Abstract
Neointima formation causes the failure of 60% of arteriovenous fistulas (AVFs) within 2 years. Neointima-forming mechanisms are controversial but possibly linked to excess proinflammatory responses and dysregulated Notch signaling. To identify how AVFs fail, we anastomosed the carotid artery to the internal jugular vein in normal and uremic mice and compared these findings with those in failed AVFs from patients with ESRD. Endothelial cells (ECs) of AVFs in uremic mice or patients expressed mesenchymal markers (FSP-1 and/or α-SMA) and exhibited increased expression and nuclear localization of Notch intracellular domain compared with ECs of AVFs in pair-fed control mice. Furthermore, expression of VE-Cadherin decreased, whereas expression of Notch1 and -4, Notch ligands, the downstream transcription factor of Notch, RBP-Jκ, and Notch target genes increased in ECs of AVFs in uremic mice. In cultured ECs, ectopic expression of Notch ligand or treatment with TGF-β1 triggered the expression of mesenchymal markers and induced endothelial cell barrier dysfunction, both of which were blocked by Notch inhibition or RBP-Jκ knockout. Furthermore, Notch-induced defects in barrier function, invasion of inflammatory cells, and neointima formation were suppressed in mice with heterozygous knockdown of endothelial-specific RBP-Jκ. These results suggest that increased TGF-β1, a complication of uremia, activates Notch in endothelial cells of AVFs, leading to accelerated neointima formation and AVF failure. Suppression of Notch activation could be a strategy for improving AFV function in uremia.
The preferred vascular access for patients with ESRD is an arteriovenous fistula (AVF). Unfortunately, their patency is estimated to be only 60% after 1 year.1–3 AVF failure develops mainly from vascular stenosis created by neointima formation and/or thrombosis.4,5 Hyperplasia of the neointima is created by inflammatory and proliferative responses, but the role of endothelial damage in the process of neointima development is unknown.
One candidate for triggering neointima formation is the dysfunction of endothelium. In the cardiovascular system, the endothelium is not only a barrier between the circulating blood and vascular smooth muscle cell, but, also, it releases mediators that regulate vascular tone, vessel growth, platelet function, and coagulation.6 Moreover, endothelial damage/dysfunction interferes with these functions. Endothelial cells (ECs) could influence the fate of an AVF. For example, in patients with CKD, biomarkers of endothelial dysfunction (vWf and vascular cell adhesion molecule-1) are expressed in ECs, and the capacity for endothelial repair is decreased.7 However, the relationships among CKD-induced changes in ECs and neointima formation in AVFs are poorly defined.
A candidate mediator of endothelial cell dysfunction is Notch signaling. It is initiated by binding to its ligands with triggering proteolytic cleavage of the transmembrane receptor by γ-secretase and release of the Notch intracellular domain (NICD). After translocation of NICD into the nucleus, it associates with the DNA binding protein recombinant binding protein-Jκ (RBP-Jκ) plus coactivators and initiates expression of target genes, such as α-smooth muscle actin (α-SMA) or the Hes family proteins.8 Not surprisingly, the Notch pathway is involved in multiple aspects of vascular development, including regulation of endothelial function9 and control of endothelial mysenchymal transition in the heart valve.10 Increased Notch signaling has also been associated with neointima formation11 and arteriovenous malformations.12
Our goal was to identify the role of Notch signaling during the development of neointima formation in AVFs. We show that neointima formation in AVFs of mice with CKD is associated with the development of an abnormal phenotype of ECs: there are expressions of some mesenchymal markers in ECs. In response to CKD, the expressions of α-SMA and fibroblast-specific protein 1 (FSP-1) in ECs from AVFs are increased, but there is reduced endothelial barrier function. These changes in ECs were found to be activated by the Notch pathway in AVFs placed in mice with CKD. Thus, we identified a pathway that begins with activation of Notch in endothelial cells and leads to impaired barrier function of the endothelium and infiltration of inflammatory cells, ultimately resulting in the formation of a neointima. Stimulation of the Notch/RBP-Jκ signaling was increased by CKD. The pathway has the potential for identifying Notch-induced changes in the function of ECs that could be a target for preventing neointima formation in AVFs, the Achilles heel of the hemodialysis patients.
Results
In ESRD Patients, Mesenchymal Markers Are Expressed in the Endothelium of Failed AVFs
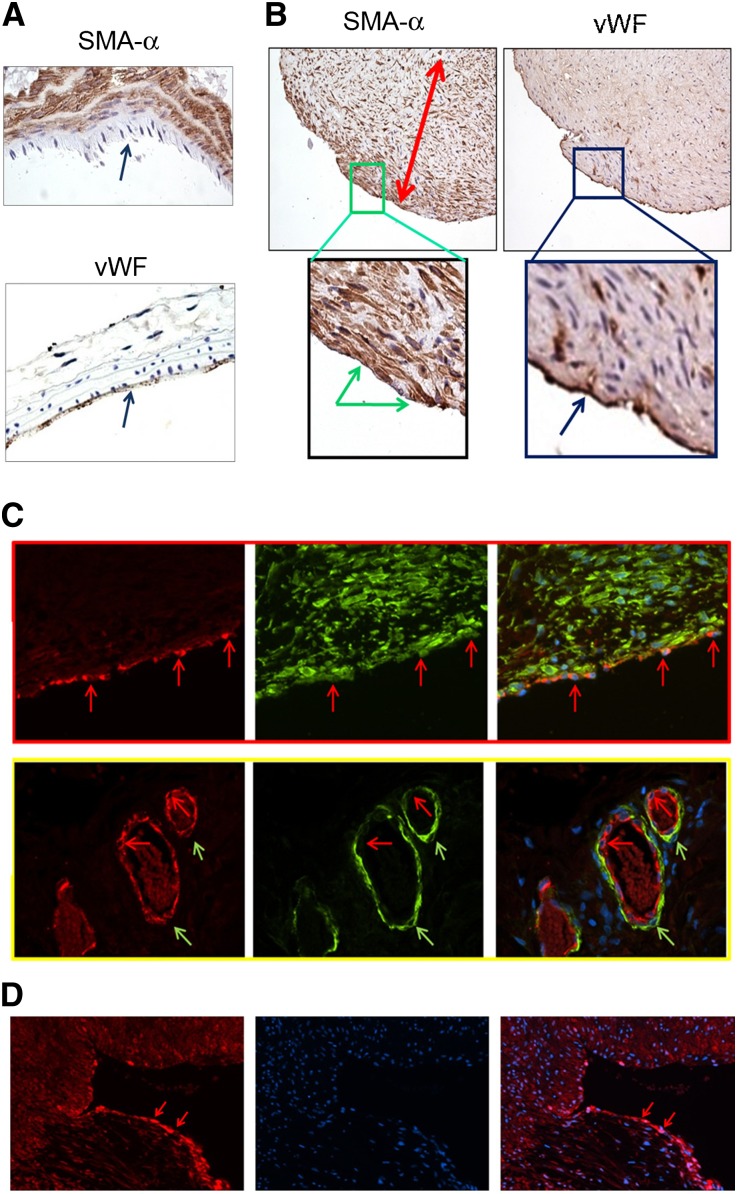
In normal arteries, α-SMA–positive cells were presented in the media, whereas positive staining of vWf was found only in the endothelium (Figure 1A). In AVFs from ESRD patients, vWf was detected on the surface of the neointima (Figure 1B). However, α-SMA was also expressed in the endothelial layer of AVFs that were characterized as having severe neointima formation (Figure 1B). Double staining of vWf and α-SMA in AVFs from ESRD patients confirmed that endothelial cells expressed vWf plus the mesenchymal marker α-SMA (Figure 1C, upper panel). FSP-1, another mesenchymal marker, was found in the endothelium of failed AVFs from ESRD patients (Figure 1D). There was no colocalization of α-SMA and vWf in microvessels of the adventitium (Figure 1C, lower panel). These results show that endothelial cells of the neointima of AVFs from ESRD patients express the mesenchymal cell marker α-SMA.
Figure 1.
Mesenchymal markers are expressed in endothelium of AVFs from ESRD patients. (A) Immunohistologic staining of α-SMA or vWf in mouse artery. (B) From a patient with ESRD, sections of AVFs reveal severe neointima hyperplasia. Blue arrows in A refer to the endothelium. Double-headed arrows indicate the thickness of the neointima, whereas green arrow in B indicates positive staining of α-SMA in the endothelium. Blue arrow in B refers to vWf-positive cells in the endothelium. (C) Double staining of α-SMA (green) and vWf (red) in AVFs from ESRD patients. The upper panel shows the endothelial layer of the AVF, whereas the adventitial microvessels are shown in the lower panel. Red and green arrows in these panels indicate ECs and smooth muscle cells, respectively. Original magnification, ×400. (D) FSP-1 immunostaining of ECs in failed AVFs of ESRD patients; arrows show FSP-1 expression. Representative AVFs are presented.
CKD Enhances Endothelial Expression of Mesenchymal Markers and Neointima Formation in Mouse AVFs
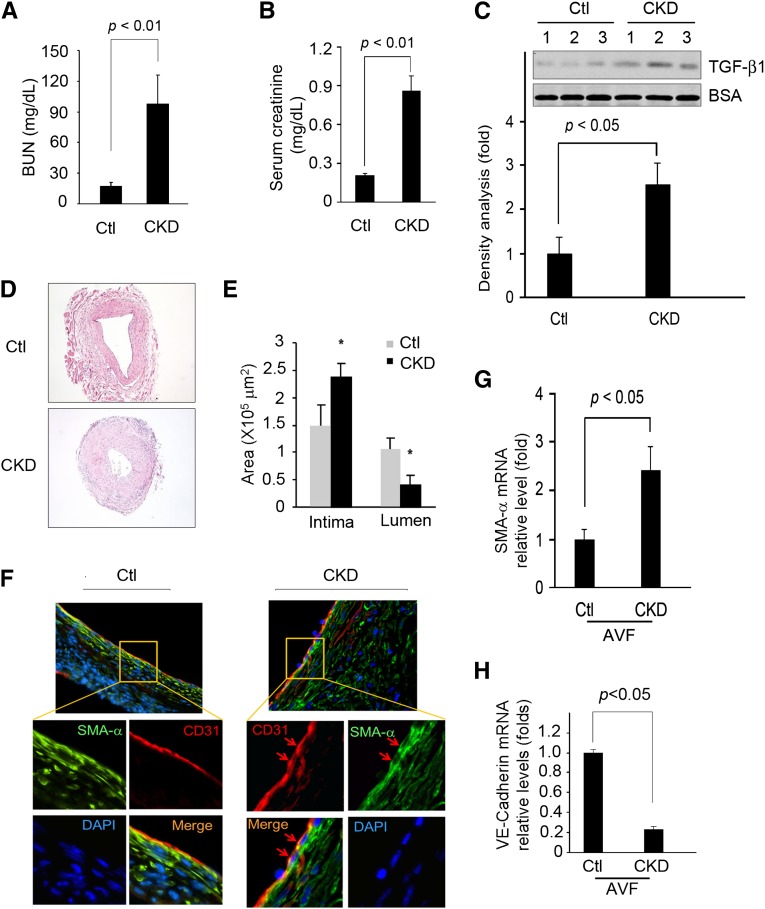
The levels of BUN, creatinine, and TGF-β1 were all elevated in this mouse model of CKD (Figure 2, A–C). In AVFs created in mice with CKD, there was substantially more neointima formation versus results in AVFs of nonuremic, pair-fed mice (Figure 2D). Other than the increase in neointima in AVFs placed in CKD mice, α-SMA was expressed in the endothelial layer (Figure 2F). Double staining showed colocalization of the endothelial marker (CD31) and α-SMA (Figure 2F). These observations are similar to the findings in AVFs from ESRD patients; notably, the endothelium of AVFs from control mice did not stain positively for α-SMA (Figure 2F). Our results show that advanced CKD leads to the expression of mesenchymal markers in ECs of AVFs from uremic mice.
Figure 2.
Mesenchymal markers are expressed in endothelium of AVFs from CKD mice. (A) BUN, (B) serum creatinine, and (C) TGF-β1 levels were measured (P<0.05) from five mice with CKD. Ctl, control. (D) Hematoxylin & eosin staining of sections from AVFs of control or CKD mice. Neointima formation was increased in AVFs from CKD mice versus control pair-fed mice. Representative pictures of AVFs from each group of five mice are presented. (E) The areas of neointima and lumen of AVFs from control and CKD mice (*P<0.05, compared with control). (F) Coimmunofluorescence staining of α-SMA or CD31 in AVF sections from control or CKD mice. Red arrows refer to double staining in the endothelium. (G and H) VE-Cadherin and α-SMA mRNA expression in AVF of mice with CKD are compared with levels in AVF of control mice. DAPI, 4′,6-diamidino-2-phenylindole.
In AVFs from CKD mice, vascular endothelial (VE)-Cadherin mRNA was reduced compared with results in the AVFs from control mice (Figure 2G). α-SMA mRNA was significantly increased in AVFs from CKD versus control mice (Figure 2H), and >95% of the VE-Cadherin–positive cells were located in the endothelium. These cells also stained the endothelial marker CD31 positively (Supplemental Figure 1). We conclude that virtually all VE-Cadherin–positive cells are endothelial cells, and that VE-Cadherin is downregulated in mice with CKD.
CKD Activates Notch Signaling in AVFs from ESRD Patients and Uremic Mice
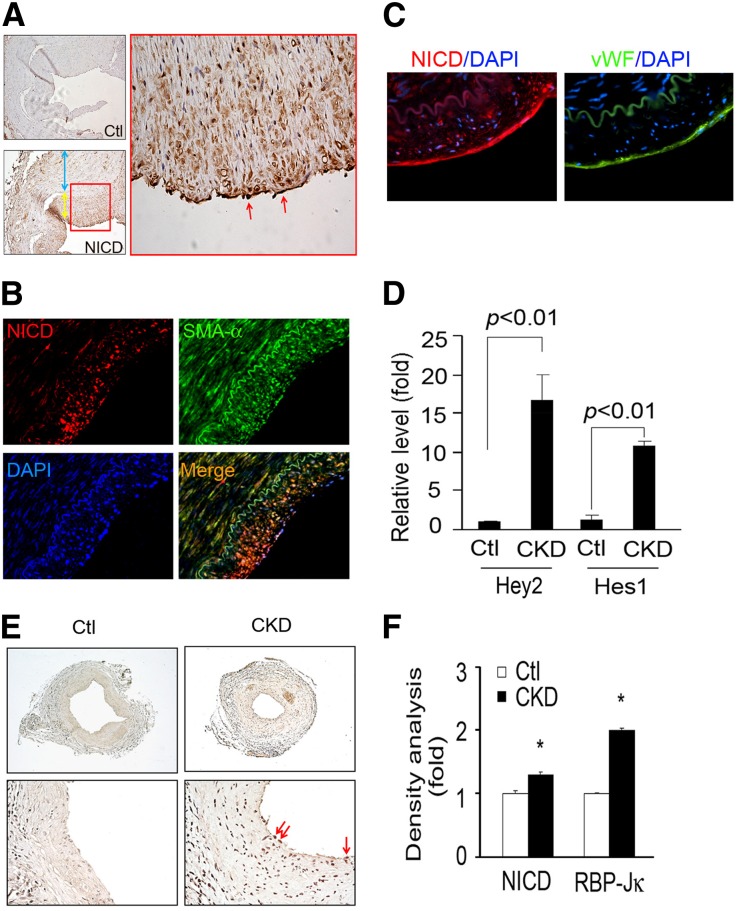
Because Notch signaling regulates cell fate determination and differentiation13 and is activated in CKD and other kidney diseases,14 we found that the expression of intracellular domain of Notch1 (N1ICD) in ECs in AVFs from ESRD patients (Figure 3A). By double staining of N1ICD with α-SMA or vWf, we found that N1ICD was expressed in both neointima and ECs of failed AVFs of ESRD patients (Figure 3, B and C). In fact, N1ICD colocalized with the endothelial marker vWf (Figure 3C). Similar findings were present in AVFs from CKD mice (data not shown).
Figure 3.
The expressions of Notch signaling mediators are increased in AVFs from ESRD patients and CKD mice. (A) Notch activation (N1ICD) in human AVFs was detected in ECs (red arrows) and neointima cells (yellow arrow) but not smooth muscle cells of the media (blue arrow). (B) Coimmunofluorescence staining of N1ICD and α-SMA in human AVFs. DAPI, 4′,6-diamidino-2-phenylindole. (C) Serial sections from human AVFs were stained with N1ICD (red) and vWf (green). DAPI staining is shown in blue. (D) CKD induces Notch target genes, Hey2 and Hes1, in AVFs of CKD mice. RNAs from AVFs of CKD or control mice were analyzed (n=5 mice per group). (E) In CKD, RBP-Jκ expression was increased in ECs of AVF versus control mice. (F) Density analysis of the immunostaining for N1ICD and RBP-Jκ (*P<0.05, compared with control).
Other than an increased expression of N1ICD in the nuclei of ECs, there was a dramatic upregulation in the mRNAs of the Notch target genes, Hey2 and Hes1, compared with results in AVFs from sham-control mice (Figure 3D). We also found that Notch1 and -4 and their ligands (Jagged1 and DLL4) were increased in AVFs from CKD mice versus results from control mice (Supplemental Figure 2). Likewise, immunostaining revealed enhanced N1ICD and RBP-Jκ expression in the endothelium of AVFs from CKD mice (Figure 3, E and F).
Notch Activation Induces the Expression of Mesenchymal Markers in ECs
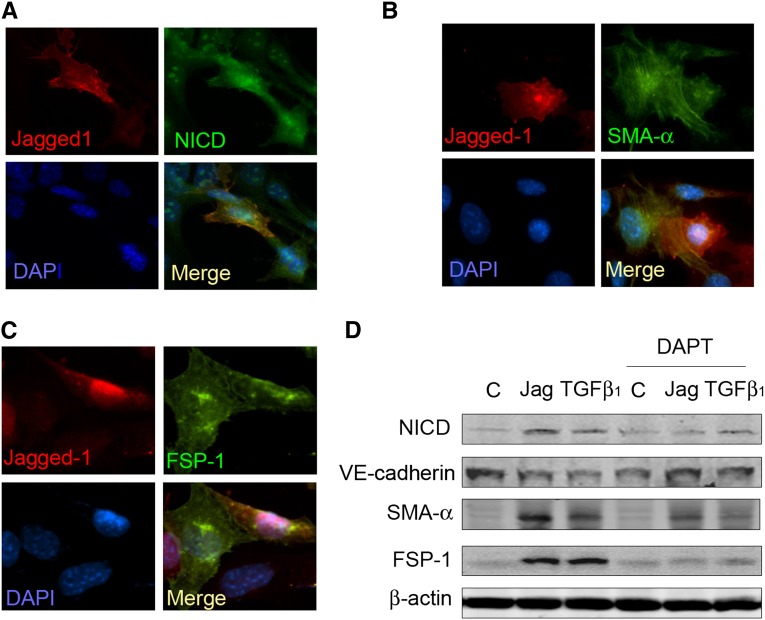
To examine whether ligand-induced Notch activation affects mesenchymal marker expression in ECs, we cocultured ECs that had been infected with adenovirus to express Notch ligand (AdJagged1) or a control vector in a 1:1 ratio. The response to increased expression of the Notch ligand, Jagged1, included nuclear translocation of N1ICD in both ECs expressing Jagged1 ECs and ECs in contact with Jagged1-expressing cells (Figure 4A). In addition, overexpression of Jagged1 induced α-SMA and FSP-1 expressions in both groups of cells (Figure 4, B and C).
Figure 4.
Activation of Notch pathway in ECs induces expression of mesenchymal markers. (A–C) Lung ECs were infected with AdJagged1 or control vector (AdVector) and cocultured for 24 hours before immunostaining; Jagged1-expressing cells (red) were also positive for (A) N1ICD, (B) α-SMA, or (C) FSP-1 (nuclei were labeled with DAPI). (D) Western blot analysis shows that Notch activation was associated with increased expression of α-SMA and FSP-1. ECs were infected with AdJagged1 or treated with TGF-β1 for 48 hours with or without pretreatment of DAPT (10 µM). The loading control was β-actin. Representative data from three repeated experiments are shown. C, control; DAPI, 4′,6-diamidino-2-phenylindole; Jag, Jagged.
To determine the relationship between Notch activation and expression of α-SMA and FSP-1, we expressed Jagged1 in ECs or treated them with TGF-β1 in the presence or absence of a γ-secretase inhibitor, DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester). In TGF-β1–treated cells, there was an increase in the level of the N1ICD protein. This response was abolished by DAPT, suggesting that TGF-β1 stimulates Notch signaling (Figure 4D). Likewise, in ECs infected with Jagged1 or after treatment with TGF-β1, VE-Cadherin expression was decreased, and α-SMA or FSP-1 expressions were increased. Both types of response were blocked by DAPT (Figure 4D). These results are consistent with the premise that activation of Notch signaling in ECs results in expression of mesenchymal markers.
Notch Signaling Regulates Endothelial Barrier Dysfunction
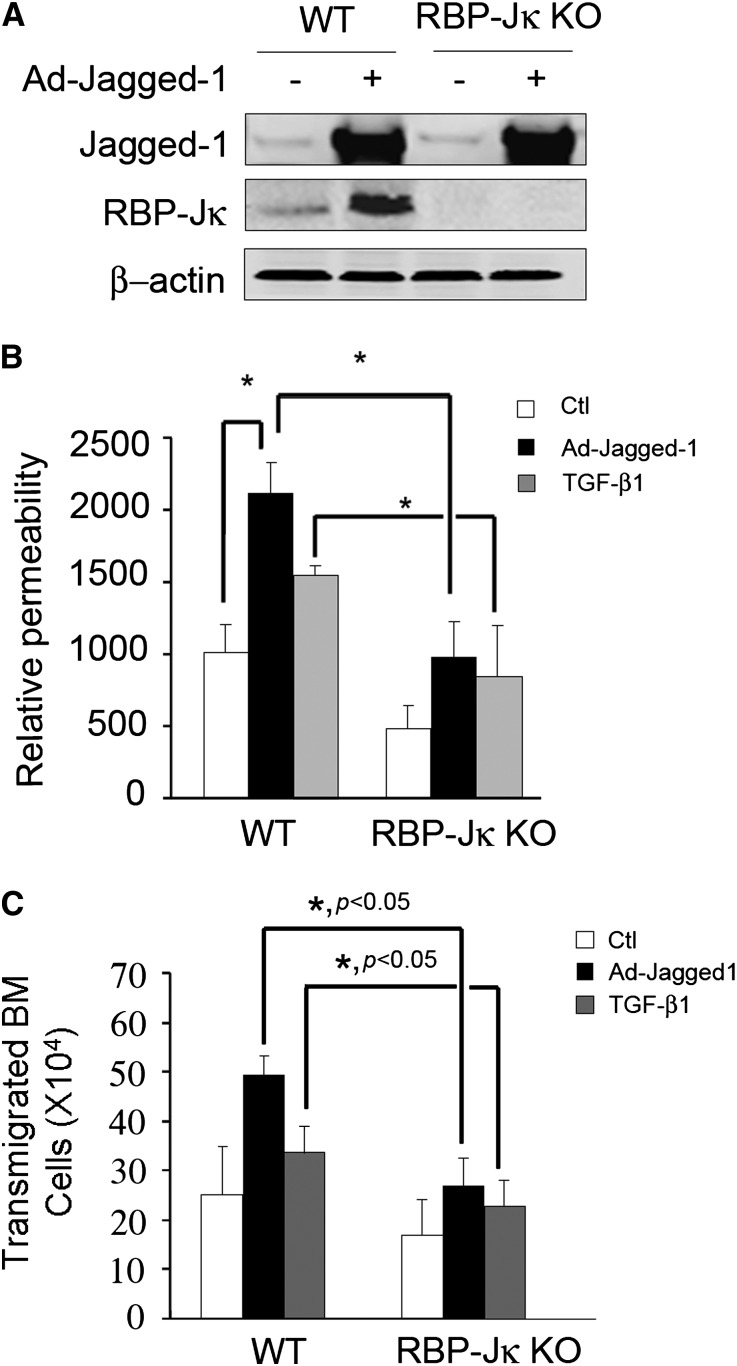
To examine how Notch signaling affects EC function, we assessed the penetration of dextran polymers through monolayers of ECs obtained from wild-type (WT) or RBP-Jκ knockout (KO) mice. After overexpression of Jagged1 or treatment with TGF-β1, EC monolayers exhibited an increase in dextran flux. This response was significantly suppressed in ECs obtained from RBP-Jκ KO mice (Figure 5, A and B). Similarly, there was increased migration of bone marrow cells through the EC monolayers in response to Jagged1 overexpression or TGF-β1 treatment. RBP-Jκ KO significantly blocked the increase in transendothelial leakage (Figure 5C).
Figure 5.
Notch signaling regulates EC barrier function. (A) Western blotting shows the absence of RBP-Jκ protein in ECs from the RBP-Jκ KO mice. (B) RBP-Jκ KO blocks Notch activation-induced leakage of dextran-FITC. ECs from WT and RBP-Jκ KO mice were infected with AdJagged1 or treated with 2 ng/ml TGF-β1 for 48 hours. FITC-labeled dextrans were added, and their accumulation in the lower chamber was measured after 30 minutes using a fluorescent spectrophotometer (*P<0.05, compared with control). (C) RBP-Jκ KO blocks Notch activation-induced transendothelial migration of bone marrow cells. A monolayer of WT and RBP-Jκ KO ECs was coated in the top insert of the Boyden chamber and infected with AdJagged1 or treated with TGF-β1 for 48 hours. Bone marrow (BM) cells (1×107) were added to the insert, and 24 hours later, the number of the bone marrow cells crossing into the lower chamber was counted. Representative data from three repeated experiments are shown.
In AVFs from Mice with CKD, RBP-Jκ Knockdown in the Endothelium Suppresses Infiltration of Inflammatory Cells
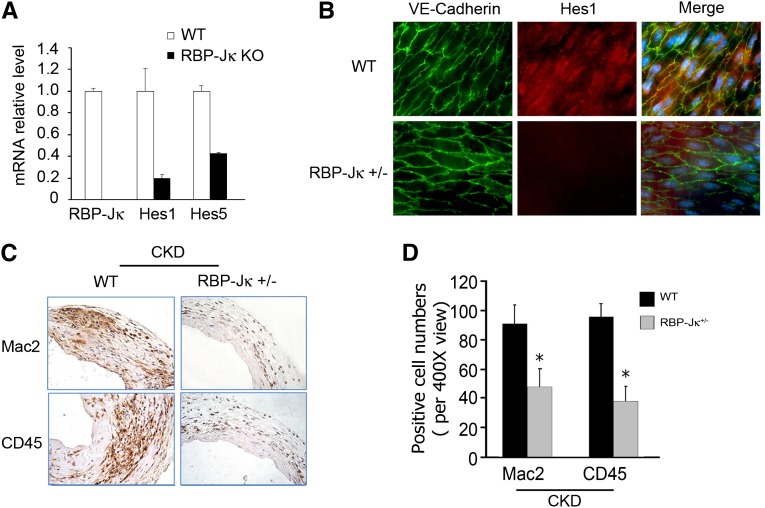
To examine how endothelial barrier function changes in AVFs, we created RBP-Jκflox/flox/VE-Cadherin-Cre+ transgenic mice that do not express the RBP-Jκ gene in ECs. Unfortunately, these mice only survive for ∼14 days, and, therefore, we isolated ECs from these mice to study how the absence of RBP-Jκ affects EC function. In ECs isolated from RBP-Jκ KO mice, mRNA levels of Hes1 and -5 were decreased versus results in ECs from WT mice (Figure 6A). Because the Hes1 protein in the endothelium of the transgenic mice, RBP-Jκflox/+/VE-Cadherin-Cre+, was decreased, we conclude that knockdown of RBP-Jκ suppresses the expression of its target genes (Figure 6B). In addition, there was infiltration of macrophages (Mac2+) and mononuclear cells (CD45+) into AVFs in WT mice with CKD. This infiltration was inhibited in AVFs placed in RBP-Jκ knockdown mice (Figure 6, C and D).
Figure 6.
RBP-Jκ knockdown suppresses inflammatory cell infiltration into AVFs of mice with CKD. (A) mRNA levels of Hes1 and -5 in ECs lacking RBP-Jκ were significantly lower than in WT mice. (B) Immunofluorescent staining of an en face section of the vein of an AVF reveals loss of the Notch target gene, Hes1 (red), in veins from RBP-Jκflox/+/VE-Cadherin-Cre+ mice compared with results with WT mice. Nuclei were stained with DAPI. (C) RBP-Jκ knockdown in mice with CKD inhibits infiltration of inflammatory cells. After 2 weeks, both Mac2- and CD45-positive cells were detected in AVFs from WT or RBP-Jκ knockdown mice with CKD (n=4 mice per group). The number of positive cells was counted and summarized in D (*P<0.05, compared with WT).
RBP-Jκ Knockdown Suppresses CKD-Induced Neointima Formation in AVFs
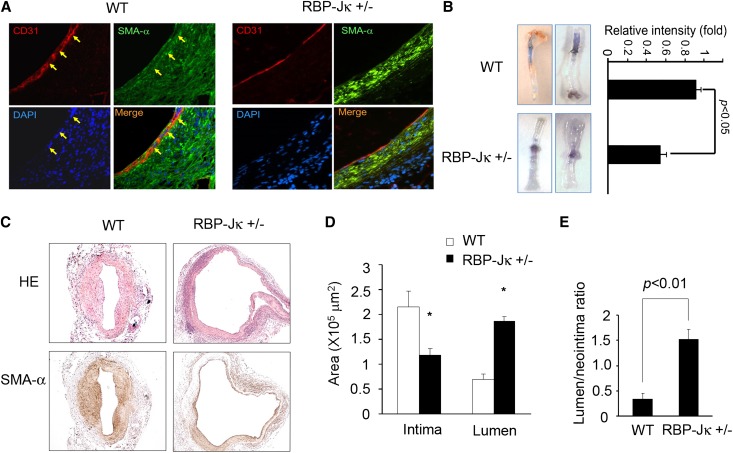
We evaluated the effect of RBP-Jκ knockdown on mesenchymal marker expression in the endothelium of AVFs created in CKD mice. Both WT and RBP-Jκflox/+/VE-Cadherin-Cre+ mice with CKD were studied. The expression of α-SMA in the endothelium of AVFs from RBP-Jκ knockdown mice was significantly decreased versus results in WT mice with CKD (Figure 7A). The barrier function of the endothelium in AVFs was impaired. There was leakage of Evans Blue dye in AVFs created in RBP-Jκflox/+/VE-Cadherin-Cre+ mice versus results in control mice (Figure 7B).
Figure 7.
RBP-Jκ knockdown inhibits CKD-induced neointima formation in AVFs. (A) Double staining of EC markers (CD31) or mesenchymal cell marker (α-SMA) was present in AVFs of WT or RBP-Jκ knockdown mice with CKD. Yellow arrows indicate coimmunostaining of CD31 and α-SMA. (B) At 2 weeks after placing the AVF, Evans Blue was administrated intravenously. The vein was perfused with PBS. The intensity of Evans Blue leak was analyzed. The data are presented as mean±SEM (n=4). (C) Hematoxylin & eosin (HE) and α-SMA staining showed that the neointima was significantly smaller (P<0.05), whereas the lumen was larger (P<0.05) in AVFs from RBP-Jκ knockdown mice with CKD compared with WT CKD mice. (D and E) In mice with CKD, the neointima area (α-SMA+) and the ratio of neointima to lumen areas in AVFs created in RBP-Jκflox/+/VE-Cadherin-Cre+ mice are compared with WT mice. The data are presented as mean±SEM (n=5) (*P<0.05, compared with control).
We compared neointima formation in AVFs from RBP-Jκflox/+/VE-Cadherin-Cre+ and WT control mice (both with CKD). RBP-Jκ knockdown in ECs suppressed the CKD-induced neointima formation: there was an increase in the lumen and the lumen/intima ratio in RBP-Jκflox/+/VE-Cadherin-Cre+ mice versus results in WT CKD mice (Figure 7, C–E). These results indicate that inhibition of Notch signaling can suppress both CKD-induced endothelial barrier dysfunction and the formation of a neointima.
Discussion
Endothelial dysfunction, a hallmark of CKD, can begin in the early stages of CKD.7 In mice with advanced CKD, we found that Notch signaling in ECs is activated by cell–cell interaction, leading to the expression of mesenchymal proteins in ECs of the AVFs. One possible trigger of Notch activation is TGF-β1 in uremic mice. In support of this possibility, we found that treating ECs with TGF-β1 stimulated expression of N1ICD. It was followed by endothelial dysfunction and interruption of the EC barrier function, resulting in infiltration of inflammatory cells into the AVFs. Notably, this sequence of events in our mouse model of CKD is similar to results in failed AVFs from patients with ESRD. Both are associated with endothelial dysfunction and neointima formation (Figures 1 and 2). Specific novel results that we uncovered include evidence that Notch signaling is a critical component of the pathogenesis of endothelial barrier dysfunction. It promotes expression of mesenchymal markers in EC, supporting neointima formation, especially when there is CKD. Our results suggest that novel therapeutic interventions targeted at Notch signaling could improve AVF patency.
We did not use transgenic mice created to label endothelial lineages, and we did not study the origin of neointima cells in AVFs. Instead, we focused on determining how a major complication of AVF (impaired barrier function) occurs. The impairment was induced by Notch activation, which also stimulated the expression of mesenchymal markers in ECs of the AVFs.
The endothelial monolayer of cells plays a crucial role in vascular homeostasis.15 After vascular injury or EC loss, vascular permeability increases, which promotes the influx of neutrophils and monocytes into the vessel.16,17 These inflammatory cells are involved in neointima cell migration and proliferation.18 Alternatively, a CKD-induced increase in reactive oxidative stress leads to dysfunction of the endothelium and impaired barrier function.19,20 How CKD induced events affecting the AVF is complicated.21,22 Our results indicate that neointima formation in AVFs of ESRD patients or mice with CKD increased expression of mesenchymal cell markers (α-SMA and FSP-1) and decreased VE-Cadherin in ECs (Figures 1 and 2). These changes cause leakage of the endothelium (Figure 5), promoting infiltration of bone marrow–derived inflammatory cells into AVFs (Figure 6C). The inflammatory cells secrete factors that stimulate smooth muscle cell proliferation, which has been reported for MCP-1,23,24 SDF-1,25 or other chemokines after balloon- or stent-induced endothelial dysfunction.26 Likewise, in a vein graft model, we found that bone marrow-derived FSP-1–positive cells can increase smooth muscle cell proliferation and neointima formation.18
We recognize that certain mesenchymal markers (e.g., α-SMA and collagen I) are regulated in non-ECs by multiple intracellular signaling pathways, including TGF-β1 and Wnt, as well as Notch.8,27,28 In cultured epithelial cells, it has been reported that TGF-β1 stimulates the expression of the Notch ligand Jagged1 and induces epithelial–mesenchymal transition.29 Reportedly, activated Notch1 or -4 can cause similar responses in cultured ECs.30 It also has been reported that expression of soluble Jagged1 interferes with Notch signaling and can reduce the neointima formation induced by balloon injury.31 The present results indicate that CKD plays a role in Notch-induced responses, because it increases the expression of the Notch receptors in AVFs and the expression of the Notch target gene, Hes1, in AVFs (Figure 3B). Our results provide the first evidence for a mechanism by which CKD activates Notch signaling in AVFs, especially in the endothelium (Figure 3).
RBP-Jκ is the major transcription factor that responds to activation of Notch signaling that leads to expression of mesenchymal markers in ECs, such as α-SMA, calponin, and N-cadherin.32–34 This finding was confirmed in cultured ECs with Jagged1 overexpression or exposure to TGF-β1, because there was expression of mesenchymal proteins and an increase in EC permeability with more transendothelial migration of bone marrow cells. The key role of Notch signaling also showed that RBP-Jκ knockdown in ECs suppresses expression of mesenchymal proteins, as well as leakage of the endothelium and the infiltration of inflammatory cells (Figures 4 and 5). These changes limited neointima formation in AVFs (Figures 6 and 7).
In mammalian cells, there are four Notch receptors (Notch1, -2, -3, and -4) and five Notch ligands (DLL1, -3, and -4 and Jagged1 and -2). All are expressed in ECs except DLL335,36; Notch1 and -4 receptors are expressed by ECs.8 This finding is relevant, because Notch1 is the primary functional Notch receptor during developmental angiogenesis12; also, dysfunction of Notch4 is associated with arteriovenous malformations.37,38 Interestingly, in the AVFs from mice with CKD, the most robust increase in mRNAs was from Jagged1 and Notch1 versus other Notch receptors and their ligands (Supplemental Figure 2). Therefore, we used them and the transcription factor RBP-Jκ to study Notch signaling. Because RBP-Jκ conducts all Notch signaling, we cannot exclude the involvement of activated Notch4 or even other Notch ligands as contributors to EC dysfunction. Studies of transgenic mice with KO of specific Notch receptors or their ligands will be needed to clarify whether other Notch receptors or ligands affect CKD-induced AVF failure (Figure 8).
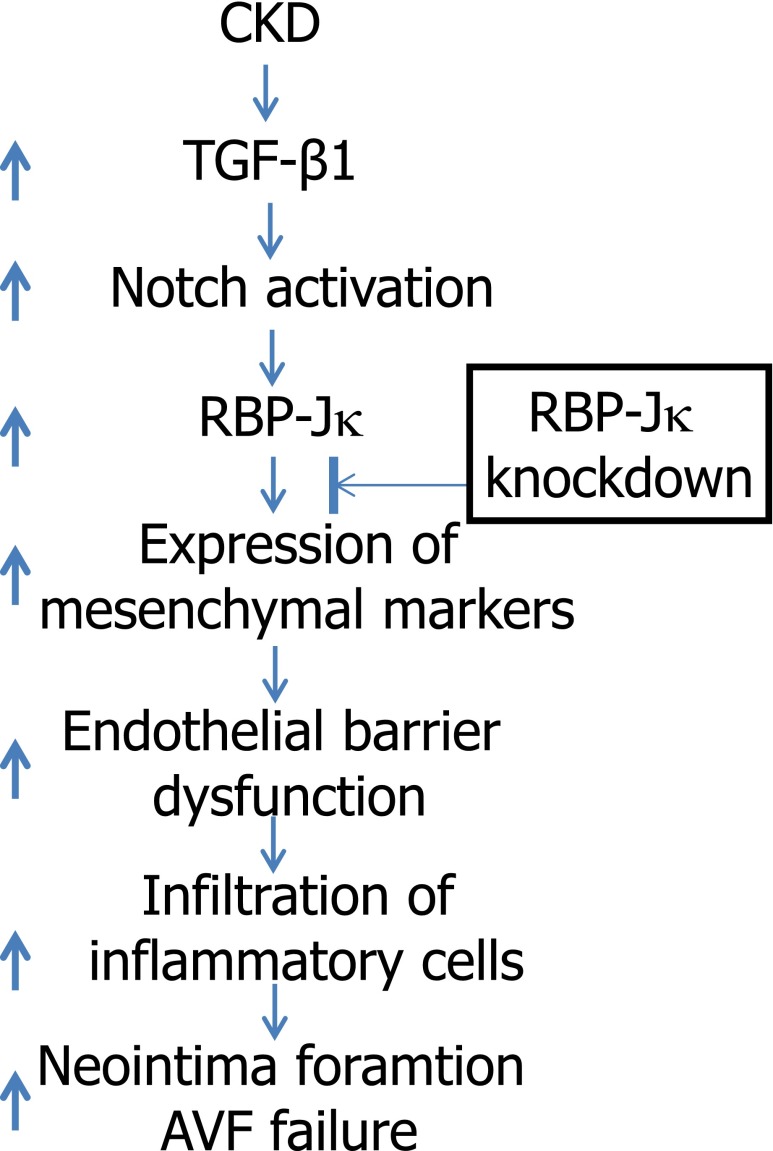
Figure 8.
Activated Notch signaling in ECs mediates CKD-induced neointima formation. Complications of CKD (such as TGF-β1) induce activation of Notch signaling that stimulates the expression of mesenchymal markers in ECs of AVFs. This leads to EC barrier dysfunction and enhances infiltration of inflammatory cells. These responses promote the formation of neointima and loss of dialysis access. Knockdown of Notch pathway in ECs suppresses CKD-induced neointima formation.
We conclude that Notch activation is a critical step in the progressive failure of AVFs in mice. The processes that we uncovered are accelerated by CKD, and, specifically, TGF-β1, a circulating cytokine associated with CKD, can stimulate Notch activation and expression of mesenchymal markers in ECs in AVFs.39 These changes contribute to dysfunction of the endothelial barrier, permitting invasion by inflammatory cells and creation of a neointima. Notably, knockdown of RBP-Jκ blocks this pathway; because Notch signaling is a key activator of a regulatory pathway of endothelial function and neointima formation, we suggest that inhibiting Notch signaling will yield strategies to patency of AVFs in CKD.
Concise Methods
Mice and CKD
All studies were approved by the Institutional Animal Care and Use Committee of Baylor and performed in accordance with National Institutes of Health guidelines. Male mice were kept in a 12-hour light/dark cycle. RBP-Jκ floxed mice were provided by K. Susztak (Albert Einstein College of Medicine, NY). VE-Cadherin-Cre transgenic mice were purchased from The Jackson Laboratory. RBP-Jκflox/flox mice were identified by genotyping using the primers 5′-TAACTATCTTGGAAGGCTAAAAT-3′; 5′-AAGAGGGACATTGCATTTTCACAT-3′. PCR products of 850 bp corresponded to the RBP-Jκ floxed gene. CKD was induced by subtotal nephrectomy in anesthetized mice as described.39,40 Briefly, mice were fed 20% protein chow, and, after matching for body weight, subtotal nephrectomy was performed in anesthetized mice in a two-step surgery method (ketamine, 125 mg/kg body wt; and xylazine, 6.4 mg/kg body wt). First, the left kidney was decapsulated to avoid ureter and adrenal damage, and approximately three quarters of the left kidney was removed. During the recovery, the mice were given two doses of buprenorphine (0.1–2.5 mg/kg body wt s.c.) after surgery and 12 hours later. The diet was changed to 6% Protein Rodent Diet Chow (Harlan Teklad, Madison, WI) ad libitum to reduce mortality and limit hypertrophy. Second, the right kidney was removed 1 week later, and after 1 week, the mice with CKD were pair-fed 40% protein chow with sham-operated control mice. The BUN was measured by the Comparative Pathology Laboratory Center at Baylor College of Medicine. The serum creatinine level was detected by using the QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA). After 2–3 weeks, AVFs were created in control and CKD mice; other control and CKD mice underwent sham surgery.
Reagents and Virus
Penicillin, streptomycin, DMEM, and FBS were obtained from Invitrogen Life Technologies (Carlsbad, CA). The γ-secretase Notch inhibitor DAPT was from Calbiochem (San Diego, CA). Human TGF-β1 was purchased from R&D Systems (Minneapolis, MN). The protein assay kit was from Bio-Rad (Hercules, CA), and dextran-FITC was from Sigma-Aldrich (St. Louis, MO). The FSP-1 antibody was obtained from DAKO (Carpinteria, MA), and the rat antibodies against VE-Cadherin and CD31 were from BD Biosciences (San Jose, CA). Antibodies against N1ICD, vWf, and α-SMA antibodies were from Abcam (Cambridge, MA), the RBP-Jκ antibody was obtained from Millipore (Billerica, MA), and Jagged1, Hes1 and -5, TGF-β1, and β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The fluorescent-700 or -800 secondary antibodies were obtained from Invitrogen (Carlsbad, CA). The full-length Jagged1 recombinant adenovirus was provided by M.J. Post (Maastricht University, The Netherlands).
Human AVF Samples and Mouse AVF Model
We studied samples of AVFs from five hemodialysis patients ranging in age from 58 to 77 years. In each case, samples of failed AVFs were collected at surgery with the approval of the Baylor Institutional Review Board. Mouse AVFs were created as described.41 Briefly, mice of 12 weeks of age were anesthetized, and the right internal jugular vein was isolated using a dissecting microscope (Leica MZ6; Leica, Germany). Its distal end was clamped and ligated, the common carotid artery was ligated below its bifurcation, and the proximal end was clamped. An end-to-end anastomosis was created using 11–0 nylon suture with an interrupted stitch. After unclamping, patency was confirmed visually. The mice were kept warm after surgery, and the analgesic (buprenorphine) was given two times 12 hours apart. At 2 and 4 weeks after surgery, the mice were anesthetized by intraperitoneal injection and euthanized by perfusing the left ventricle with PBS and 10% formalin for 10 minutes (to maintain the endothelium and morphology of the AVF). AVFs were collected, and slides from 0.5 to 1 mm from the venous anastomosis were collected for hematoxylin/eosin staining.
The neointima and media were defined as the regions between the lumen and the adventitia. The vessel wall thickness was determined by measuring the difference between the area of the lumen and the neointima using the NIS-Elements BR 3.0 Program (Nikon, Melvile, NY). Five cross-section slides were obtained by selecting the first of every 10 sections from each AVF. These slides were used to evaluate neointima formation. We recognized that our end-to-end anastomosis in the mouse AVF model is different from the end-to-side AVF performed commonly in patients with ESRD. The 2-week time point for evaluating the AVF was selected, because both control and CKD mice were recovered from surgical placement of the AVF. Therefore, we evaluated results in the AVFs from both control and CKD mice (e.g., inflammatory cell infiltration and Evans Blue leakage).
Immunohistochemistry
For histologic analysis, AVFs were perfused through the left ventricle with 10% phosphate-buffered formaldehyde and processed as described.42 Sections were blocked with 10% goat serum (Vector Laboratories, Burlingame, CA) for 30 minutes and then incubated with primary antibodies (NICD, 1:500; RBP-Jκ, 1:500; FSP-1, 1:1000; α-SMA, 1:2000). Sections were washed in 0.5% Tween 20 in PBS and incubated with a biotinylated secondary antibody (Vector Laboratories) at room temperature. After washes in 0.5% Tween 20 in PBS, tissue sections were incubated with an Elite ABC reagent (Vector Laboratories) followed by instructions as described in a peroxidase substrate kit (Vector Laboratories). The sections were counterstained by hematoxylin. For double immunofluorescent staining of samples, fluorescent secondary antibodies were applied to sections; 4′,6-diamidino-2-phenylindole was used in counterstaining. Pictures were recorded using a Nikon Eclipse 80i Fluorescence Microscope (Nikon).
En Face Analysis of AVF
We analyzed the endothelium in AVFs using an en face technique with immunostaining as described.43 AVF segments were cut longitudinally, mounted on glass slides with the endothelium facing up, and air dried for 1–2 hours. AVFs were incubated with antibodies against VE-Cadherin and Hes1 followed by immunofluorescent-labeled secondary antibodies (Rockland, Gilbertsville, PA); 4′,6-diamidino-2-phenylindole was used to stain nuclei.
Real-Time RT-PCR
Total RNAs from control vein or AVF were isolated using the RNeasy Kit (Qiagen, Valencia, CA). Real-time RT-PCR was performed using the Opticon Real-Time RT-PCR Machine (MJ Research, Waltham, MA). The specificity of real-time RT-PCR was confirmed by agarose gel electrophoresis and melting curve analysis. The primers for real-time RT-PCR are listed in Supplemental Table 1.
Mouse EC Isolation
Because only small numbers of ECs can be isolated from the AVFs, we studied pathways in cultured ECs from the lung. This study was done because not only are lung ECs plentiful but also, ECs from different tissues maintain major EC characteristics, such as barrier function. We recognize that there may be varied responses to external signals. However, we have found that activated Notch is associated with EC barrier dysfunction in vitro and in vivo. Primary cultures of mouse ECs were isolated from the lungs as described.44 The lung ECs were maintained in EGM-2 SingleQuot Kit Supplement & Growth Factors (Lonza) plus 20% FBS. ECs with RBP-Jκ KO were isolated from RBP-Jκflox/flox/VE-Cadherin-Cre mice within 7 days after birth (mice do not survive after 14 days).
Cell–Cell Interaction
ECs were infected with AdJagged1 or the vector alone for 24 hours. These ECs were trypsinized and cocultured at a 1:1 ratio. After confluence, the ECs were fixed, and immunostaining was performed.
Western Blot Analysis
ECs were lysed in radioimmunoprecipitation assay buffer, and ∼20 μg proteins were separated by SDS-PAGE. After transferring to nitrocellulose membranes, antibodies were added.45
Transendothelial Migration Assay
WT ECs or ECs with RBP-Jκ KO were seeded in 24-well Boyden Transwell inserts (2×105/well). After a confluent monolayer was formed, ECs were infected with AdJagged1 or AdFSP-1 for 24 hours. For the transendothelial migration assay, bone marrow cells (2×106) from WT mice were added to the inserts and cultured for 24 hours. The total number of bone marrow cells in the lower chamber was counted. Each experiment was repeated at least three times.
Endothelium Permeability Assay
WT or RBP-Jκ KO ECs treated as described were kept in 25 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-DMEM (pH 7.4) for 15 minutes. The HEPES-DMEM was then removed from the top of the well; 100 µl FITC-labeled dextran (12,000 molecular weight; 1 mg/ml in 25 mM HEPES-DMEM) was added to the top of each well. Aliquots (50 µl) were removed from the bottom well at the times specified for each experiment and collected in white 96-well plates. FITC-labeled dextran was measured in a luminescence spectrometer (LS 50B; PerkinElmer) using 480 and 530 nm as the excitation and emission wavelengths, respectively.
In Vivo Analysis of Endothelial Barrier Function of AVFs (Evans Blue Assay)
Before collecting AVFs, 50 µl 5% Evans Blue in saline was injected into the tail vein, and 10 minutes later, mice were perfused with PBS and then 10% neutral buffered formalin through the left ventricle. AVFs were removed and photographed; the contralateral jugular vein was used as control. Evans Blue accumulated in the extracellular matrix after disruption of the endothelium. The density of the stained AVFs was evaluated with ImageJ.
Statistical Analyses
All data are presented as mean±SEM. Results were analyzed using t test when results from two groups were compared or two-way ANOVA when data from over three groups were studied; P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Health Grant R01DK095867 (to J.C.), and R37DK37175 (to W.E.M.), American Heart Association Grants 10SDG2780009 (to J.C.), and a generous grant from Dr. and Mrs. Harold Selzman.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Notch Ties a Knot on Fistula Maturation,” on pages 648–650.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050490/-/DCSupplemental.
References
- 1.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI, Dialysis Access Consortium Study Group : Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsapenko MV, d’Uscio LV, Grande JP, Croatt AJ, Hernandez MC, Ackerman AW, Katusic ZS, Nath KA: Increased production of superoxide anion contributes to dysfunction of the arteriovenous fistula. Am J Physiol Renal Physiol 303: F1601–F1607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotmans JI, Pasterkamp G, Verhagen HJ, Pattynama PM, Blankestijn PJ, Stroes ES: Hemodialysis access graft failure: Time to revisit an unmet clinical need? J Nephrol 18: 9–20, 2005 [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Butt M, Dwivedi G, Blann A, Khair O, Lip GY: Endothelial dysfunction: Methods of assessment & implications for cardiovascular diseases. Curr Pharm Des 16: 3442–3454, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P: Vascular incompetence in dialysis patients—protein-bound uremic toxins and endothelial dysfunction. Semin Dial 24: 327–337, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Iso T, Hamamori Y, Kedes L: Notch signaling in vascular development. Arterioscler Thromb Vasc Biol 23: 543–553, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH: The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137: 1124–1135, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Chang AC, Fu Y, Garside VC, Niessen K, Chang L, Fuller M, Setiadi A, Smrz J, Kyle A, Minchinton A, Marra M, Hoodless PA, Karsan A: Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev Cell 21: 288–300, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Takeshita K, Liu P-Y, Satoh M, Oyama N, Mukai Y, Chin MT, Krebs L, Kotlikoff MI, Radtke F, Gridley T, Liao JK: Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation 119: 2686–2692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs LT, Starling C, Chervonsky AV, Gridley T: Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis 48: 146–150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK: Essential role of endothelial Notch1 in angiogenesis. Circulation 111: 1826–1832, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K: The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 14: 290–298, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM: Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91: 3527–3561, 1998 [PubMed] [Google Scholar]
- 16.Vaziri ND, Ni Z, Oveisi F, Liang K, Pandian R: Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension 39: 135–141, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Wever R, Boer P, Hijmering M, Stroes E, Verhaar M, Kastelein J, Versluis K, Lagerwerf F, van Rijn H, Koomans H, Rabelink T: Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 19: 1168–1172, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, Wang Y, Liang A, Jia L, Du J: FSP-1 silencing in bone marrow cells suppresses neointima formation in vein graft. Circ Res 110: 230–240, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Bugnicourt JM, Da Silveira C, Bengrine A, Godefroy O, Baumbach G, Sevestre H, Bode-Boeger SM, Kielstein JT, Massy ZA, Chillon JM: Chronic renal failure alters endothelial function in cerebral circulation in mice. Am J Physiol Heart Circ Physiol 301: H1143–H1152, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Zhang D, Zheng J, Feng Y, Zhang Y, Liu W: Actin cytoskeleton-dependent pathways for ADMA-induced NF-κB activation and TGF-β high expression in human renal glomerular endothelial cells. Acta Biochim Biophys Sin (Shanghai) 44: 918–923, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Kokubo T, Ishikawa N, Uchida H, Chasnoff SE, Xie X, Mathew S, Hruska KA, Choi ET: CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol 20: 1236–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer S, Kokozidou M, Heiss C, Kranz J, Kessler T, Paulus N, Krüger T, Jacobs MJ, Lente C, Koeppel TA: Chronic kidney disease aggravates arteriovenous fistula damage in rats. Kidney Int 78: 1312–1321, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Egashira K, Nakano K, Ohtani K, Funakoshi K, Zhao G, Ihara Y, Koga J-i, Kimura S, Tominaga R, Sunagawa K: Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol 27: 2563–2568, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, Nath KA: MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol 22: 43–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Möpps B, Mericskay M, Gierschik P, Biessen EA, Weber C: SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res 96: 784–791, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Jabs A, Okamoto E, Vinten-Johansen J, Bauriedel G, Wilcox JN: Sequential patterns of chemokine- and chemokine receptor-synthesis following vessel wall injury in porcine coronary arteries. Atherosclerosis 192: 75–84, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Arciniegas E, Frid MG, Douglas IS, Stenmark KR: Perspectives on endothelial-to-mesenchymal transition: Potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 293: L1–L8, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Armstrong EJ, Bischoff J: Heart valve development: Endothelial cell signaling and differentiation. Circ Res 95: 459–470, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23: 1155–1165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A: Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res 94: 910–917, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Caolo V, Schulten HM, Zhuang ZW, Murakami M, Wagenaar A, Verbruggen S, Molin DGM, Post MJ: Soluble Jagged-1 inhibits neointima formation by attenuating Notch-Herp2 signaling. Arterioscler Thromb Vasc Biol 31: 1059–1065, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, Manabe I, Arai M, Nagai R, Kurabayashi M: Jagged1-selective notch signaling induces smooth muscle differentiation via a RBP-Jkappa-dependent pathway. J Biol Chem 281: 28555–28564, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X: Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 20: 291–302, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Luna-Zurita L, Prados B, Grego-Bessa J, Luxán G, del Monte G, Benguría A, Adams RH, Pérez-Pomares JM, de la Pompa JL: Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest 120: 3493–3507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheppke L, Murphy EA, Zarpellon A, Hofmann JJ, Merkulova A, Shields DJ, Weis SM, Byzova TV, Ruggeri ZM, Iruela-Arispe ML, Cheresh DA: Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood 119: 2149–2158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann JJ, Iruela-Arispe ML: Notch signaling in blood vessels: Who is talking to whom about what? Circ Res 100: 1556–1568, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Murphy PA, Kim TN, Lu G, Bollen AW, Schaffer CB, Wang RA: Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci Transl Med 4: 117ra8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy PA, Lam MTY, Wu X, Kim TN, Vartanian SM, Bollen AW, Carlson TR, Wang RA: Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A 105: 10901–10906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE: Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 25: 1653–1663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Wang XH, Wang H, Du J, Mitch WE: Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol 21: 419–427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang A, Wang Y, Han G, Truong L, Cheng J: Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol 304: F1413–F1420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J, Du J: Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol 27: 1744–1751, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Xu Q, Zhang Z, Davison F, Hu Y: Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res 93: e76–e86, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC: Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115: 2119–2127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng J, Wang Y, Ma Y, Chan BT, Yang M, Liang A, Zhang L, Li H, Du J: The mechanical stress-activated serum-, glucocorticoid-regulated kinase 1 contributes to neointima formation in vein grafts. Circ Res 107: 1265–1274, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.