 0.0368398 | -0.00024 | 0.000595 | 0.67 |
| Gestational age (weeks) | 1.364831 | 0.043841 | -0.0004 | 0.00178 | 0.3798969 |
| HOMA-IR | 0.53508 | 0.05 | -0.00551 | 0.003307 | 0.45 |
| SBP | 0.50792 | 0.619418 | -0.00024 | 0.000382 | 0.23 |
| baseline glucose | 0.49281 | 0.629785 | -0.01147 | 0.007187 | 0.15 |
| HDL cholesterol | 0.300814 | 0.767978 | -0.01232 | 0.016335 | 0.33 |
DBP-diastolic blood pressure, SBP-systolic blood pressure
DISCUSSIONS
Regarding the distribution of obese patients according to birth weight, as expected, it appears that SGA group is lower than AGA group, accounting for about a quarter of it.
Recent reports indicate that the presence of obesity in childhood is associated with increased adult CIMT (4,5). CIMT is a well-known marker of subclinical atherosclerosis and it also can indicate future cardio-cerebrovascular disease (6,7,13). In our study we measured the CIMT in obese SGA and non SGA subjects. We found that CIMT in obese children born SGA was significantly increased as compared with obese children born AGA similar age, sex and BMI. Several reports suggest increased CIMT in obese children, to date very few studies regarding CIMT in SGA children have been carried out. We found two other studies that are in accordance with our study (14,15), one that could not demonstrate an association between birth weight and CIMT (16).
In our study, CIMT was significantly related to IR, triglycerides and HDL-cholesterol a in both groups, suggesting a link between IMT, insulin resistance and dyslipidemia.
Baseline glucose levels were almost similar in both groups. A difference in HOMA has been noticed.
Fasting insulin and HOMA-IR levels were significantly related to CIMT. We could not say the same for fasting blood glucose. We speculated that an early phase of increased insulin level during childhood might precede the onset of insulin resistance in young adult SGA subjects. Atabek et al (17) demonstrated an association between IR and premature carotid atherosclerosis in children obesity. Insulin not only directly stimulates the expression of vascular cell adhesion molecule (18), but disrupts the balance between the production of NO and ET-1 leading to endothelial dysfunction (19).
Dyslipidemia is related to cardio-cerebrovascular disease (20,21). According to Pearson correlation analysis triglycerides and HDL-cholesterol were related to IMT. Hypertension was the less frequent component of MetS in our study. It was equal distributed. There is also a significant correlation between DBP and CIMT but not between SBP and CIMT and this finding is in accordance to other studies did (22,23). Perry and colleagues (24) found that in subjects aged under 40 years, DBP is a stronger predictor of cardiovascular risk than SBP. ❑
LIMITATIONS
Data from our small clinical samples and the limited number of SGA group may not be representative for general populations. The CIMT may also probably be influenced by other risk factors which have not been tested in our study. ❑
CONCLUSION
High tryglyceride level and low HDL-cholesterol level, IR and blood pressure, which are all components of MetS are strong predictors of increased CIMT in obese children. Being born SGA increases the atherogenic risk.
Metabolic impairment in SGA children is amplified by weight gain and influenced by fetal programming; developing intrauterine IR as a prenatal surviving mechanism is a risk factor for postnatal MetS. IR is a significant determinant of mean CIMT level, which indicates that it is closely related to cardio-cerebrovascular disease.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Roede MJ, Van Wieringen JC. Growth diagrams 1980. Netherlands third nation-wide survey. Tijdschrift voor Sociale Gezondheidszorg. 1985;63(suppl):1–34. [Google Scholar]
- 2.Benson CB, Doubilet PM, Saltzman DH. Intrauterine growth retardation: predictive value of US criteria for antenatal diagnosis. Radiology. 1986;160:415–417. doi: 10.1148/radiology.160.2.3523593. [DOI] [PubMed] [Google Scholar]
- 3.Davis PH, Dawson JD, Riley WA, et al. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 4.Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Chen W, Srinivasan SR, et al. Childhood cradiovascular risk factors and carotid vascular changes in adulthood the Bogalusa Heart Study. JAMA. 2003;290:2271–6. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 6.Csiba L. Carotid intima-media thickness measured by ultrasonography: effect of different pharmacotherapies on atherosclerosis progression. Orv Hetil. 2005;146:1239–44. [PubMed] [Google Scholar]
- 7.Lorenz MW, Markus HS, Bots ML, et al. Prediction of Clinical Cardiovascular Events With Carotid Intima-Media Thickness. a systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics, Centers for Disease Control and Prevention growth charts: United States National Center for Health Statistics, Hyattsville, MD, United States, 2000. Avaliable from www.cdc.gov/growthcharts.
- 9.Niklasson A, Ericson A, Fryer JG, et al. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981). Acta Paediatrica Scandinavica. 1991;80:756–762. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 10.National High Blood Pressure Education Program. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 11.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 12.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 13.Urbina EM, Srinivasan SR, Tang R, et al. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study). Am J Cardiol. 2002;90:953–8. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 14.Crispi F, Figueras F, Cruz-Lemini M, et al. Cardiovascular programming in children born small for gestational age and relationship with prenatal signs of severity. Am J Obstet Gynecol. 2012;207:121e1–9. doi: 10.1016/j.ajog.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Trevisanuto D, Avezzù F, Cavallin F, et al. Arterial wall thickness and blood pressure in children who were born small for gestational age: correlation with umbilical cord high-sensitivity C-reactive protein. Arch Dis Child. 2010;95:31–4. doi: 10.1136/adc.2008.150326. [DOI] [PubMed] [Google Scholar]
- 16.Dratva J, Breton CV, Hodis HN, et al. Birth weight and carotid artery intima-media thickness. J Pediatr. 2013;162:906–11.e1-2. doi: 10.1016/j.jpeds.2012.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atabek ME, Pirgon O, Kivrak AS. Evidence for association between insulin resistance and premature carotid atherosclerosis in children obesity. Pediatr Res. 2007;61:345–9. doi: 10.1203/pdr.0b013e318030d206. [DOI] [PubMed] [Google Scholar]
- 18.Kim I, Moon S-O, HOON Kim S, et al. Vascular endothelial growth factor expression of intercellular adhesion molecule 1(ICAM-1), vascular cell adhesion molecule 1(VCAM-1), and E-selectin through nudear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;76:7614–20. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 19.Pandolfi A, Solini A, Pellegrini G, et al. Selection insulin resistance affecting nitric oxide release but not plasminogen activator inhibitor-1 synthesis in fibroblasts from insulin–resistance individuals. Arterioscler Thromb Vasc Biol. 2005;25:2392–7. doi: 10.1161/01.ATV.0000185831.13559.a2. [DOI] [PubMed] [Google Scholar]
- 20.Magnussen CG, Venn A, Thomson R, et al. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol. 2009;53:860–9. doi: 10.1016/j.jacc.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis. 2008;196:489–96. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Reinehr T, Kiess W, de Sousa G, et al. Intima media thickness in children obesity relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006;55:113–8. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Zanchetti A, Crepaldi G, Bond MG, et al. Systolic and pulse blood pressures (but not diastolic blood pressure and serum cholesterol) are associated with alterations in carotid intima-media thickness in the moderately hypercholesterolaemic hypertensive patients of the Plaque Hypertension Lipid Lowering Italian Study. PHYLLIS study group. J Hypertens. 2001;19:79–88. doi: 10.1097/00004872-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Perry MP, et al. Pretreatment blood pressure as a predictor of 21 year mortality. Am J Hypertens. 2000;13:724–733. doi: 10.1016/s0895-7061(99)00214-9. [DOI] [PubMed] [Google Scholar]