Abstract
Previously, we showed that some podocytes in juvenile mice are recruited from cells lining Bowman’s capsule, suggesting that parietal epithelial cells (PECs) are a progenitor cell population for podocytes. To investigate whether PECs also replenish podocytes in adult mice, PECs were genetically labeled in an irreversible fashion in 5-week-old mice. No significant increase in labeled podocytes was observed, even after 18 months. To accelerate a potential regenerative mechanism, progressive glomerular hypertrophy was induced by progressive partial nephrectomies. Again, no significant podocyte replenishment was observed. Rather, labeled PECs exclusively invaded segments of the tuft affected by glomerulosclerosis, consistent with our previous findings. We next reassessed PEC recruitment in juvenile mice using a different reporter mouse and confirmed significant recruitment of labeled PECs onto the glomerular tuft. Moreover, some labeled cells on Bowman’s capsule expressed podocyte markers, and cells on Bowman’s capsule were also directly labeled in juvenile podocyte-specific Pod-rtTA transgenic mice. In 6-week-old mice, however, cells on Bowman’s capsule no longer expressed podocyte-specific markers. Similarly, in human kidneys, some cells on Bowman’s capsule expressed the podocyte marker synaptopodin from 2 weeks to 2 years of age but not at 7 years of age. In summary, podocyte regeneration from PECs could not be detected in aging mice or models of glomerular hypertrophy. We propose that a small fraction of committed podocytes reside on Bowman’s capsule close to the vascular stalk and are recruited onto the glomerular tuft during infancy to adolescence in mice and humans.
A major cause of ESRD is the progression of CKD with secondary glomerulosclerosis resulting from injury and, eventually, loss of podocytes.1–3
Under physiologic conditions, differentiated podocytes are unable to proliferate and, therefore, cannot appropriately compensate cell depletion.4,5 A potential mechanism for podocyte replacement from bone marrow–derived stem cells has been proposed in the Alport mouse model as well as kidney transplants.6–8 However, regeneration of podocytes from an extrarenal source could not be confirmed by other groups.9–11 Recently, we have shown using lineage tracing that, in juvenile mice, cells from Bowman’s capsule (presumptive parietal epithelial cells [PECs]) migrate onto the vascular tuft and differentiated into podocytes.12 This finding has raised hope that PECs are good candidate cells to act as intrarenal progenitors for podocytes, even in adult mammalian kidneys. In fact, at the same time, Ronconi et al.13 identified human PECs using the markers CD24 and a differentially glycosylated form of CD133. The work by Ronconi et al.13 showed that expression of these markers diminishes to the vascular stalk and that podocyte marker proteins (podocalyxin) are, in turn, upregulated. From these findings, it was hypothesized that progressive differentiation of PECs into podocytes on Bowman’s capsule may occur and that these cells were in the process of migrating onto the capillary tuft through the vascular stalk. In addition, recent studies have reported in models for FSGS that repopulation of podocytes occurs after treatment with high-dose angiotensin-converting enzyme inhibitors or prednisone treatment.14,15 In a leptin-deficient model of diabetic nephropathy, repopulation of podocytes was suggested as one possible explanation for an improved renal function and structure after leptin treatment.16 These latter studies proposed PECs as a possible source of regenerating podocytes. However, these studies relied on indirect observations, such as expression of proliferation markers in PECs or other expression markers proteins, which may not be stable in disease. Furthermore, without specific labeling of cells, it is impossible to study the actual migration and differentiation between two distinct cell populations, such as PECs and podocytes. Therefore, at present, the in vivo evidence for a repopulation or regeneration of podocytes by PECs in adult mammalian kidneys is still nondefinitive.
The current study examined the regenerative potential of PECs in the adult mouse kidney. Using lineage tracing models, the fate of PECs was traced in aging mice and models of glomerular hypertrophy.
Results
Tracing of Genetically Tagged PECs in Aging Mice
We previously reported that podocytes are recruited from Bowman’s capsule in juvenile mice.12 To investigate whether this recruitment continues to occur in adult mice, PECs were labeled in triple transgenic PEC-rtTA/LC1/R26R mice in a doxycycline-inducible irreversible fashion at 5 weeks of age and killed at different time points (1.5, 3, 6, 12, and 18 months after induction of labeling) (Figure 1A). Across the entire observation period of 18 months, a similar distribution of β-galactosidase (β-gal)–positive cells was observed. Genetic labeling of PECs persisted even after 12 months (Figure 1, D–D″, arrowheads). Overall, the absolute number of β-gal–positive cells on the glomerular tuft at all time points was negligible (Figure 1, B–D″, arrows with tails); β-gal–positive cells were virtually always localized at the vascular stalk (transitional cells) and genetically labeled by the PEC-rtTA transgenic mouse.12 These cells were counted as β-gal–positive cells localized on the glomerular tuft and constitute the low but constant presence of β-gal–positive cells on the glomerular tuft (Figure 1E). More than five β-gal–positive cells were never observed within 100 glomeruli of each evaluated kidney. Mice ages 19.5 months (i.e., 18 months after induction) showed (ultra) structural alterations with protein casts, tubular dilatation, glomerular basement membrane (GBM) thickening and mesangial expansion (Figure 1, F–H). Enlargement and formation of filopodial protrusions were observed in some podocytes (Figure 1H, open arrow). There was segmental foot process effacement, but the majority of the podocytes showed normal foot processes.
Figure 1.
Absence of PEC recruitment in aging mice. (A) Timeline of the experiment. Mice were killed after 1.5, 3, 6, 12, and 18 months as indicated by the arrows (1.5–12 months, n=5; 18 months, n=3). Dox, doxycycline. (B–D) Representative histologic images of X-gal/eosin-stained kidney sections at different time points. At all time points, X-gal staining persisted in PECs (arrowheads). β-Gal–positive cells were rarely observed within the glomerular tuft and almost always localized to the vascular pole (arrows with tails). These cells are transitional cells, which are directly labeled by the PEC-rtTA mouse.12 Within the tubules, scattered tubular cells were labeled (arrows). (E) Statistical analysis of β-gal–positive cells in 100 glomerular cross-sections of X-gal–stained frozen sections in aging PEC-rtTA/LC1/R26R mice shows no significant changes over time. Glom., glomeruli. (F and G) Periodic acid–Schiff (PAS)-stained kidney of an 18-month-old PEC-rtTA mouse shows (F, arrow) protein casts, (G, arrow) mesangial expansion/sclerosis, and (G, arrowhead) a thickened irregular GBM. (H) Mesangial expansion and sclerosis were confirmed by transmission electron microscopy (arrow). In addition, the GBM was thickened with spikes/humps (arrowheads) and covered by podocytes with filopodial protrusions (open arrow).
Induction of Glomerular Hypertrophy after Partial Nephrectomy to Stimulate Podocyte Regeneration
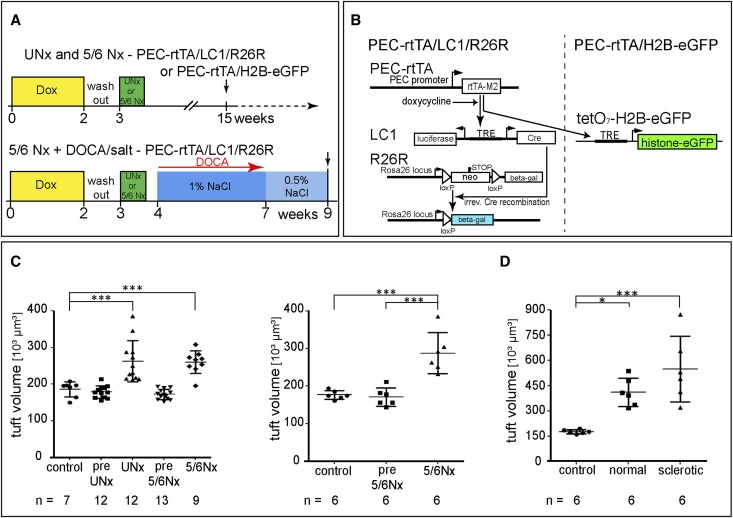
Podocyte recruitment in aging mice might be absent or too low to be detected. For this reason, an effort was undertaken to accelerate potential recruitment of podocytes from PECs. Uninephrectomy (UNx) or 5/6 nephrectomy (5/6Nx) were performed using two different reporter mice: PEC-rtTA/LC1/R26R or PEC-rtTA/H2B-eGFP transgenic mice (Figure 2, A and B). In both reporter strains, not a single labeled cell was detected on the glomerular tuft in more than 20 mice that had received doxycycline, which labels ∼72% of PECs as shown previously.12 After a washout period, glomerular hypertrophy was successfully induced after UNx and 5/6Nx in both transgenic mouse lines (Figure 2, C and D). Because of limited amounts of kidney tissue after 5/6Nx, podocyte numbers could only be estimated indirectly on 80 random glomerular cross-sections per experimental mouse. As shown in Supplemental Figures 1–3, progressive podocytopenia was observed after UNx and 5/6Nx. Because UNx alone was not sufficient to induce significant podocytopenia in PEC-rtTA/LC1/R26R mice, 5/6Nx exclusively was performed in the second reporter mouse (PEC-rtTA/H2B-eGFP), and again, it resulted in significant glomerular hypertrophy (Figure 2C).
Figure 2.
Induction of progressive glomerular hypertrophy. (A) Experimental setups and timeline to induce glomerular hypertrophy using two alternative reporter mice: R26R and H2B-eGFP. All experiments included 14 days of doxycycline treatment and a 7-day washout before UNx or 5/6Nx. The 5/6Nx + DOCA salt model included 25 mg DOCA over 3 weeks combined with supplementation of 1% NaCl and 2 additional weeks with 0.5% NaCl by drinking water until euthanized. Arrow, time of euthanization. (B) Two alternative reporter genes were used in this study, which were both induced by the PEC-specific PEC-rtTA mouse. (Left) In the PEC-rtTA/LC1/R26R mouse, administration of doxycycline induces reversible expression of Cre recombinase from the LC1 transgene, which mediates irreversible activation of β-gal expression. (Right) Administration of doxycycline induces reversible expression of eGFP-tagged histone from the tetO7-H2B-eGFP transgene, which is deposited in the nucleus, where it persists because of a long-half life. (C) Glomerular hypertrophy after partial nephrectomy. Glomerular tuft volume was deduced from measured glomerular tuft areas of 80 glomerular cross-sections in kidneys of sham-operated (control) mice, resected kidneys (pre-UNx and pre-5/6Nx), and residual kidneys after 3 months (UNx and 5/6Nx) using the formula VG=(β/k)(Am)3/2.14 n, number of mice as indicated (one-way ANOVA followed by Bonferroni test). (D) PEC-rtTA/LC1/R26R mice were subjected to the 5/6Nx+DOCA/salt model to induce the most significant glomerular hypertrophy and glomerulosclerosis in a fraction of the glomeruli. Glomerular size was evaluated in histologically normal and sclerotic glomeruli individually. *P<0.05; *** P<0.001.
Histologic examination of all experimental kidneys after 5/6Nx did not show FSGS lesions in any of the experimental mice. To further aggravate the stimulus for potential podocyte regeneration, 5/6Nx with deoxycorticosterone acetate (DOCA)/salt treatment was performed (Figure 2A, lower schematic). This model induces FSGS lesions in both reporter mice as we reported previously.17 In the current study, the 5/6Nx DOCA/salt model induced FSGS in 47±11% of the glomerular cross-sections. Both morphologically normal and (segmentally) sclerotic glomerular cross-sections showed marked glomerular tuft hypertrophy (Figure 2D). Among sclerotic glomerular cross-sections, a large variance in the glomerular tuft area was observed, which reflects the focal and segmental damage of the affected glomeruli, ranging from small segmental sclerotic lesions to global sclerosis. Podocytopenia was significant in both the normal and sclerotic glomerular cross-sections (Supplemental Figure 1).
Tracing of PECs after Induction of Hypertrophy and Podocytopenia
To study whether PECs regenerate podocytes in our models of progressive glomerular hypertrophy, cell fate tracking of labeled PECs was performed and quantified. When using the β-gal reporter line PEC-rtTA/LC1/R26R, no significant increase of irreversibly labeled PECs was observed on the glomerular tuft cross-sections in any of our experimental groups (Figure 3, A–C). Even after longer time periods of up to 12 months, no significant recruitment of PECs was observed (Figure 3D). Among the glomeruli with positive β-gal staining on glomerular tuft cross-sections, positive cells per glomerular cross-section never exceeded two (Figure 3C). Like in nonmanipulated mice, positive cells were mostly located at the vascular stalk near the transition between Bowman’s capsule and GBM. When two cells were visible in one single glomerular cross-section, both cells were usually located adjacent to each other in close proximity of the vascular stalk. They were not dispersed and located in the periphery of the glomerular tuft in any case, indicating that these cells are labeled transitional cells12 rather than regenerated podocytes (Figure 3A4).
Figure 3.
Tracing of labeled PECs after induction of glomerular hypertrophy in (A–D) PEC-rtTA/LC1/R26R or (F–H) rtTA/H2B-EGFP reporter mice. (A1–A3) X-gal–stained frozen sections of (A1) control (n=7) and (A2 and A3) experimental remnant kidneys (UNx, n=12; 5/6Nx, n=9). Staining quality is reduced, because no perfusion fixation was performed (for accurate kidney weights). Original magnification, ×400. (A4) β-Gal–positive cells close to the vascular pole (arrows). (B) Statistical analysis of 100 random glomerular cross-sections per animal including data from resected control kidneys (pre-UNx, n=12; pre-5/6Nx, n=13). Virtually no β-gal–positive cells were detected on the glomerular tuft (black bars, glomeruli with β-gal–positive cells within the tuft; error bars, SD). (C) Absolute numbers of β-gal–positive cells exclusively in those glomerular cross-sections showing β-gal positivity on the tuft. Error bars, SD. (D) Analysis of PEC to podocyte regeneration in aging PEC-rtTA/LC1/R26R mice after UNx shows no significant changes of time (1.5 and 3 months, n=5; 6 months, n=4; 12 months, n=3). (E) Costainings of synaptopodin or claudin-1 (red) and the reporter eGFP-histone (green) on paraffin sections of control and experimental remnant kidneys 3 months after 5/6Nx using PEC-rtTA/H2B-EGFP reporter mice. No significant numbers of eGFP-labeled cells were detected within the glomerular tuft in any of the experimental or control groups. Arrowheads mark eGFP-positive cells along the vascular stalk. Original magnification, ×400. (F) Statistical analysis of eGFP-positive cells on the glomerular tuft 3 months after 5/6Nx (control, sham operated; pre-5/6Nx, resected kidney; 5/6Nx, contralateral remnant kidney after 3 months; n=6 in each group). (G) The absolute number of eGFP-positive cells on the tuft in glomeruli, with eGFP positivity on the tuft ranging only between 1 and 1.5. Error bars, SD. (H) Absolute number of eGFP-positive PECs on glomerular cross-sections, with eGFP positivity on the capsule showing that GFP-histone labeling persisted in PECs. Error bars, SD.
To confirm our findings, the experiments were repeated using a different reporter strain, PEC-rtTA/H2B-eGFP, in which PEC nuclei are labeled by the expression of enhanced green fluorescent protein (eGFP)-histone. Again, no increase in the number of eGFP-labeled cells on the glomerular tuft was observed, even after 5/6Nx, which was similar to our findings using the β-gal reporter line (Figure 3, E and F). The absolute number of eGFP-positive cells in glomeruli with positive cells did not exceed two cells per glomerulus (Figure 3G), consistent with direct labeling of transitional cells (Figure 3E, arrowheads). eGFP-histone labeling persisted in PECs even after 3 months (Figure 3H). A small decrease of eGFP-positive PECs on the Bowman’s capsule was noted 3 months after 5/6Nx compared with control kidneys (pre-5/6Nx) that were removed from the same animal (Figure 3H). Although this difference was not significant compared with sham-operated control kidneys, nevertheless, it suggests that PECs are distributed over an enlarged Bowman’s capsule in glomerular hypertrophy and that this finding is not fully compensated by PEC proliferation alone.
PEC Invasion onto the Glomerular Tuft in FSGS Lesions
Genetically labeled PECs were only observed in more than 80% of glomeruli affected by sclerotic lesions in the most aggravated model of 5/6Nx and DOCA/salt. The formation of FSGS lesions and the participation of PECs have already been studied by us previously.17–19 In those studies, we showed, in different models of FSGS, that PECs do not replace podocytes functionally. Rather, these cells expressed the activation marker CD44 and remained positive for classic PEC markers. In accordance with these previous studies, the genetically labeled PECs present on the glomerular tuft in the current study were also positive for PEC activation marker CD44 (Figure 4, A and A′, arrows) and LKIV69 (staining parietal extracellular matrix) (Figure 4, B and B′, arrows). Within the glomerulus, CD44 can only be expressed by leukocytes other than activated PECs, and LKIV69 is specific for PECs.17,20 Of note, this result was only seen in glomeruli containing FSGS lesions, whereas hypertrophied glomeruli that were still morphologically normal with no visible FSGS lesion did not show migration of genetically tagged PECs onto the glomerular tuft (not shown).17
Figure 4.
Sclerotic lesions are populated by PECs in β-gal reporter mice. Serial cryosections of a glomerulus (A and B) stained with X-gal/eosin and immunostained for (A′) CD44/hemalaun or (B′) extracellular matrix derived from PECs (LKIV69). Although labeled PECs reside on Bowman’s capsule, activated PECs invade the glomerular tuft in several locations (arrows).
Recruitment of Podocytes from Cells on Bowman’s Capsule in Juvenile Mice
Given the above results in adult kidneys in aging mice or glomerular hypertrophy, the experiments in juvenile mice were repeated using a different reporter strain PEC-rtTA/H2B-eGFP. Labeling of PECs was induced by two doxycycline injections on days 3 and 4 after birth, and the mice were euthanized at day 8 to verify specificity of labeling (Figures 5, A–C″, and 6, A–D″). After 4 days, eGFP-labeled cells were still localized on Bowman’s capsule, and not a single cell within the periphery of the glomerular tuft was labeled. Of note, about 20% of glomerular cross-sections showed labeled transitional cells (Figure 6F), all of which were localized at the vascular stalk (Figure 6G). These eGFP-labeled cells expressed increased amounts of the podocyte marker Wilms tumor 1 (WT-1) compared with the relatively low expression of WT-1 in PECs, which has been described previously.21 These results also support that these cells are the previously described transitional cells (Figures 5, B–C″, arrows, and 6F).12
Figure 5.
Tracking PECs in juvenile PEC-rtTA/H2B-eGFP mice. (A) Mice were induced with doxycycline 3 and 4 days after birth and analyzed 8 days or 6 weeks after birth (arrows). (B–C″) Immunofluorescent costainings for (B′ and C′) eGFP and (B and C) WT-1 of paraffin sections of 8-day-old PEC-rtTA/H2B-eGFP mice confirm labeling of PECs and absence of labeling in podocytes. At the vascular pole, WT-1–positive cells are also labeled (arrows). Original magnification, ×400. (D–E″) At 6 weeks, eGFP–labeled cells can be found along the hilus of the glomerular tuft and also, within the periphery of the glomeruli (arrows). Coexpression of WT-1 and location on the glomerular tuft suggests that these eGFP-positive cells are fully differentiated podocytes. Arrowheads mark labeled cells that have migrated onto the tuft but did not fully differentiate into podocytes (WT-1–negative and eGFP-positive). Nuclei counterstaining with Hoechst. Original magnification, ×400.
Figure 6.
Direct labeling of synaptopodin-positive cells on Bowman’s capsule in juvenile PEC-rtTA/H2B-eGFP mice. (A–B″) When costaining for podocytes marker synaptopodin (red) and the reporter transgene eGFP-histone (green) 8 days after birth, weak expression of synaptopodin is noted in cells on Bowman’s capsule, mostly located close to the vascular pole (arrows). These synaptopodin-positive cells are also eGFP-labeled by the PEC-rtTA/H2B-eGFP mouse. *eGFP-labeled and synaptopodin-negative cells on Bowman’s capsule (classical PECs). Prominent synaptopodin expression was present in eGFP-positive cells at the vascular stalk (presumptive transitional cells with the round nucleus characteristic for podocytes; arrowheads). (C–D″) In 6-week-old mice, synaptopodin-positive cells were no longer present on Bowman’s capsule (arrows). Again, significant numbers of eGFP-histone–tagged cells were present within the glomerular capillary tuft and expressed synaptopodin, indicating that these cells represented differentiated podocytes recruited from Bowman’s capsule. (E) Total number of PECs and eGFP-labeled PECs in 60 random glomerular cross-sections at 8 days and 6 weeks of age as detected by Hoechst/eGFP double staining (n=4 mice per group; error bars, SD; no sign of differences between time points). (F) Recruitment of eGFP-labeled differentiated podocytes on the glomerular tuft was evaluated by eGFP/Hoechst/WT-1 or -/p57 triple staining of mice ages 8 days or 6 weeks (30 glomerular cross-sections in n=4 mice each; error bars, SD). ***P<0.0001. (G) The percentage of glomerular cross-sections with eGFP-positive transitional cells (as defined by their location at the vascular stalk) was about 20% in both age groups (n=4; 60 glomeruli per mouse).
At 6 weeks of age, significant recruitment of labeled cells onto the glomerular tuft was observed (Figure 6F). eGFP-positive cells were localized along the vascular stalk and also found within the periphery of the glomerular tuft. This distribution is suggestive of the migratory path of these cells along the vascular stalk into the periphery of the glomerular tuft. eGFP-positive cells on the glomerular tuft coexpressed the podocyte marker WT-1 (Figure 5, D and E), p57 (not shown), and synaptopodin (Figure 6B), indicating that they are fully differentiated podocytes. Expression of podocyte markers was confirmed by detection of synaptopodin expression in cells on Bowman’s capsule 8 days after birth (Figure 6, A and B). Synaptopodin-positive cells on Bowman’s capsule were localized close to the vascular pole and more prominent in the smaller cortical glomeruli with dense glomerular tufts. Juxtamedullary glomeruli, which develop sooner than the cortical glomeruli, rarely showed synaptopodin-positive PECs on Bowman’s capsule (data not shown). The PEC-rtTA/H2B-eGFP reporter line marked about 80% of all cells on Bowman’s capsule, similar to the labeling frequency in adult mice12 (Figure 6E), regardless of their coexpression of podocyte marker proteins. At 6 weeks of age, significant synaptopodin expression was no longer observed on Bowman’s capsule (Figure 6, C–D″).
The above-described findings are suggestive that a fraction of the cells on Bowman’s capsule are committed to differentiate into podocytes. To verify this notion, we tested whether the podocyte-specific transgenic mouse Pod-rtTA also directly labels putative podocytes on Bowman’s capsule in juvenile mice. In the Pod-rtTA mouse, transgene expression is driven under control of the podocin promoter; podocin is considered one of the most specific marker proteins for podocytes.22 After labeling 3 and 4 days after birth, cells were, indeed, directly labeled on Bowman’s capsule (Figure 7, B and E″). In contrast, in adult PEC-rtTA/H2B-eGFP reporter mice exclusively, the visceral podocytes were but the cells on Bowman’s capsule were not directly labeled (not shown).
Figure 7.
The podocyte-specific Pod-rtTA/H2B-eGFP mouse directly labels cells on Bowman’s capsule in juvenile mice. (A) Labeling was induced on days 3 and 4 after birth in Pod-rtTA/H2B-eGFP mice. Kidneys were analyzed 8 days after birth (arrow). (B) Nuclear eGFP immunostaining (brown, PAS counterstaining) shows eGFP-histone–labeled cells on Bowman’s capsule (arrows). (C–E″) WT-1 (red) is expressed in podocytes on the glomerular tuft and to a lesser extent, the cell on the Bowman’s capsule. The reporter transgene eGFP-histone (green) is expressed in visceral podocytes and in addition, also by some WT-1–positive cells on Bowman’s capsule (arrows).
Are There Podocytes-Committed Cells in Human Kidneys?
To verify whether cells on Bowman’s capsule also express podocyte marker proteins in humans (i.e., presumptive committed podocytes), synaptopodin expression was investigated in human kidneys at various ages (2 weeks, 5 months, and 2 and 7 years of age). Similar to mice, synaptopodin-positive cells were observed on Bowman’s capsule at 2 weeks (Figure 8, A1 and A2), 5 months (Figure 8, B1 and B2), and 2 years of age (Figure 8C3). These cells showed the typical morphology of parietal epithelial cells with a thin cytoplasm and a flat nucleus (Figure 8, A2, B1, B2, and C3, arrowheads). At younger ages, glomeruli were small with unfolded capillary loops and closely packed with podocytes (Figure 8A2, arrows). In 2-year-old kidney, a mixture of small glomeruli was observed with synaptopodin-positive cells on Bowman’s capsule (Figure 8C3) along with bigger glomeruli, in which the synaptopodin expression was restricted to the glomerular tuft. The latter were the juxtamedullary glomeruli. The glomeruli of a 7-year-old kidney showed no synaptopodin-expressing cells on Bowman’s capsule (Figure 8, D1 and D2). Costainings for the podocyte marker nestin showed very similar expression patterns on Bowman’s capsule (Supplemental Figures 5–7), whereas the expression of GLEPP1 seemed to be restricted to fewer cells on Bowman’s capsule (Supplemental Figures 8–10). As shown in our quantitative analysis of synaptopodin-positive cells, presumptive committed podocytes were more numerous on Bowman’s capsule at younger age (Figure 8E). In addition, committed podocytes were more numerous in the smaller subcortical glomeruli, which mature later, independent of age (Figure 8F).
Figure 8.
Synaptopodin expression is also in cells on Bowman’s capsule in juvenile human kidney. Staining of synaptopodin (green) and Hoechst (blue) on paraffin sections of kidneys derived from juvenile humans at different ages (A1 and A2, 2 weeks; B1 and B2, 5 months; C1–C3, 2 years; D1 and D2, 7 years of age). Arrowheads mark synaptopodin expression in cells on Bowman’s capsule. Visceral podocytes are densely packed on the glomerular tuft at younger ages (arrows). Scale bars, 50 µm. (E) Area covered by synaptopodin-positive cells on Bowman’s capsule in 20 random glomerular cross-sections for each time point shows a progressive decrease with increasing age. (F) Similarly, more area of Bowman’s (Bm’s) capsule was covered by synaptopodin-positive cells in smaller (and presumably, less mature) glomeruli. Tuft area was only measured in glomerular cross-sections, which also included the vascular stalk.
Discussion
The current study is a follow-up of our previous study showing the recruitment of podocytes from cells of Bowman’s capsule in juvenile mice.12 The present study investigates whether recruitment of podocytes from PECs occurs in the adult mouse kidney under physiologic conditions (i.e., normal aging) or after induction of glomerular hypertrophy and/or relative podocytopenia.
Our first major finding was that permanently tagged PECs in aging mice did not migrate onto the glomerular tuft to replenish podocytes. Loss of renal function and development of glomerulosclerosis are associated with aging in humans as well as rodents.23–27 Several studies have indicated that loss of podocytes plays an important role in age-related glomerulosclerosis.26,28–30 It was unclear until now whether lost podocytes were at least partially regenerated and if age-related glomerulosclerosis was, thus, caused by an insufficient replacement of podocytes.31 The aged PEC-rtTA mice showed typical changes within the glomerulus associated with kidney aging27 (i.e., mesangial matrix expansion, thickening of the GBM with formation of spikes/humps, proteinuria, and podocyte hypertrophy). Despite these structural changes, no replacement of podocytes by PECs was detected, even in these very old mice.
The second major finding of this study was the lack of regeneration of podocytes from PECs after induction of glomerular hypertrophy. Although podocyte numbers were not precisely determined for technical reasons (limited renal tissue after 5/6Nx), there was a significant trend to fewer podocytes per glomerular area or glomerular cross-section in the 5/6NX and 5/6Nx with DOCA/salt models, but again, no migration of PECs on the glomerular tuft was detected using two different reporter mice. The same result is also corroborated by another independent study using FACS analysis to quantify podocyte turnover.32 Combined with our observations in aging mice, these data strongly argue against a significant regeneration of podocytes by PECs in adult mice, and it may be premature to denote PECs as progentior cells.13,33,34
The only situation in which we observed migration of PECs on the glomerular tuft in adult mice was in glomeruli affected by glomerulosclerosis. However, in these glomeruli, the PECs were activated and deposited foreign extracellular matrix. Therefore, they are involved in the formation of the FSGS lesions and not beneficial to the glomerulus. This finding is consistent with our previous reports.17–19,27
In the current study, we examined podocyte regeneration under physiologic conditions and in models of glomerular hypertrophy. The choice to use these models was made for several reasons. First, the models are based on a nontoxic physiologic stress and/or injury. A similar spectrum of glomerular injury can be achieved in, for instance, the adriamycin model35,36 or models in which podocytes are specifically injured/depleted (Diphtheria toxin2,37 and LMB2/NEP25 models38) by injection of the different doses of the specific toxins. Using toxin-induced models, one cannot rule out that signaling necessary for a proper regeneration is also affected in the surviving podocytes. Furthermore, the present study reveals that the podocin promoter (at least in the Pod-rtTA mouse) also targets the transitional cells at the vascular stalk when used for podocyte-specific depletion models. Transitional cells may be of importance as a minor reserve for podocytes during adolescence. Using less specific toxins, like the adriamycin model, toxic effects on other glomerular cells, including the PECs, cannot be ruled out. Second, the models that we used reflect the pathology observed in human glomerular disease. Should a significant turnover of podocytes from PECs exist under physiologic conditions, it should have been detected in normal aging mice over time. Furthermore, many human diseases are associated with an increase in single nephron GFR and subsequent glomerular hypertrophy (e.g., obesity, aging, diabetes, hypertension, partial renal mass ablation, or CKD independent of the primary insult).39 It is well established that glomerular hypertrophy predisposes to secondary glomerulosclerosis (FSGS) and progression to ESRD.40–44 Third, by analyzing UNx, 5/6Nx, and the 5/6Nx with DOCA/salt models, a broad injury spectrum from mild glomerular hypertrophy ranging from no significant reduction of podocytes per glomerular area (UNx) to a marked glomerular hypertrophy and relative podocytopenia (5/6Nx) was covered. As shown by the induction of FSGS lesions, the injury spectrum also covered excessive glomerular hypertrophy exceeding any potential regenerative mechanisms (5/6Nx and DOCA/salt model).
The fourth major finding in the current study was a simple, but consistent, explanation of why migration of cells from the Bowman’s capsule to the glomerular tuft was only observed in juvenile mice. Using markers specific for PECs or podocytes and using the PEC (PEC-rtTA)- and podocyte (Pod-rtTA)-specific reporter mice, the development of glomeruli after the capillary loop stage was investigated in more detail. Only in young, still growing glomeruli, a population of cells was detected on Bowman’s capsule expressing both PEC and podocyte markers. These cells were no longer detected in 6-week-old adult mice. Similar observations were made in kidneys of newborn and juvenile humans. The cell fate decision to differentiate into PECs or podocytes is made before the capillary loop stage.45 We postulate that a number of cells on Bowman’s capsule commit to become podocytes and that these cells can be detected by coexpression of podocyte markers (the so-called transitional cells). These precommitted cells are still located on the Bowman’s capsule at birth, when the glomerular tufts are still very small and crowded by podocytes (Figure 8). At this early stage, the capillary surface may not be sufficient to hold all podocytes, which are required later in life when the body (and plasma volume, which determines glomerular tuft size) is fully grown. As the glomeruli enlarge when the mice or humans grow, these cells are then recruited onto the glomerular tuft (Figure 9). This podocyte reserve is rather small (less than 10% of all podocytes). We speculate that this finding may also provide an explanation why obesity predisposes not only to glomerular hypertrophy but also, secondary FSGS. We may be running out of our small reserve of podocyte-committed cells, and because no additional regenerative mechanism may exist in adults, we develop renal disease as adults.
Figure 9.
Proposed schematic of differentiation and subsequent migration during postnatal development in mouse and human. Induction of labeling in the PEC reporter mouse marks all cells on Bowman’s capsule: classic PECs as well as cells closer to the vascular pole destined to differentiate into podocytes. At this stage, these cells coexpressing markers were PECs as well as podocytes. (Left) They represent a reservoir of podocytes while there is not enough space on the relatively small glomerular tuft, which is closely packed by visceral podocytes (Figure 8, A1 and A2). During adolescence, glomeruli undergo progressive hypertrophy, and the densely packed podocytes need to cover an increasing filtration area. (Center panel) At this stage, genetically labeled (mouse) and synaptopodin-expressing (mouse and human) cells migrate from the Bowman’s capsule onto the tuft through the vascular stalk. (Right) Fully developed glomeruli show fully differentiated podocytes on the glomerular tuft and PECs on the Bowman’s capsule.
Concise Methods
Transgenic Mice
Triple transgenic PEC-rtTA/LC1/R26R mice12 were used for UNx, 5/6Nx, and aging experiments. These mice express β-gal after administration of doxycycline under a rabbit podocalyxin promoter specifically in the PECs. To stain these cells, a dye labeled substrate (X-gal) was given on frozen sections, which is metabolized by recombinant β-gal so that the unlabeled dye stained transgenic cells irreversibly blue.
For additional experiments, PEC-rtTA/LC1/R26R/H2B-eGFP mice (short PEC-rtTA/H2B-eGFP) were used. These mice carry the same transgenes as the PEC-rtTA/LC1/R26R mice but have an additional transgene expressing eGFP fused to histone H2B under a doxycycline-dependent transactivator-activated promoter.46
For the experiments with juvenile mice, PEC-rtTA/LC1/R26R/H2B-eGFP and the podocytes-labeling Pod-rtTA/LC1/R26R/H2B-eGFP mice (short Pod-rtTA/H2B-eGFP) were used.22 Although the Cre recombinase- and β-gal–encoding transgenes are not required for eGFP labeling of the PECs, these mice were also named PEC-rtTA/H2B-eGFP and Pod-rtTA/H2B-eGFP. Mice were housed in the facility of the Aachen University Hospital under specific pathogen-free conditions. Animals received regular feeding and water ad libidum. Animal studies were approved by the local state government authorities Landesamt für Natur, Umwelt und Verbraucherschutz Cologne: #50.203.2 AC 7; 10508A4; 9.93.2.10.35.07.041; 8.87–50.10.35.08.254; 8.87–51.04.20.09.314; 8.87–51.04.20.09.312; 8.87–51.05.20.09.251; 8.87–51.04.20.09.345; 84–02.04.2012.A289.
UNx and 5/6Nx
The PEC-rtTA/LC1/R26R mice and PEC-rtTA/LC1/R26R/H2B-eGFP mice received doxycycline hydrochloride by drinking water for a total of 14 d (5% sucrose and 1 mg/ml doxycycline protected from light), which was exchanged every 2 d. After a washout phase of 7 d, mice were divided into the different groups, and UNx, 5/6Nx, or sham operation was performed. Mice were anesthetized with ketamine-xylazine (100 mg/ml ketanest and 20 mg/ml xylazine in normal saline [0.9%]; 0.1 ml/10 g body wt), and after shaving the abdominal area, a laparotomy was made. The hilus of the left kidney was ligated, the capsule was removed, and the kidney was excised near the hilus. In addition, for 5/6Nx, the right kidney was occluded with a vascular clamp, the capsule was removed at the upper and lower poles of the kidney, and both poles were cut off as well. Bleeding was stopped using gelatin sponges (Gelastypt; Sanofi-Aventis GmbH, Frankfurt, Germany). The cut was sutured, and the mice were monitored 3 months after surgery until they were killed to harvest the residual kidneys. Therefore, mice were anesthetized as described above. The kidneys were recovered and apportioned; one part was snap-frozen in Tissue-Tek (Miles, Inc., Iowa City, IA), whereas the other part was immersion-fixed and embedded in paraffin.
5/6Nx and DOCA Salt
PEC-rtTA/LC1/R26R mice underwent 5/6Nx as described above after 14 days of doxycycline treatment and a 7-day washout phase. Furthermore, 7 days after surgery, a 25-mg pellet of DOCA (Innovative Research, FL) was implanted subcutaneously and released over the following 3 weeks; 1% NaCl was added to the drinking water. After 3 weeks of DOCA release, the mice received 0.5% NaCl by drinking water for an additional 2 weeks and were monitored the whole time. Then, the mice were perfused with 3% paraformaldehyde, and their kidneys were harvested as previously described.
Induction of Genetic Tagging in Juvenile Mice
For proliferation and migration studies in developing mice, the previous experimental protocols were repeated with juvenile PEC-rtTA/LC1/R26R/H2B-eGFP and Pod-rtTA/LC1/R26R/H2B-eGFP mice.12 To induce the specific labeling, 3-day-old animals were injected intraperitoneally with 50 µg/g body wt doxycycline dissolved in 0.45% saline over a total of 2 days. For early time points, some control mice were killed on day 8 by decapitation, and the kidneys were harvested for cryo- and paraffin embedding (after immersion fixation in 4% formalin). The other group was killed after 6 weeks as described above.
Human renal tissues were obtained from the database of the Institute of Pathology in Aachen, Germany. Use of these tissues was approved by the local ethics committee.
For enzymatic X-gal staining, 5-µm cryosections were incubated for 5 minutes in 4°C cold postfixation solution (2% glutaraldehyde, 0.01% sodium deoxycholate, 0.02% IGEPAL-CA630 [Sigma-Aldrich], and 1 mM MgCl2 in PBS [pH 7.8]) and then washed for 20 minutes in PBS. The sections were incubated overnight at 37°C in a humidified atmosphere in staining solution (1 mg/ml X-gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS [pH 7.8]). The next day, sections were counterstained with eosin, washed in tap water, and mounted (Immu-Mount; Thermo Scientific, Waltham, MA.).
Immunohistochemistry and immunofluorescence stainings were performed with both 5-µm cryosections and 3-µm paraffin sections (Table 1).
Table 1.
Antibodies used in this study
| Dilution | ID Number | Company | |
| Primary antibodies | |||
| Rabbit anti-eGFP | 1:100 | 632377 | Clontech |
| Mouse anti-eGFP | 1:100 | 632381 | Clontech |
| Rabbit anti-claudin 1 | 1:100 | RB-9209- P1 | Thermo Scientific |
| Goat anti-synaptopodin | 1:200 | sc 21537 | Santa Cruz Biotechnology |
| Rat anti-CD44 | 1:100 | 553131 | BD Pharmingen |
| Rabbit anti–WT-1 | 1:50 | sc192 | Santa Cruz Biotechnology |
| Rabbit anti-nestin | 1:200 | AB5922 | Millipore |
| Mouse anti-GLEPP1 | 1:100 | AM336–5M | BioGenex |
| Secondary antibodies | |||
| Donkey anti-mouse DyLight 488 | 1:200 | 715–485–151 | Dianova |
| Donkey anti-mouse AlexaFluor 546 | 1:200 | A10036 | Invitrogen |
| Donkey anti-rabbit DyLight 488 | 1:200 | 711–485–152 | Dianova |
| Donkey anti-rabbit DyLight 549 | 1:200 | 711–505–152 | Dianova |
| Donkey anti-goat DyLight 549 | 1:200 | 705–505–147 | Dianova |
| Donkey anti-goat DyLight 488 | 1:200 | 705–485–147 | Dianova |
| Rabbit biotinylated anti-rat IgG | 1:300 | BA 4001 | Vector Laboratories |
Immunohistochemical stainings were counterstained with hemalaun.
Light and Fluorescence Microscopy
Fluorescent stainings were examined using a Leica DMRX microscope together with a JVC KY-F1030 camera. Images were analyzed using DISKUS software (Carl H. Hilgers, Königswinter, Germany) for measuring glomerular tuft areas as well as counting podocytes and eGFP-positive cells and scoring β-gal–stained cryosections.
For transmission electron microscopy, small fragments of cortex were fixed in 2.5% glutaraldehyde dissolved in 0.1 M sodium cacodylate buffer (pH 7.4) overnight at 4°C and washed in the same buffer. The tissue fragments were postfixed in palade-buffered 2% OsO4 for 1 hour, dehydrated, and embedded in Epon812 using Luft’s procedure (Merck, Darmstadt, Germany). Ultrathin serial sections were contrasted with 4% uranyl acetate for 45 minutes and subsequently, lead citrate for 5 minutes at room temperature. Sections were examined in a Jeol 1200 EX2 Electron Microscope (JEOL, Tokyo, Japan).
Statistical Analyses of Glomerular Tuft Areas and Podocytes
To reveal hypertrophic processes, the tuft areas of 80 glomeruli of periodic acid–Schiff-stained sections of each control were removed, and residual kidney was measured using the DISKUS software. Estimation of mean glomerular volume (VG) was performed on digital images of cortical tissue stained with periodic acid–Schiff. VG was calculated using the formula VG=(β/k)(Am)3/2, where Am is the mean glomerular cross-sectional area, k=1.01 is a size distribution coefficient, and β=1.38 is the shape coefficient for glomeruli that are assumed to be spherical.47 Additionally, the number of WT-1–positive nuclei of visceral podocytes was counted in each control, and residual kidney was removed. To assess podocyte loss, the number of podocytes per millimeter2 was calculated and normalized to the hypertrophied tuft area (i.e., the number of podocytes per millimeter2 was divided by the factor by which the area of glomerular cross-sections was increased).
Evaluation of β-Gal–Positive Cells within the Glomerulus
To evaluate regeneration potential of PECs after injury, β-gal–stained frozen sections of each control were removed, and residual kidney was examined in a blinded manner; 100 glomeruli of each kidney were evaluated by scoring the X-gal–stained PECs within the tuft.
Evaluation of eGFP-Positive Cells within the Glomerulus
To evaluate regenerative potential of PECs in PEC-rtTA/LC1/R26R/H2B-eGFP mice, paraffin sections of control and 5/6Nx residual kidneys were immunohistochemically stained for eGFP. Furthermore, eGFP-positive cells on the Bowman’s capsule and the glomerular tuft of 80 glomeruli per sample were counted.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the TP17 and TP25 Sonderforschungsbereich/Transregio 57 of the Deutsche Forschungsgemeinschaft (P.B., J.F., and M.J.M.) and NephCure Foundation Grant F001 (to B.S. and M.J.M.).
P.B., J.F., and M.J.M. are members of the SFB/Transregio 57 DFG Consortium Mechanisms of Organ Fibrosis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050481/-/DCSupplemental.
References
- 1.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Nagata M, Yamaguchi Y, Komatsu Y, Ito K: Mitosis and the presence of binucleate cells among glomerular podocytes in diseased human kidneys. Nephron 70: 68–71, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Pabst R, Sterzel RB: Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int 24: 626–631, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G: Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross O, Borza DB, Anders HJ, Licht C, Weber M, Segerer S, Torra R, Gubler MC, Heidet L, Harvey S, Cosgrove D, Lees G, Kashtan C, Gregory M, Savige J, Ding J, Thorner P, Abrahamson DR, Antignac C, Tryggvason K, Hudson B, Miner JH: Stem cell therapy for Alport syndrome: The hope beyond the hype. Nephrol Dial Transplant 24: 731–734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer-Schwesinger C, Lange C, Bröcker V, Agustian PA, Lehmann U, Raabe A, Brinkmeyer M, Kobayashi E, Schiffer M, Büsche G, Kreipe HH, Thaiss F, Becker JU: Bone marrow-derived progenitor cells do not contribute to podocyte turnover in the puromycin aminoglycoside and renal ablation models in rats. Am J Pathol 178: 494–499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A: Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ: Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichaiwong W, Hudkins KL, Wietecha T, Nguyen TQ, Tachaudomdach C, Li W, Askari B, Kobayashi T, O’Brien KD, Pippin JW, Shankland SJ, Alpers CE: Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol 24: 1088–1102, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smeets B, Moeller MJ: Parietal epithelial cells and podocytes in glomerular diseases. Semin Nephrol 32: 357–367, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Smeets B, Te Loeke NA, Dijkman HB, Steenbergen ML, Lensen JF, Begieneman MP, van Kuppevelt TH, Wetzels JF, Steenbergen EJ: The parietal epithelial cell: A key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol 15: 928–939, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabgani N, Grigoleit T, Schulte K, Sechi A, Sauer-Lehnen S, Tag C, Boor P, Kuppe C, Warsow G, Schordan S, Mostertz J, Chilukoti RK, Homuth G, Endlich N, Tacke F, Weiskirchen R, Fuellen G, Endlich K, Floege J, Smeets B, Moeller MJ: Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS One 7: e34907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB: Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998–2003, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Goyal VK: Changes with age in the human kidney. Exp Gerontol 17: 321–331, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Kaplan C, Pasternack B, Shah H, Gallo G: Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol 80: 227–234, 1975 [PMC free article] [PubMed] [Google Scholar]
- 25.Razzaque MS, Shimokawa I, Koji T, Higami Y, Taguchi T: Life-long caloric restriction suppresses age-associated Fas expression in the Fischer 344 rat kidney. Mol Cell Biol Res Commun 1: 82–85, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Yumura W, Sugino N, Nagasawa R, Kubo S, Hirokawa K, Maruyama N: Age-associated changes in renal glomeruli of mice. Exp Gerontol 24: 237–249, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Camici M, Carpi A, Cini G, Galetta F, Abraham N: Podocyte dysfunction in aging—related glomerulosclerosis. Front Biosci (Schol Ed) 3: 995–1006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teiken JM, Audettey JL, Laturnus DI, Zheng S, Epstein PN, Carlson EC: Podocyte loss in aging OVE26 diabetic mice. Anat Rec (Hoboken) 291: 114–121, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Wiggins J: Podocytes and glomerular function with aging. Semin Nephrol 29: 587–593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu ZH, Abrass CK, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB: Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol, in press [DOI] [PMC free article] [PubMed]
- 33.Romagnani P, Remuzzi G: Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol Metab 24: 13–20, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P: Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grond J, Weening JJ, van Goor H, Elema JD: Application of puromycin aminonucleoside and adriamycin to induce chronic renal failure in the rat. Contrib Nephrol 60: 83–93, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wang YP, Tay YC, Harris DC: Progressive adriamycin nephropathy in mice: Sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Guo JK, Marlier A, Shi H, Shan A, Ardito TA, Du ZP, Kashgarian M, Krause DS, Biemesderfer D, Cantley LG: Increased tubular proliferation as an adaptive response to glomerular albuminuria. J Am Soc Nephrol 23: 429–437, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ: Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S: Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65: 1870–1876, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Mallamaci F, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Remuzzi G, Zoccali C, REIN Study Group : ACE inhibition is renoprotective among obese patients with proteinuria. J Am Soc Nephrol 22: 1122–1128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grouls S, Iglesias DM, Wentzensen N, Moeller MJ, Bouchard M, Kemler R, Goodyer P, Niggli F, Gröne HJ, Kriz W, Koesters R: Lineage specification of parietal epithelial cells requires β-catenin/Wnt signaling. J Am Soc Nephrol 23: 63–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E: Defining the epithelial stem cell niche in skin. Science 303: 359–363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weibel ER: Stereological methods. In: Practical Methods for Biological Morphometry, London, Academic Press, 1979, pp 40–116 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.