Abstract
Podocytes are an essential component of the glomerular filtration barrier and cover the outer aspect of glomerular capillaries. They form a complex actin-based cytoskeleton in vivo and show prominent motility in vitro, but whether podocytes are stationary or mobile in vivo is debated. To address this question, the pronephros of translucent zebrafish larvae (casper) expressing enhanced green fluorescent protein (eGFP) specifically in podocytes (wt1a:eGFP larvae) was observed by intravital two-photon microscopy over extended periods of time. Podocyte cell bodies and the interdigitating branching pattern of major processes could be resolved with a resolution of approximately 1 µm in the xy-plane. Time-lapse imaging of zebrafish larvae at 5–7 days after fertilization demonstrated that podocytes neither migrated nor changed the branching pattern of their major processes over a time period of up to 23 hours. In summary, we show by extended intravital two-photon microscopy that podocytes are stationary cells in the intact glomerulus of a translucent zebrafish with fluorescently-labeled podocytes.
Podocytes cover the outer aspect of the glomerular capillaries forming actin-based interdigitating foot processes (FPs) with neighboring podocytes in a zipper-like manner.1 This unique three-dimensional organization of FPs is required for proper filtration of the blood and an intact filtration barrier. Ultrastructural changes of this sophisticated morphology of FPs, such as effacement or retraction of FPs, lead to severe glomerular diseases, often resulting in ESRD. Previous studies showed that FPs express actin and actin-associated proteins, such as cortactin, formin, cofilin, and RhoGTPases that are involved in the nucleation, polymerization, and depolymerization of actin filaments.2–6 It is still not completely understood why podocytes possess such a prominent actin cytoskeleton. For many years, researchers have debated, mainly stimulated by in vitro findings,7–11 whether podocytes in vivo are actively moving cells or rather stationary. Recent observations in mice suggest that podocytes may migrate several micrometers within minutes.12 This would imply that there is a coordinated migration of neighboring podocytes to guarantee a continuously formed filtration slit. Resolving this question is important to understand the role and the influence of the actin-cytoskeleton for the function of podocytes in vivo.
For this purpose, podocytes need to be visualized in living animals with high resolution. In the past, two groups observed kidney tissue in living mice by the use of multiphoton microscopy.12–14 However, because podocytes were only negatively stained (by dye exclusion), primary and secondary processes of podocytes have not been visualized with sufficient resolution. More recently, Khouri et al. have used transgenic mice expressing green fluorescent protein (GFP) in a podocyte-specific fashion to resolve podocytes by multiphoton microscopy in more detail.15 By using transgenic mice that expressed GFP only in a fraction of podocytes, Grgic et al. have even succeeded in visualizing the structural details down to the FPs by standard laser-scanning confocal microscopy.16 Likewise, Höhne et al. could visualize FPs by two-color labeling of podocytes in transgenic mice.17 However, in the latter studies, the kidney tissue was explanted or fixed, or glomeruli were isolated before imaging.
The goal of the present study was to visualize podocytes in living zebrafish larvae and to study the dynamics of podocyte processes in high resolution for several hours. For this purpose, the translucent zebrafish (mitfaw2/w2;roya9/a9 [casper]) lacking black melanophores and reflecting iridophores18 was intercrossed with the wt1a:eGFP transgenic zebrafish (Tg[-35.1wt1a:EGFP]),19 resulting in mitfaw2/w2;roya9/a9;Tg(−35.1wt1a:eGFP). The zebrafish strain was abbreviated as ET (ET-EGFP/Translucent). The genetically modified zebrafish larvae were imaged at age 27 hours postfertilization (hpf) up to 7 days postfertilization (dpf).
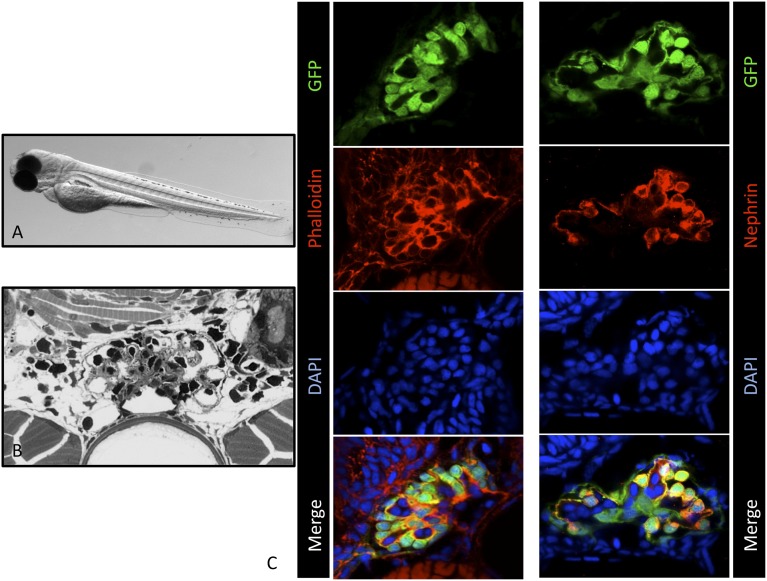
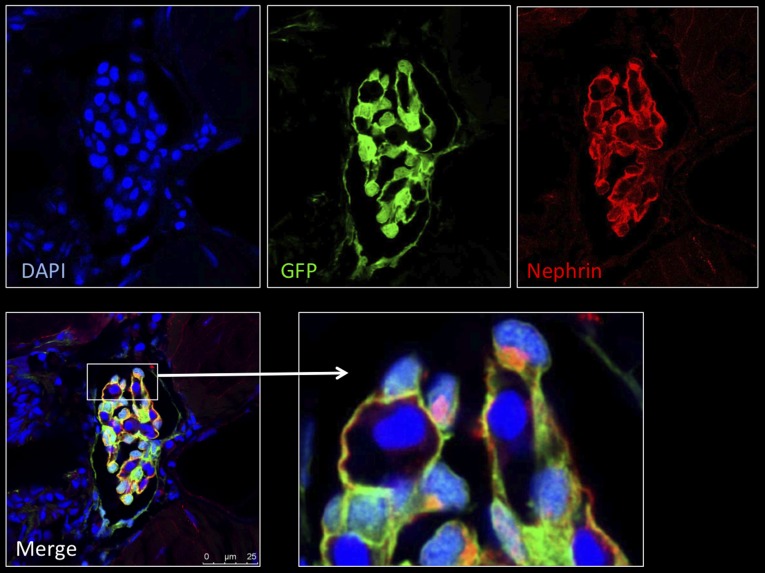
First, we verified that the morphology of the glomerulus and of enhanced GFP (eGFP)-labeled podocytes of the ET larvae was normal. Semithin sections demonstrated a regular morphology of the pronephric glomerulus and of glomerular cells (Figure 1B). In addition, we stained histologic sections of larvae (3 dpf) with Alexa Fluor 546–labeled phalloidin to visualize the actin cytoskeleton and with an antibody against nephrin, a podocyte-specific protein. The histologic staining showed the expression of F-actin in podocytes along the capillaries, consistent with its localization in FPs, and in mesangial cells (Figure 1C, left). For nephrin, we found staining exclusively in podocytes, in agreement with the literature (Figure 1C, right).20 Further, ET zebrafish develop normally and show no abnormalities compared with other zebrafish strains.
Figure 1.
ET larvae show regular glomerular morphology and nephrin expression. (A) ET larva at 3 dpf. (B) Semi-thin section of ET larva at 7 dpf. (C) Cryosections of ET zebrafish larvae at 3 dpf. Staining of F-actin by Alexa Fluor 546-conjugated phalloidin is shown in the left panel. The right panel shows the colocalization (yellow) of eGFP-positive podocytes (green) and the nephrin staining (red). Scale bars: 10 µm. DAPI, 4′,6-diamidino-2-phenylindole.
To visualize podocyte processes with high resolution, the larvae were anesthetized with tricaine and mounted by embedding in 0.8% agarose dissolved in E3 medium. To avoid evaporation of the E3 medium, the surface of the agarose gel was covered with parafilm. The temperature of the animals was kept at 22°C. The blood circulation was monitored at regular time points until the end of the experiments. Only zebrafish larvae with a proper blood circulation throughout the entire experiment were included in the study.
For better visualization of the capillaries, quantum dots (Qdots) were injected intravenously into the tail region of zebrafish larvae just before embedding in some experiments. With use of two-photon microscopy, z-stacks were recorded (Supplemental Movie 1) and the podocytes of the zebrafish glomerulus were reconstructed in three dimensions (Supplemental Movie 2). These movies demonstrate that the primary and secondary podocyte processes covering the capillaries can be clearly visualized by our in vivo approach. Furthermore, Supplemental Movie 2 shows a three-dimensional reconstruction of a glomerulus of a living animal for the first time.
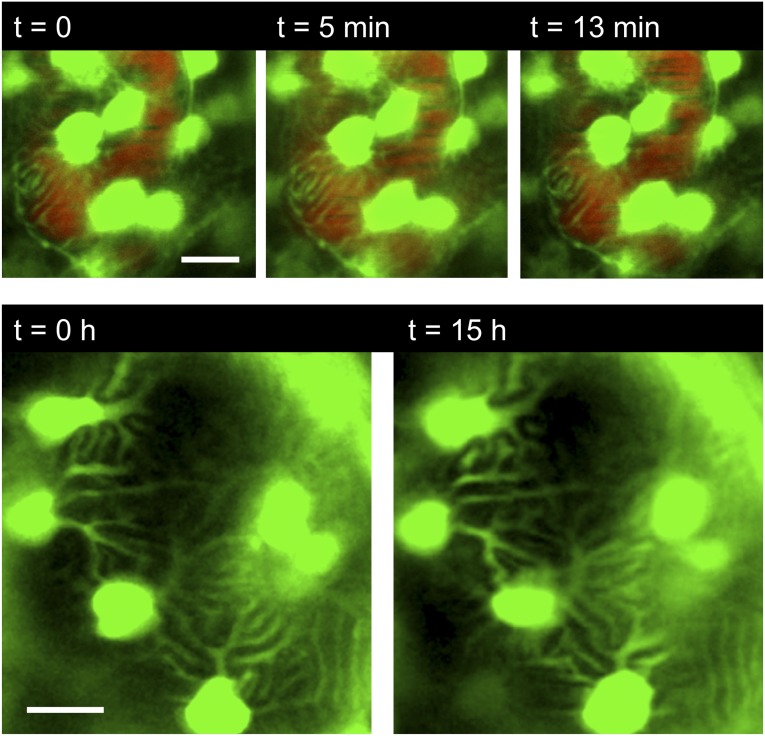
After we verified that zebrafish larvae could be maintained and imaged up to 23 hours, we studied the dynamics of primary and secondary podocyte processes. To detect rapid movements, images were acquired every 6 seconds for 13–20 minutes (n=3). As shown in Figure 2 and Supplemental Movie 3, podocyte cell bodies, as well as the major processes, always remained at the identical position during the entire observation time.
Figure 2.
Podocyte processes can be visualized in vivo over time. Upper panel: ET larva injected with Qdots (red) for visualization of the capillaries in the pronephros. Short-time series showing the processes of podocytes attached to glomerular capillaries (images were taken every 60 seconds). No dynamics of the processes were observed. (Bottom) ET larva recorded for 15 hours. Images were taken every 30 minutes. No notable changes of the position of the primary and secondary processes were observed. Scale bars: 10 µm.
To reveal whether podocytes perform movements over a longer time range, the observation time was increased to 2, 3, 6–13, and 18–23 hours (n=8 for 2–5 hours, n=27 for 6–23 hours). Long-time experiments were performed without injection of Qdots to exclude side effects on the motility of podocytes. As stated above, the circulation of the larvae was monitored at regular time intervals, excluding compromised perfusion or viability. Cell bodies and major processes of podocytes remained static over many hours (Figure 2). In particular, we never observed podocytes migrating to, from, or along the glomerular tuft.
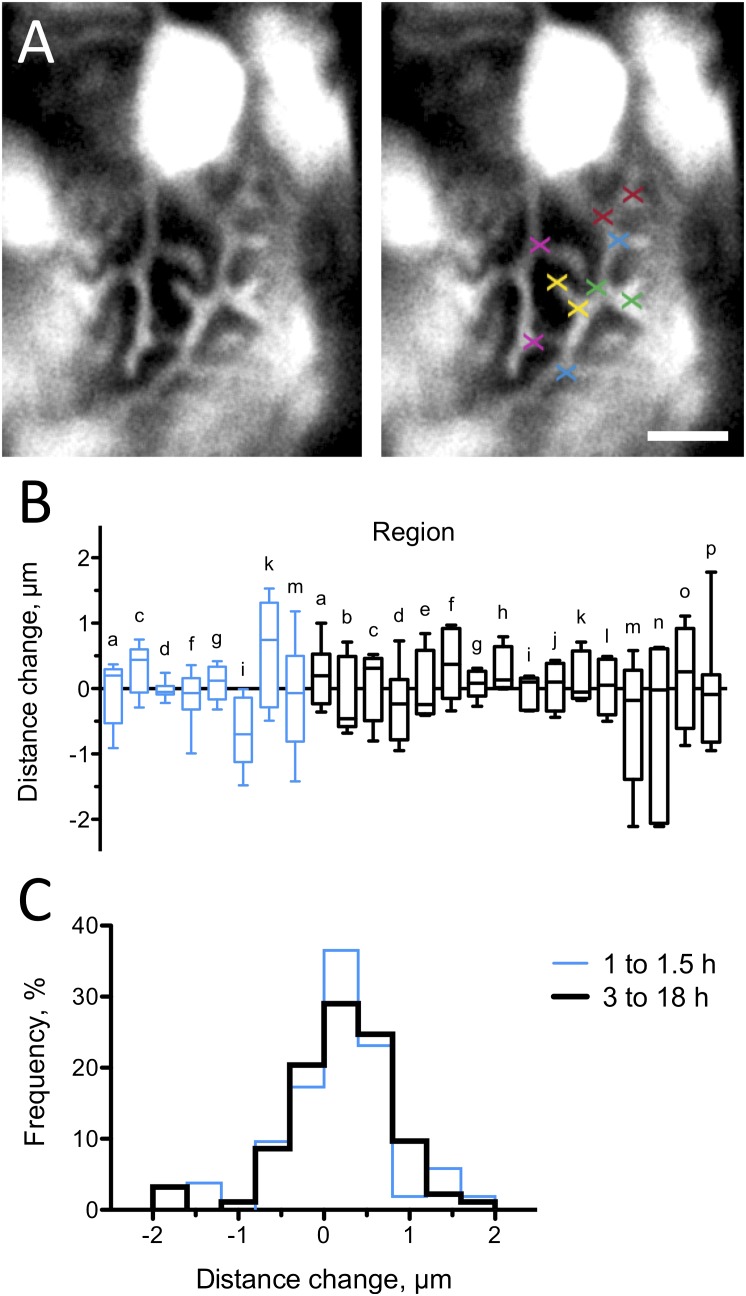
To find out whether podocyte processes are static over a prolonged period, we measured the distances between characteristic branching points of podocyte processes at two different time points (Figure 3). In total, we measured 142 distances (mean±SD, 4.2±1.6 µm at baseline) in 16 different regions of 11 larvae. The mean distances did not change significantly in any of the 16 regions after 3–18 hours (6.1±4.1 hours) (black boxes in Figure 3B). Thus, processes in a region neither expanded nor contracted during a period of several hours. In addition, we measured the distances in 8 of the 16 regions after 1–1.5 hours (1.2±0.3 hours) (blue boxes in Figure 3B). The mean distances did not change significantly at earlier time points, showing a similar scatter as the later measurements. Figure 3C demonstrates that the distribution of the distance changes measured at later time points is nearly identical to that of the distance changes measured at earlier time points. Because one would expect that the distribution of distance changes broadens over time if podocyte processes are motile, our results demonstrate that podocyte processes do not exhibit motility within an experimental error of about 1.5 µm. This error probably results from limited optical resolution, from subtle tilting of living larvae, from minute capillary movements at the heart rate, and from the uncertainty in identifying the exact same branching points at different time points.
Figure 3.
Podocyte processes are stationary over time. Motility of podocyte processes was assessed by measuring several distances between characteristic branching points per region at two time points. (A) As an example, five different distances were measured in one region and are indicated by crosses of the same color. (B) Box plot of the distance changes in 16 regions (regions a–p) in 11 larvae after a mean time of 6.1 hours (black boxes; n=93 measured distances). Distance changes in eight regions were measured additionally after a mean time of 1.2 hours (blue boxes; n=49 measured distances). None of the regions showed a significant positive or negative mean change in distances, corresponding to expansion or contraction of processes, respectively. (C) The distribution of distance changes after 3–18 hours is similar to that after 1–1.5 hours, indicating that the motility of podocyte processes over several hours is below our experimental detection limit of about 1.5 µm. Scale bar: 10 µm.
We have not found any dynamics, either of podocyte cell bodies or of podocyte processes, in living zebrafish larvae in continuous observation times of up to 23 hours. Because our experiments spanned a total observation time of more than 1000 podocyte-hours (4–5 podocytes per experiment×about 250 hours of total observation time), vigorous migration of podocytes is a rare event, if it occurs under physiologic conditions at all.
To investigate whether podocyte processes exhibit dynamics on a timescale of seconds, as reported recently in isolated glomeruli,17 we recorded podocyte processes in living zebrafish larvae with a frame rate of 4.5–7.1 Hz, about two to three times faster than the heart rate (n=15 experiments). Depending on the position of the capillary, we observed slight shifting of the region of interest that was synchronous with the heart rate (Supplemental Movie 4). However, podocyte processes neither contracted nor expanded. Note that aortic pressure in 5-dpf zebrafish larvae is about 0.5 mmHg,21 resulting in hemodynamic forces in zebrafish that are about 100-fold lower than those in mammals.
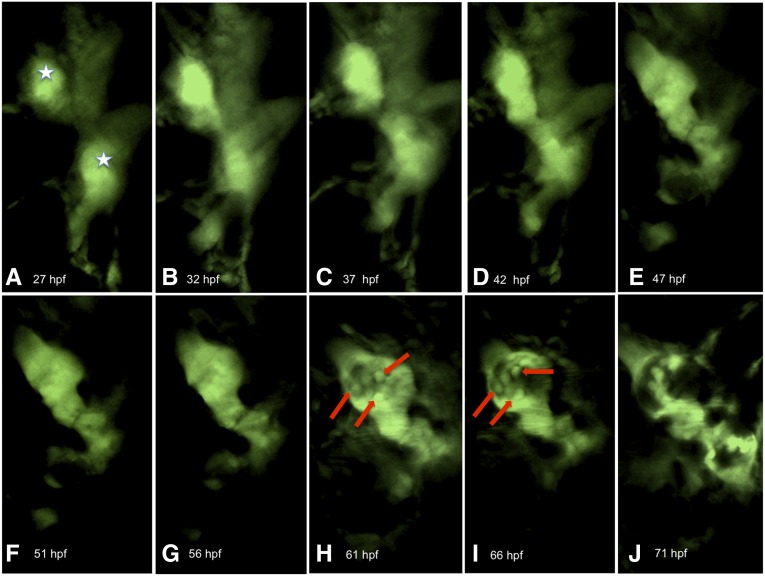
To exclude the possibility that the static behavior of podocytes in the developed pronephric glomerulus was due to a phototoxic effect of the pulsed laser light, we performed three types of control experiments. In the first, we observed the development of the glomerulus in a 27-hpf larva over 44 hours. At the beginning of the experiment, two pronephric primordia were seen (Figure 4, A–C, white stars), which started to move toward the midline at 42 hpf (Figure 4, D and E). At 61 hpf, the cell bodies of podocytes became visible (arrows in Figure 4, G and H). At 71 hpf, both glomerular primordia had fused in the midline and the glomerular tuft was well developed (Figure 4J). In the second control experiment, we imaged cells adjacent to the glomerulus. As shown in Supplemental Movie 5, these cells were highly motile, advancing and retracting processes during an observation period at least of 1 hour. In the third control experiment, we fixed the agarose-embedded zebrafish larva immediately after the experiment (4-dpf larva, 1 day of observation time) and sections were stained with an antibody against nephrin. Our in vivo observation protocol affected neither glomerular tuft morphology nor nephrin expression in podocytes (Figure 5). From these control experiments we concluded that our in vivo observation protocol does not affect cell function or integrity. Thus, if podocytes in the fully-developed pronephric glomerulus were motile, we would have been able to detect their motile behavior.
Figure 4.
Podocytes are motile during formation of the pronephric glomerulus. The development of the pronephros of a zebrafish larva (27 hpf) was followed over 44 hours (A–J). The white stars mark the pronephric primordia (A). In H and I, the first podocytes (arrows) can be distinguished.
Figure 5.
Long-term two-photon microscopy does not affect glomerular morphology or nephrin expression. A zebrafish larva (4 dpf) embedded in agarose was fixed after 1 day’s observation time and stained with DAPI (4′,6-diamidino-2-phenylindole) for the nuclei and with an antibody against nephrin. The higher magnification of the glomerulus shows the well-formed glomerular tuft and podocytes expressing nephrin (white box).
Multiphoton microscopy is a technique for imaging living cells in various organs, such as the brain, the skin, and the kidney.22,23 However, in the past, the resolution of multiphoton imaging was insufficient to visualize the morphology and dynamic behavior of primary and secondary podocyte processes. In this report, we show that with a new generation of two-photon microscopy in combination with a specific transgenic zebrafish larva it is now possible to study the dynamics of primary and secondary podocyte processes in vivo over short- as well as long-term periods for the first time. The transgenic zebrafish larvae expressed eGFP specifically in podocytes and did not form pigmented cells (melanophores and iridophores) that interfere with the two-photon excitation. Two-photon imaging of podocyte processes in zebrafish larvae might also be a potential novel tool to visualize the development of podocyte-associated diseases in vivo.
Concise Methods
Zebrafish Stocks
Zebrafish were reared and maintained as previously described.24 The wt1a:eGFP zebrafish strain19 expresses eGFP under the wt1a promoter in podocytes and parietal epithelial cells (kindly provided by Dr. C. Englert, Jena, Germany). The translucent zebrafish strain casper18 (kindly provided by Dr. U. Strähle, Karlsruhe, Germany) was crossed with the wt1a:eGFP strain. The zebrafish larvae were selected for transparency and green fluorescent podocytes. All zebrafish were grown and mated at 28.5°C. Embryos were kept and handled in E3 solution. The experiments were done at 22°C.
Histology and Immunohistochemistry
For fluorescence microscopy of zebrafish cryosections, larvae were fixed in 2% paraformaldehyde in 1× PBS, pH 7.4, for 3 hours at room temperature. The fixed larvae were incubated in 30% sucrose at 4°C overnight and snap frozen in liquid nitrogen using Tissue-Tek (Sakura, Staufen, Germany). Cryostat sections (60 μm) were permeabilized with 0.3% Triton X-100 (Merck, Darmstadt, Germany) for 30 seconds and stained with phalloidin (Alexa Fluor 546–conjugated phalloidin; Invitrogen) and 1 mg/100 ml Hoechst 33342 (Sigma-Aldrich) for 30 minutes. For the nephrin staining, rabbit anti-zebrafish nephrin antibody (gift of Dr. A. Majumdar, Uppsala, Sweden) was incubated overnight at 4°C. After washing three times with 1× PBS, the Cy3-conjugated anti-rabbit secondary antibody (1:250; Dianova, Hamburg, Germany) was applied for 30 minutes. The samples were washed and mounted in Mowiol for microscopy (Carl Roth, Karlsruhe, Germany). Semithin sections (0.7 µm) embedded in Epon 812 (Serva, Heidelberg, Germany) were stained with methylene blue.
Imaging
Confocal microscopy was performed with a Leica TCS SP5 microscope (Leica Microsystems, Wetzlar, Germany). For the stained cryosections, 40× and 63× oil-immersion objectives were used. For in vivo observation, transgenic zebrafish larvae (27 hpf/7 dpf) were anesthetized by tricaine (0.1%–0.5%; Sigma-Aldrich), embedded in molten 0.8% agarose (Biozym LE Agarose, Germany) and positioned in a dorsal-up position by an eyelash-pencil. Immediately after the agarose was solid, the embedded larvae were covered by E3 medium supplemented with tricaine. To visualize blood vessels, Qdots (655 ITK amino [PEG], 8 µM; Life Technologies) were mixed with 0.5% phenol red (Sigma-Aldrich) at a final concentration of 0.8 µM. A volume of 3 nl was injected intravenously with an injection needle using a microinjector (transjector 5246; Eppendorf AG, Hamburg, Germany) in the anesthetized zebrafish larvae at 5 dpf. Images were recorded at different time points (every 6 seconds to 90 minutes and every 140–223 milliseconds, respectively) by a Zeiss LSM 7MP (Carl Zeiss Microimaging, Jena, Germany), using 20×, 40×, and 63× water immersion objectives. The excitation wavelength was 900 nm. The images for Movie 2 were deconvolved with ImageJ supported by the iterative deconvolution three-dimensional plugin to reduce the background. Three-dimensional reconstruction was performed with Volocity software (Improvision, Coventry, UK). To quantify motility of podocyte processes, 4–8 distances between characteristic branching points were measured per region (Figure 3A) at baseline and at a later time point using ImageJ with an average resolution of 11 pixels per µm.
Disclosures
None.
Supplementary Material
Acknowledgments
The excellent technical assistance of Regina Maciejewski and Oliver Zabel is gratefully acknowledged. We thank Dr. Eric Schordan for the three-dimensional reconstruction of the glomerulus.
This study was supported by a grant of the European Union within the seventh framework program to K.E. (project EnVision, grant agreement no. 264143), by grants of the German Federal Ministry of Education and Research to M.J.M. and K.E. (project PodoRePro, grant no. 01GN0804 and 01GN0805) and to N.E. and M.J.M. (E-Rare project Rare-G, grant no. 01GM12088 and 01GM1208A), and by a grant of the Forschungsverbund Molekulare Medizin at the University Medicine Greifswald to N.E. and K.E.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013020178/-/DCSupplemental.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ashworth S, Teng B, Kaufeld J, Miller E, Tossidou I, Englert C, Bollig F, Staggs L, Roberts IS, Park JK, Haller H, Schiffer M: Cofilin-1 inactivation leads to proteinuria—studies in zebrafish, mice and humans. PLoS ONE 5: e12626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besse-Eschmann V, Le Hir M, Endlich N, Endlich K: Alteration of podocytes in a murine model of crescentic glomerulonephritis. Histochem Cell Biol 122: 139–149, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Sun H, Schlondorff JS, Brown EJ, Higgs HN, Pollak MR: Rho activation of mDia formins is modulated by an interaction with inverted formin 2 (INF2). Proc Natl Acad Sci U S A 108: 2933–2938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T: Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K: Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem 286: 26003–26015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstädt H, Hsu HH, Schlondorff J, Ramos A, Greka A: Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3: ra77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duning K, Schurek EM, Schlüter M, Bayer M, Reinhardt HC, Schwab A, Schaefer L, Benzing T, Schermer B, Saleem MA, Huber TB, Bachmann S, Kremerskothen J, Weide T, Pavenstädt H: KIBRA modulates directional migration of podocytes. J Am Soc Nephrol 19: 1891–1903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P: Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P: Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem 279: 34827–34832, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Peti-Peterdi J, Sipos A: A high-powered view of the filtration barrier. J Am Soc Nephrol 21: 1835–1841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA: Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905–C916, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Peti-Peterdi J, Morishima S, Bell PD, Okada Y: Two-photon excitation fluorescence imaging of the living juxtaglomerular apparatus. Am J Physiol Renal Physiol 283: F197–F201, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Khoury CC, Khayat MF, Yeo TK, Pyagay PE, Wang A, Asuncion AM, Sharma K, Yu W, Chen S: Visualizing the mouse podocyte with multiphoton microscopy. Biochem Biophys Res Commun 427: 525–530, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grgic I, Brooks CR, Hofmeister AF, Bijol V, Bonventre JV, Humphreys BD: Imaging of podocyte foot processes by fluorescence microscopy. J Am Soc Nephrol 23: 785–791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höhne M, Ising C, Hagmann H, Völker LA, Brähler S, Schermer B, Brinkkoetter PT, Benzing T: Light microscopic visualization of podocyte ultrastructure demonstrates oscillating glomerular contractions. Am J Pathol 182: 332–338, 2013 [DOI] [PubMed] [Google Scholar]
- 18.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, Zon LI: Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2: 183–189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bollig F, Perner B, Besenbeck B, Köthe S, Ebert C, Taudien S, Englert C: A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development 136: 2883–2892, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, Majumdar A: A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol 334: 1–9, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Hu N, Sedmera D, Yost HJ, Clark EB: Structure and function of the developing zebrafish heart. Anat Rec 260: 148–157, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V: Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487: 496–499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan F, Gan WB: Two-photon imaging of dendritic spine development in the mouse cortex. Dev Neurobiol 68: 771–778, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Müller T, Rumpel E, Hradetzky S, Bollig F, Wegner H, Blumenthal A, Greinacher A, Endlich K, Endlich N: Non-muscle myosin IIA is required for the development of the zebrafish glomerulus. Kidney Int 80: 1055–1063, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.