Abstract
Soluble fms-like tyrosine kinase 1 (sFlt1), a circulating antiangiogenic protein, is elevated in kidney diseases and contributes to the development of preeclampsia. Hydrogen sulfide is a vasorelaxant and proangiogenic gas with therapeutic potential in several diseases. Therefore, we evaluated the potential therapeutic effect and mechanisms of action of hydrogen sulfide in an animal model of sFlt1-induced hypertension, proteinuria, and glomerular endotheliosis created by adenovirus-mediated overexpression of sFlt1 in Sprague-Dawley rats. We injected sFlt1-overexpressing animals intraperitoneally with the hydrogen sulfide–donor sodium hydrosulfide (NaHS) (50 µmol/kg, twice daily) or vehicle (n=7 per group). Treatment with NaHS for 8 days significantly reduced sFlt1-induced hypertension, proteinuria, and glomerular endotheliosis. Measurement of plasma protein concentrations with ELISA revealed a reduction of free plasma sFlt1 and an increase of free plasma vascular endothelial growth factor (VEGF) after treatment with NaHS. Renal VEGF-A mRNA expression increased significantly with NaHS treatment. In vitro, NaHS was proangiogenic in an endothelial tube assay and attenuated the antiangiogenic effects of sFlt1. Stimulation of podocytes with NaHS resulted in both short-term VEGF release (120 minutes) and upregulation of VEGF-A mRNA levels (24 hours). Furthermore, pretreatment of mesenteric vessels with a VEGF receptor 2–neutralizing antibody significantly attenuated NaHS-induced vasodilation. These results suggest that hydrogen sulfide ameliorates sFlt1-induced hypertension, proteinuria, and glomerular endotheliosis in rats by increasing VEGF expression. Further studies are warranted to evaluate the role of hydrogen sulfide as a novel therapeutic agent for vascular disorders such as preeclampsia.
Elevated soluble fms-like tyrosine kinase 1 (sFlt1, also referred to as soluble vascular endothelial growth factor receptor 1) levels are associated with several diseases, including preeclampsia, vasculitis, and CKD.1–3 In preeclampsia, a consistent line of evidence has shown that increased sFlt1 is one of the major contributors to the development of hypertension and proteinuria.4–7 sFlt1 is a splice variant of the vascular endothelial growth factor (VEGF) receptor lacking the transmembrane and cytoplasmic domains and acts as a powerful antagonist of VEGF, thereby inhibiting VEGF signaling in the vasculature.8–10 Increased levels of circulating sFlt1 lead to functional VEGF deficiency, causing endothelial dysfunction, decreased angiogenesis, impaired capillary repair, and consequently hypertension and proteinuria.5,11 Indeed, VEGF inhibitors used as part of cancer chemotherapy are associated with significant hypertension and proteinuria.12–14 Moreover, genetic models of glomerular VEGF deficiency are also associated with proteinuria and glomerular endothelial damage.13,15 Thus far, no targeted inventions to reduce circulating sFlt1 are available in the clinic. Although recombinant VEGF or placental growth factor have proven to be effective in experimental models, no clinical trials have yet been conducted .16–19 sFlt1 removal using dextran sulfate apheresis has recently shown to be a possible new strategy in patients with very severe preterm preeclampsia.20
Hydrogen sulfide (H2S) belongs to a family of gasotransmitters, along with nitric oxide and carbon monoxide. The endogenously produced gas is important in physiologic processes, such as regulating arterial diameter, blood flow, and leukocyte adhesion.21 H2S has been shown to enhance vasorelaxation through an endothelial cell–independent mechanism by acting on ATP-sensitive potassium (KATP) channels.22,23 However, KATP channel blockers do not completely abolish H2S-induced relaxation, and H2S stimulates vasorelaxation in an endothelial cell–dependent manner at lower doses.24–27 This suggests that H2S likely stimulates vasorelaxation through additional pathways that have not yet been elucidated.
In several animal models, endogenous activity of H2S-producing enzymes and exogenous H2S donors or precursors protect against ischemia and reperfusion injury and exhibit anti-inflammatory activity.28–31 The proangiogenic effect of H2S was first described by Cai et al., who showed that an exogenously administrated H2S donor (sodium hydrosulfide [NaHS]) promoted proliferation, migration, and tube-like structure formation in endothelial cells in vitro.32 In vivo, it was demonstrated that H2S is a proangiogenic factor in a model of hind limb ischemia.33 These effects were associated with an increase in VEGF expression and activation of vascular endothelial growth factor receptor (VEGFR)2 signaling in vascular endothelial cells, suggesting that the effects of H2S may be mediated by VEGF and its receptor VEGFR2.33 Bir et al. recently showed that H2S-stimulated ischemic vascular growth is dependent on augmented expression and activity of VEGF.34
We therefore hypothesized that by upregulating VEGF, H2S may directly antagonize the detrimental effects of sFlt1 on the endothelium, consequently attenuating the development of hypertension and proteinuria. In this study, we demonstrate a protective effect of exogenous H2S administration in a rat model with overt hypertension and proteinuria induced by sFlt1 overexpression.
Results
H2S Ameliorates Hypertension and Proteinuria in sFlt1-Transfected Rats
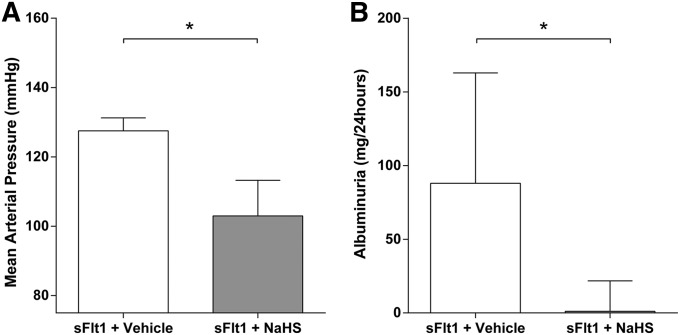
To evaluate the in vivo effect of exogenous H2S on BP and proteinuria, we administered 50 μmol/kg NaHS (H2S-donor) twice daily to sFlt1-overexpressing rats. BPs measured by invasive means were significantly lower in the NaHS-treated group (103 mmHg; interquartile range [IQR], 98–113 mmHg) compared with the control vehicle-treated group (128 mmHg; IQR, 124–131) (P<0.003; Figure 1A). These findings were also confirmed by continuous telemetry measurements during 8 days of NaHS or placebo treatment (Supplemental Figure 1). Similarly, we noted a significant and dramatic reduction in proteinuria after treatment with NaHS (Figure 1B). The sFlt1-injected rats developed a median albuminuria of 88 mg/24 h (IQR, 10–163), whereas albuminuria was almost normalized at 1 mg/24 h in the sFlt1-injected rats treated with NaHS (IQR, 0.3–22) (P<0.05).
Figure 1.
Effect of NaHS on hypertension and proteinuria in rats overexpressing sFlt1. (A) Mean arterial pressure is measured via carotid catheterization under anesthesia after 8 days of treatment with either vehicle or 50 µM/kg NaHS. BP is significantly lower in the NaHS-injected group compared with vehicle. (B) After 7 days of treatment with vehicle or NaHS, rats are placed in metabolic cages to collect 24-hour urine and total albumin is determined. NaHS-treated rats show significant lower albuminuria than vehicle-treated rats. Data are presented as the median (IQR). n=7 per group. *P<0.05.
NaHS Improves Renal Glomerular Endotheliosis
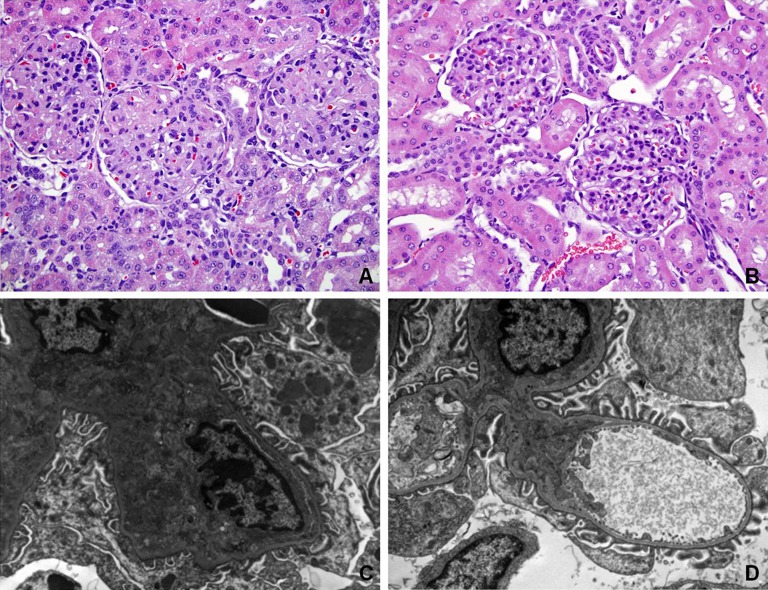
Similar to previously published data, animals overexpressing sFlt1 demonstrated marked glomerular endotheliosis with occlusion of capillary lumens (Figure 2A).5,16 Interestingly, kidneys from NaHS-treated animals had more open capillaries and less glomerular damage with absence of proteinaceous deposits and distinct mesangium (Figure 2B). Electron microscopy confirmed that glomerular endotheliosis was ameliorated in NaHS-treated animals compared with vehicle-treated rats (Figure 2, C and D).
Figure 2.
NaHS reverses glomerular endotheliosis in rats overexpressing sFlt1. (A) Histopathologic analysis of renal tissue from one representative vehicle-treated rat overexpressing sFlt1 shows marked glomerular endotheliosis with occlusion of capillary lumens. (B) Histopathologic analysis of renal tissue from one representative NaHS-treated rat overexpressing sFlt1 (50 µM/kg) shows open capillaries with absence of proteinaceous deposits and mesangium that is now distinct (stained with hematoxylin and eosin). Electron microscopy is performed for the same rats as shown in A and B. (C) Representative electron micrographs of glomeruli from a vehicle-treated rat overexpressing sFlt1 confirm glomerular endotheliosis. (D) Representative electron micrographs of glomeruli from a NaHS-treated rat overexpressing sFlt1 show ameliorated glomerular endotheliosis. Original magnification, ×40 in B; ×10,000 in D.
Proangiogenic Effects of Hydrogen Sulfide In Vitro
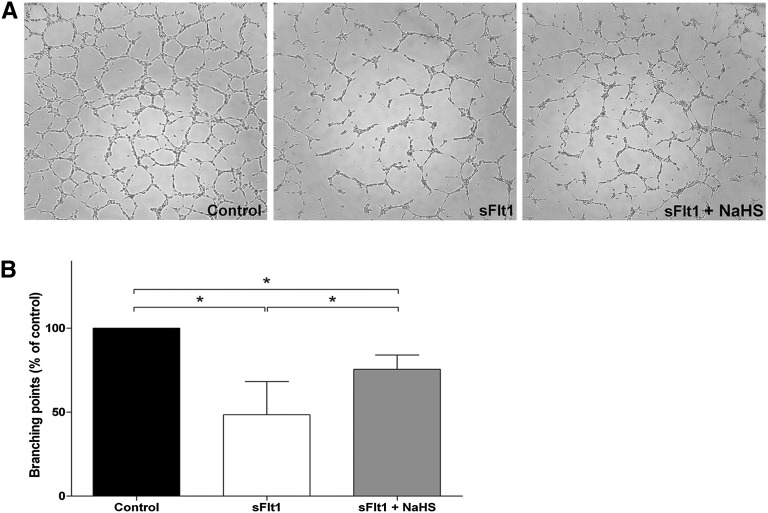
sFlt1 is able to induce an antiangiogenic state in vitro.5,6 Figure 3 shows that incubation of human umbilical vascular endothelial cells (HUVECs) with 1 µg sFlt1 resulted in a significantly reduced capacity to form tube-like structures (52.6% reduction with respect to the control; IQR, 80.2–25.0) (P<0.05). Treatment of sFlt1-incubated HUVECs with 600 µM NaHS enhanced capillary-like structure formation by 27.0% (IQR, 81.3–15.7) compared with sFlt1 (P<0.05).
Figure 3.
NaHS is proangiogenic in vitro. (A) An endothelial tube assay is performed using recombinant sFlt1 or NaHS (600 µM) to treat the cells. A representative experiment is shown for the following conditions: control, recombinant sFlt1, and recombinant sFlt1 in combination with NaHS. (B) Quantification of the endothelial tube assay. The tubes are quantified by counting branching points and are normalized to the control condition (100%). Statistical analysis is performed using the Kruskal–Wallis test. After administration of recombinant sFlt1 to the endothelial cells, significantly decreased angiogenesis is shown. NaHS is able to restore this decrease, but angiogenesis in the sFlt1 plus NaHS condition is still significantly lower compared with control. Experiments were repeated five times. Data are presented as the median (IQR). *P<0.05.
Proangiogenic Effects of Hydrogen Sulfide In Vivo
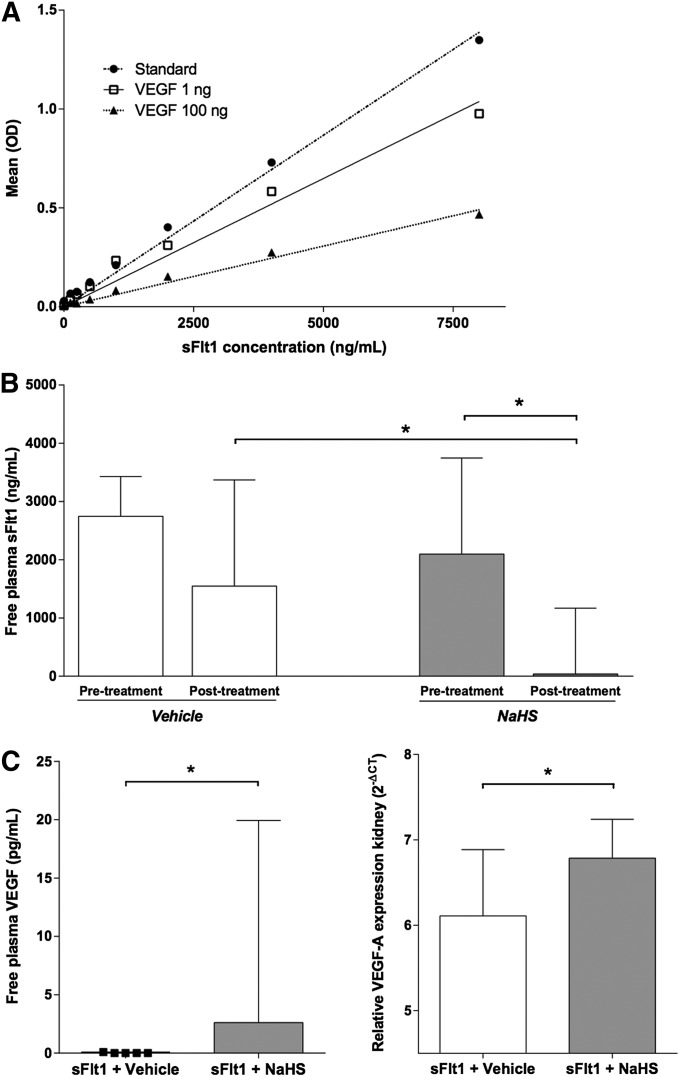
We first confirmed that the sFlt1 ELISA kit used in our studies measured only unbound mouse sFlt1 by generating a standard curve for sFlt1 protein in the presence of recombinant rat VEGF (Figure 4A). Using this ELISA system, we measured free sFlt1 in the plasma of the NaHS- and vehicle-treated rats (Figure 4B). Before treatment, similar concentrations of free sFlt1 in the plasma of both groups were shown (2097 ng/ml [IQR, 1297–3746] versus 2745 ng/ml [IQR, 2383–3430]) (P = NS). After treatment, free sFlt1 concentrations significantly decreased in the NaHS-treated group compared with the levels before the start of treatment. On the other hand, free sFlt1 concentrations in the vehicle-treated group did not differ from those before treatment. Whereas the free sFlt1 in the plasma of the NaHS-treated rats decreased to 39 ng/ml (IQR, 10–1168), plasma sFtl1 in the vehicle-treated rats remained high at 1547 ng/ml (IQR, 996–3370) (P<0.05; Figure 3B). Compared with the pretreatment levels of free plasma sFlt1, levels of the NaHS-treated rats showed a reduction of 91% (IQR, 45–99), whereas there was a small reduction of 35% (IQR, 0–56) over time in the vehicle-treated rats (P<0.05). These findings were accompanied by increased circulating free VEGF levels in animals treated with NaHS (2.6 pg/ml; IQR, 0.2–19) compared with the vehicle group (0.0 pg/ml; IQR, 0.0–0.1) (P<0.05; Figure 4C, right panel). Free plasma VEGF levels after treatment with NaHS negatively correlated to the free plasma sFlt1 measured after treatment with NaHS (r=−0.79; P<0.05). Finally, we also noted upregulation of the VEGF-A mRNA level in the kidneys after treatment with NaHS. Relative renal VEGF-A mRNA expression in vehicle-treated rats was 6.1 2-ΔCT (IQR, 4.7–6.8), whereas the NaHS-treated group showed significantly higher VEGF-A mRNA levels of 6.9 2-ΔCT (IQR, 6.2–7.5) (Figure 4C, left panel) (P<0.05).
Figure 4.
Effect of NaHS on sFlt1 and VEGF expression in rats. (A) A standard curve for recombinant VEGF protein is generated in the absence (standard) or in the presence of 1 or 100 ng VEGF using a murine ELISA for measurement of sFlt1 levels as described in Concise Methods. (B) Free plasma sFlt1 (ng/ml) before and after 8 days of treatment with either vehicle or NaHS. NaHS-treated rats (50 µM/kg) show a significant reduction in free plasma sFlt1 after treatment, whereas vehicle-treated rats do not. The free plasma sFlt1 levels in the NaHS-treated rats are significantly lower compared with the vehicle group. (C) Free plasma VEGF (pg/ml) after 8 days of treatment with NaHS (left). After treatment with NaHS, VEGF levels are significantly higher compared with vehicle treatment. Renal VEGF mRNA levels are shown with respect to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT; right). Renal VEGF mRNA expression is significantly higher in NaHS-treated rats compared with the vehicle group. n=7 per group. Data are presented as the median (IQR). *P<0.05.
NaHS Causes VEGF Release and Upregulation of VEGF mRNA by Podocytes In Vitro
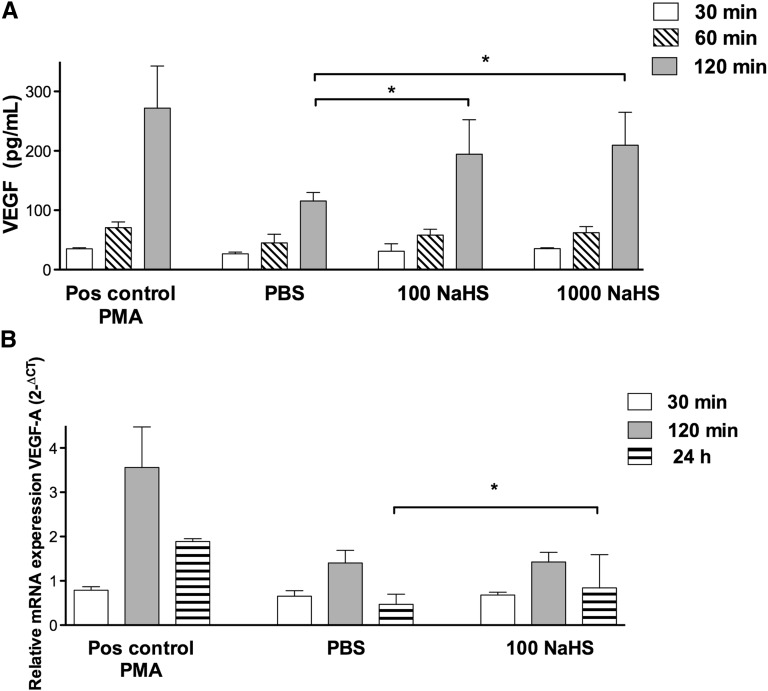
To study the short- and late-term effects of NaHS on VEGF protein and mRNA in vitro, podocytes were stimulated with NaHS. Both VEGF protein release (short term) and production of VEGF mRNA (late term) were evaluated. As shown in Figure 5A, NaHS treatment resulted in a significant increase of VEGF protein release by podocytes (210 pg/ml [IQR, 149–265] for 1000 µM NaHS compared with 97 pg/ml [IQR, 116–130] for PBS) (P<0.05 after 120 minutes). After 60 minutes, an increase was observed at both 100 and 1000 µM NaHS, but this effect was borderline significant (P=0.07). VEGF mRNA was upregulated after 24-hour stimulation with 100 µM NaHS (0.84 2-ΔCT; IQR, 0.64–1.60) compared with PBS (0.46 2-ΔCT; IQR, 0.36–0.69) (P<0.05; Figure 5B). After 60 and 120 minutes, no effects of NaHS on VEGF mRNA levels were observed.
Figure 5.
Stimulation of human podocytes with NaHS causes VEGF release and upregulation of VEGF mRNA. (A) Human podocytes treated with PMA (positive control, 50 ng/ml), PBS (control), 100 µM NaHS, or 1000 µM NaHS. VEGF (pg/ml) release is increased after 120 minutes of stimulation in the 100 and 1000 µM NaHS-treated groups compared with the PBS-treated group. (B) After 24 hours of stimulation with 100 µM NaHS, an increase in VEGF-A mRNA is present compared with PBS (control). Data are presented as the median (IQR). *P<0.05. n=5 per group. Pos, positive control; PMA, phorbol 12-myristate 13-acetate.
NaHS-Mediated Vasodilation Is Dependent on VEGF Signaling
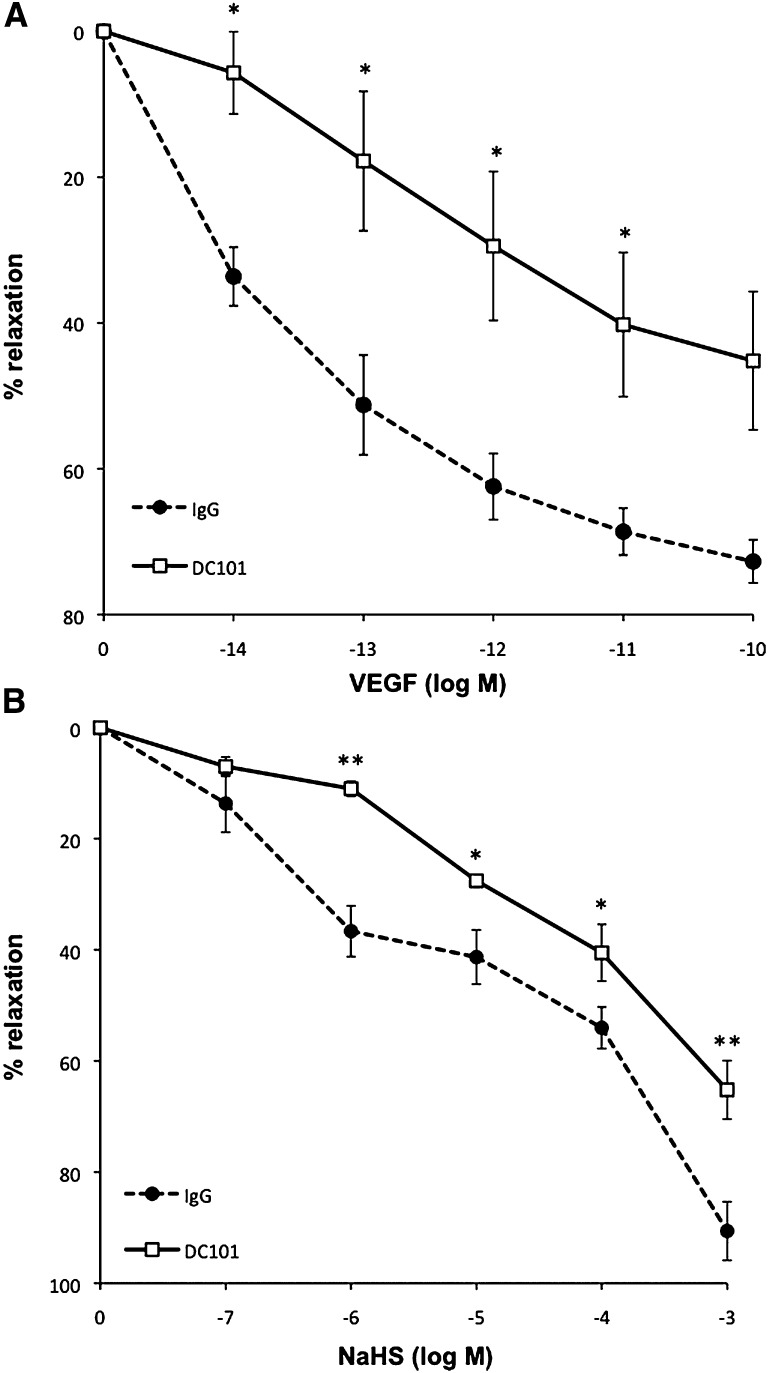
To explore whether the vascular effect of NaHS is dependent on VEGF signaling, we evaluated the vasodilatory properties of NaHS in the presence of VEGFR2-neutralizing antibody in mesenteric microvessels from mice. After precontraction of the vessels with phenylephrine, VEGF induces vasorelaxation in a dose-dependent manner (Figure 6A). This response is significantly inhibited in the presence of a VEGFR2 neutralizing antibody. Figure 6B shows that NaHS-dependent vasodilation is significantly attenuated in vessels pretreated with the same concentration of VEGFR2-neutralizing antibody.
Figure 6.
The role of VEGF signaling in NaHS-mediated vascular relaxation. (A) Treatment with the VEGFR2 neutralizing antibody DC101 (50 µg/ml) attenuates VEGF-mediated relaxation compared with vehicle-treated vessels (IgG antibody; 50 µg/ml). (B) Treatment with DC101 (50 µg/ml) attenuated NaHS-mediated relaxation compared with vehicle-treated vessels (IgG antibody; 50 µg/ml). Responses are expressed as the percentage of relaxation from preconstriction and each value represents the mean±SEM. n=3–4 mice per group. *P<0.05 vehicle-treated versus DC101-treated mice; **P<0.01 vehicle-treated versus DC101-treated mice.
Discussion
A consistent line of evidence has shown that excess sFlt1 antagonizes VEGF and consequently contributes to endothelial dysfunction.3,5,6,35,36 H2S is known for its proangiogenic, antihypertensive, and organ protective properties.28,37,38 In this study, we investigated the effects of exogenously administrated H2S in a rat model for severe endothelial dysfunction, induced by overexpression of sFlt1. As previously described, elevation of plasma sFlt1 by adenoviral overexpression resulted in hypertension, proteinuria, and glomerular endotheliosis. We noted that administration of the H2S-donor NaHS markedly attenuated hypertension and proteinuria induced by sFlt1 and that this effect was associated with upregulation of tissue and systemic VEGF by NaHS.
To the best of our knowledge, this is the first study providing evidence that H2S is able to lower free plasma sFlt1 concentrations and attenuate the detrimental vascular phenotype of high circulating sFlt1. Although H2S could have had direct effects on sFlt1, it is more likely that reduction of free sFlt1 is due to an increase of VEGF by H2S. Indeed, we found an upregulation of free plasma VEGF after treatment with H2S. These free plasma levels are negatively correlated with free plasma sFlt1 levels, suggesting that the balance can indeed be shifted from antiangiogenic to proangiogenic by H2S. In addition to increased plasma VEGF, we also found increased VEGF mRNA expression in the kidney. We are the first to show that VEGF release is increased by H2S in podocyte cell culture after 2 hours. The mechanism of upregulation requires further studies, because we found increased release of VEGF in the absence of an increased mRNA expression after 2 hours of incubation with H2S. Possible mechanisms include a role for matrix metalloproteinases, which are known to modulate VEGF release from the inside of the cell. Besides increased plasma VEGF, we found increased VEGF mRNA expression in the kidney. This may also be a direct effect of H2S, because we found increased VEGF mRNA expression in our culture model after 24 hours of H2S incubation compared with vehicle incubation. Our VEGF mRNA data are in agreement with previous experiments showing that H2S is able to induce VEGF expression; e.g., Bir et al. showed that this is mediated via hypoxia-inducible factor 1-α.33,34
In addition, we confirmed the proangiogenic effect of H2S in vitro. Maynard et al. revealed that sFlt1 inhibits capillary formation of endothelial cells in an in vitro angiogenesis assay.5 We showed that administration of H2S to sFlt1-stimulated endothelial cells prevented the disrupted tube formation. These data support previous observations that H2S has proangiogenic effects.32 When administered twice daily, H2S induced a significant reduction in BP in the adv-sFlt1 infected model. Importantly, in the majority of the rats, mean arterial pressure was determined at the end of the experiment under anesthesia. To exclude the BP-lowering effects of anesthesia, we also confirmed the antihypertensive phenotype of hydrogen sulfide using telemetry, which has been shown to be more reliable. Moreover, BP-lowering effects of H2S have also been previously reported in various other models of both experimental and spontaneous hypertension in rats.38–40 It is known that H2S-induced vasorelaxation is mainly mediated by the opening of KATP channels in vascular smooth muscle cells.22 However, in this study, the antihypertensive effect of H2S is accompanied by an increase in plasma VEGF, which itself is a vasodilator.41 Therefore, we propose that H2S provides a vasorelaxant effect by not only opening KATP channels but also through interacting with the VEGF signaling pathway. Indeed, we revealed that H2S vasodilation is dependent on VEGF signaling because blockade of VEGFR2 attenuates H2S-mediated vasodilation. Interestingly, a recent publication shows that VEGFR2 functions as a H2S-targeting receptor in promoting endothelial cell migration.42 Therefore, it is possible that H2S ameliorates hypertension in the sFlt1 overexpression model partly by upregulation of VEGF and/or direct action on VEGFR2.
Additional mechanisms by which H2S induced vasorelaxation should also be considered in our study. Vasodilation by interaction of H2S with the vasoactive agent nitric oxide (NO) has been reported.34,41,43 However, we did not find that H2S treatment caused significant changes in NO metabolite levels in plasma and urine (Supplemental Figure 2). This suggests that NO was not a major player in the vasodilatory effects of H2S in our model. However, it is unknown whether the activity of the NO synthases was altered. Furthermore, Lu et al. showed that H2S is able to lower BP by inhibiting plasma renin activity. Whether this is the case in the present model remains to be elucidated. We also do not know whether H2S will downregulate endothelin synthesis, which was recently shown to be a key downstream signaling pathway that mediates sFlt1-induced vascular disease.44 Finally, H2S is known for its antioxidative and ischemia/hypoxia protective features.45 Because massive sFlt1 production is thought to be secondary to hypoxia, we suggest that cytoprotection could be another protective effect of H2S in the sFlt1-induced phenotype.46
This study revealed a significant reduction in sFlt1-induced proteinuria and kidney damage by administration of exogenous H2S. The decrease of hypertension in the present model may have induced the reduction in proteinuria, because hypertension itself may induce kidney damage that in turn results in proteinuria. On the other hand, higher VEGF induced by NaHS could be directly protective for the kidney. This is in line with data of Eremina et al., who showed that mice with reduced production of VEGF by podocytes exhibit massive proteinuria and glomerular endotheliosis,13 suggesting the presence of a direct effect of sFlt1 on the development of proteinuria. We revealed an increased VEGF-A mRNA expression in kidneys during H2S treatment, implying a renal BP-independent protective effect on the kidney by NaHS.
In addition to an antiangiogenic state due to high sFlt1 in preeclampsia, there are also alterations in the H2S pathway in this disease.47,48 Preeclampsia is associated with elevated levels of homocysteine, a key protein in the trans-sulfurization pathway and a precursor of H2S.49,50 The enzymes cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) are involved in catalyzing homocysteine and cysteine, respectively, as well as in producing H2S. Genetic deficiency of CBS leads to homocysteinemia, which is associated with endothelial dysfunction and hypertension,51 whereas CSE-deficient mice suffer from hypertension.52 Interestingly, the expression of CBS and CSE in women with preeclampsia is decreased in the placenta.47,48 The administration of H2S had the same effect on sFlt1 levels in pregnant rats, compared with nonpregnant rats (data not shown). Importantly, pups from NaHS-treated mothers showed no apparent dysmorphic characteristics and there were no adverse effects of NaHS on placental and pup weight (Supplemental Figure 3). In view of these results, we propose that H2S could be a possible treatment for preeclampsia. Future studies should further explore the protective effects of NaHS in pregnant rats with overexpression of sFlt1.
In summary, we have demonstrated that administration of exogenous H2S ameliorates sFlt1-induced vascular disease by promoting VEGF upregulation. These findings may have important implications in developing novel therapeutics to treat preeclampsia or to reverse VEGF inhibitor side effects in cancer patients. Additional studies using H2S in pregnant models of preeclampsia and growth restriction are warranted to evaluate safety and biologic efficacy before proceeding with human trials.
Concise Methods
Animal Model and NaHS Treatment
All animal protocols were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Sprague-Dawley female rats (200–250 g) were intravenously injected into the tail vein with 1×1010 PFU/kg of adenovirus expressing sFlt1. The recombinant adenovirus expressing murine sFlt(1–3) was previously described,53 and was amplified at a commercial facility (Vector Biolabs, Philadelphia, PA). Within 72 hours, plasma mouse sFlt1 levels were confirmed using a mouse sFlt1 ELISA kit (R&D Systems, Inc., Minneapolis, MN) and animals were stratified to the treatment or control groups. Both groups were treated twice daily with intraperitoneal injections for 8 days with either 50 μM/kg NaHS (Sigma-Aldrich, St. Louis, MO) (n=7) or PBS vehicle (n=7). Twenty-four hours before termination, the rats were housed in metabolic cages. Terminal BPs were measured via carotid catheterization under anesthesia immediately before harvesting blood and kidneys, as previously described.5
In a similar design, NaHS or vehicle was administered to pregnant sFlt1-infected rats. After sFlt1 infection, rats were stratified to treatment and control groups and intraperitoneally injected twice daily for 8 days with either 50 μM/kg NaHS or PBS. Pups and placentae were analyzed to assess NaHS toxicity to pregnancy.
Histopathology and Electron Microscopy
Harvested kidneys were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. For electron microscopy, renal tissue was embedded in araldite-Epon mixture; 1-μm sections were stained with methylene blue and assessed before ultrastructural study.
Endothelial Tube Assay
HUVECs (20,000 cells per 200 µl) were plated onto Matrigel (9.0 mg/ml; BD Biosciences, Bedford, MA) coated wells with or without 1 µg recombinant sFlt (R&D Systems, Inc.) and treated with 600 µM NaHS or PBS. After 8 hours, tube formation was assessed through an inverted phase contrast microscope at ×4 (Nikon Corporation, Tokyo, Japan) and was quantitatively analyzed (number of branching points) using ImageJ software (National Institutes of Health, Bethesda, MD). The experiment was repeated five times.
Stimulation of Podocytes with NaHS In Vitro
Established lines of human glomerular visceral epithelial cells (GVECs/podocytes) were used.54 Human GVECs were seeded into 12-well plates and treated with 100 or 1000 µM NaHS or PBS. Phorbol 12-myristate 13-acetate (Sigma-Aldrich) was used as a positive control for VEGF upregulation (50 ng/ml). After 30, 60, and 120 minutes, medium was removed and VEGF concentrations were measured using ELISA (R&D Systems, Inc.). In a parallel experiment, podocytes were harvested and stored for mRNA isolation and real-time RT-PCR after 30 minutes, and 2 and 24 hours. All experiments were repeated five times.
ELISA
ELISA kits were used to measure plasma mouse sFlt-1 and rat and human VEGF (R&D Systems, Inc.). Urinary albumin was determined using the Exocell Nephrat kit (Philadelphia, PA).
RNA Isolation and Real-Time RT-PCR
Total RNA from whole rat kidneys and human GVECs was extracted using Trizol (Invitrogen, Carlsbad, CA), and cDNA was synthesized using Superscript II RT and random hexamer primers (Invitrogen). VEGF-A mRNA was quantified with TaqMan real-time quantitative RT-PCR using the VEGF-A gene expression assays (Rn01511605_m1 [rat] and Hs00900055_m1 [human]; Applied Biosystems, Foster City, CA) with hypoxanthine-guanine phosphoribosyltransferase as a reference gene in each reaction. The average Ct values for the target gene VEGF-A were divided by the average housekeeping gene, generating a 2−ΔCT value.
Mesenteric Vessel Wire Myograph Studies
Rings from second-order mesenteric resistance arteries were harvested from male wild-type C57BL/6 mice and mounted (Danish Myo Technology Aarhus, Denmark) for isometric tension recordings using PowerLab software (AD Instruments, Dunedin, New Zealand) as previously described.55 Concentration-response relaxation curves were built in the presence of VEGFR2 neutralizing antibody DC101 (50 µg/ml) or IgG antibody (50 µg/ml) by precontracting vessels with phenylephrine at 10 μM before administration of VEGF and NaHS. Data from two to four rings per mouse were averaged, with three to four mice for each wire myograph study.
Implantation of Telemetric Devices and Telemetric Data Acquisition
Systolic, diastolic, and mean arterial pressures were measured using TA11PA-C40 radiotransmitters (Data Sciences International, St. Paul, MN). The transmitter catheter was surgically secured in the lower abdominal aorta. After 7 days of recovery, continuous data collection was started using the Dataquest A.R.T. Acquisition System (Data Sciences International). All hemodynamic data were analyzed using 24-hour means.
Measurement of Plasma and Urine Nitrite/Nitrate
The stable end products of NO, nitrite and nitrate were measured in plasma and urine according to the method described by Moshage et al.56
Statistical Analyses
Results are presented as the median (IQR) or mean±SEM. Between-group comparisons were made using the Mann–Whitney U test and Wilcoxon signed rank test, and within-group differences were assessed with two-factor repeated-measures ANOVA with the Newman–Keuls post-test. Correlation coefficients were analyzed using linear regression analysis. Significant differences are reported when P<0.05.
Disclosures
S.A.K. is a coinventor on patents related to diagnosis/therapy of preeclampsia, discloses financial interest in Aggamin, LLC, and is a consultant to Siemens Diagnostics.
Supplementary Material
Acknowledgments
The authors greatly acknowledge the technical contribution of Sippie Huitema, Lisette den Boef, and Theo Borghuis.
This work was supported by a grant from the Dutch Kidney Foundation (KSBP 10.019), a Mandema Stipendium from the University Medical Center Groningen (to A.T.L.), and a grant from the Groningen University Institute of Drug Exploration. S.A.K. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013030291/-/DCSupplemental.
References
- 1.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Le Roux S, Pepper RJ, Dufay A, Néel M, Meffray E, Lamandé N, Rimbert M, Josien R, Hamidou M, Hourmant M, Cook HT, Charreau B, Larger E, Salama AD, Fakhouri F: Elevated soluble Flt1 inhibits endothelial repair in PR3-ANCA-associated vasculitis. J Am Soc Nephrol 23: 155–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marco GS, Reuter S, Hillebrand U, Amler S, König M, Larger E, Oberleithner H, Brand E, Pavenstädt H, Brand M: The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol 20: 2235–2245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maynard SE, Karumanchi SA: Angiogenic factors and preeclampsia. Semin Nephrol 31: 33–46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad S, Ahmed A: Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R: Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605–12608, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Kendall RL, Thomas KA: Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A 90: 10705–10709, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J: The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Barleon B, Reusch P, Totzke F, Herzog C, Keck C, Martiny-Baron G, Marmé D: Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis 4: 143–154, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, CPEP Study Group : Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355: 992–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD: Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: Mechanisms and potential use as a biomarker. Semin Nephrol 30: 591–601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber H-P, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD: A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst 100: 282–284, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber H-P, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS: Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50: 686–692, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M: Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A 108: 1451–1455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H, Ohkuchi A, Matsubara S, Takei Y, Murakami M, Shibuya M, Suzuki M, Sato Y: Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension 54: 1129–1135, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Gilbert JS, Gilbert SAB, Arany M, Granger JP: Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, Hemphill L, Rigby AC, Khedkar S, Lindner TH, Mallmann P, Stepan H, Karumanchi SA, Benzing T: Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124: 940–950, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Wang R: Hydrogen sulfide: A new EDRF. Kidney Int 76: 700–704, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Zhao W, Zhang J, Lu Y, Wang R: The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH: Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, Cirino G: cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS ONE 7: e53319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R: Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL: Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca²⁺-activated K⁺ channels and smooth muscle Ca²⁺ sparks. Am J Physiol Heart Circ Physiol 304: H1446–H1454, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Liu H, Sun D, Qiao W, Qi Y, Sun H, Yan C: Effects of H₂S on myogenic responses in rat cerebral arterioles. Circ J 76: 1012–1019, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Bos EM, Leuvenink HGD, Snijder PM, Kloosterhuis NJ, Hillebrands J-L, Leemans JC, Florquin S, van Goor H: Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J Am Soc Nephrol 20: 1901–1905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan L-L, Liu X-H, Gong Q-H, Zhu Y-Z: S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids 41: 205–215, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Stahl GL, Sellke FW: Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. J Thorac Cardiovasc Surg 138: 977–984, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, Moser J, Hillebrands J-L, Ploeg RJ, Yang G, Leuvenink HGD, van Goor H: Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol 24: 759–770, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai W-J, Wang M-J, Moore PK, Jin H-M, Yao T, Zhu Y-C: The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Wang M-J, Cai W-J, Li N, Ding Y-J, Chen Y, Zhu Y-C: The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065–1077, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG: Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA: Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 57: 1R–7R, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS: Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc Res 89: 671–679, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabó C: Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu M, Liu Y-H, Goh HS, Wang JJX, Yong Q-C, Wang R, Bian J-S: Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol 21: 993–1002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad FUD, Sattar MA, Rathore HA, Abdullah MH, Tan S, Abdullah NA, Johns EJ: Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Ren Fail 34: 203–210, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Tan G, Pan S, Li J, Dong X, Kang K, Zhao M, Jiang X, Kanwar JR, Qiao H, Jiang H, Sun X: Hydrogen sulfide attenuates carbon tetrachloride-induced hepatotoxicity, liver cirrhosis and portal hypertension in rats. PLoS ONE 6: e25943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, Szabo C: Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 109: 9161–9166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC: VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali MY, Ping CY, Mok Y-Y, Ling L, Whiteman M, Bhatia M, Moore PK: Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li F, Hagaman JR, Kim H-S, Maeda N, Jennette JC, Faber JE, Karumanchi SA, Smithies O, Takahashi N: eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol 23: 652–660, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Predmore BL, Lefer DJ, Gojon G: Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 17: 119–140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu FTH, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS: A systems biology perspective on sVEGFR1: Its biological function, pathogenic role and therapeutic use. J Cell Mol Med 14: 528–552, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cindrova-Davies T, Herrera EA, Niu Y, Kingdom J, Giussani DA, Burton GJ: Reduced cystathionine γ-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: Hydrogen sulfide as a placental vasodilator. Am J Pathol 182: 1448–1458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holwerda KM, Bos EM, Rajakumar A, Ris-Stalpers C, van Pampus MG, Timmer A, Erwich JJHM, Faas MM, van Goor H, Lely AT: Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta 33: 518–521, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Li L, Rose P, Moore PK: Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51: 169–187, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen AL, Ueland PM: Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: The Hordaland Homocysteine study. Am J Clin Nutr 71: 962–968, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Miles EW, Kraus JP: Cystathionine beta-synthase: Structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem 279: 29871–29874, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R: H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC: Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A 98: 4605–4610, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnamurti U, Chen Y, Michael A, Kim Y, Fan WW, Wieslander J, Brunmark C, Rondeau E, Sraer JD, Delarue F, Tsilibary EC: Integrin-mediated interactions between primary/T-sv40 immortalized human glomerular epithelial cells and type IV collagen. Lab Invest 74: 650–657, 1996 [PubMed] [Google Scholar]
- 55.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ: Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 18: 1429–1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moshage H, Kok B, Huizenga JR, Jansen PL: Nitrite and nitrate determinations in plasma: A critical evaluation. Clin Chem 41: 892–896, 1995 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.