Abstract
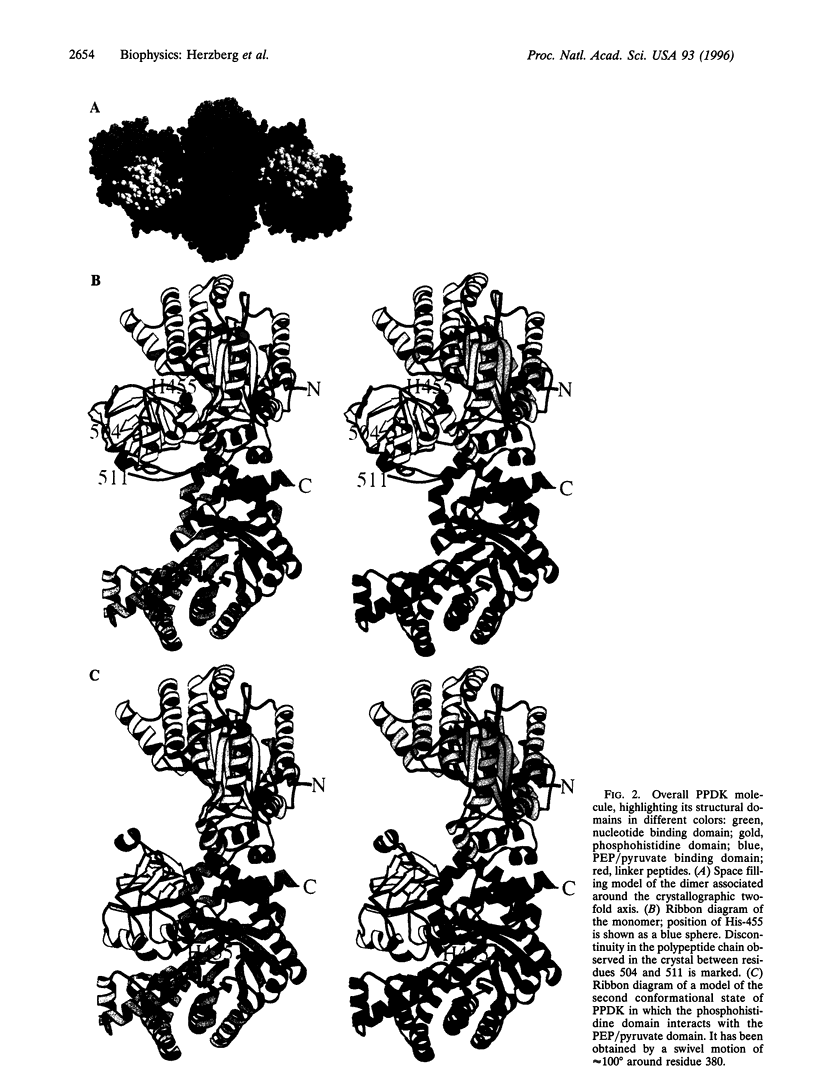
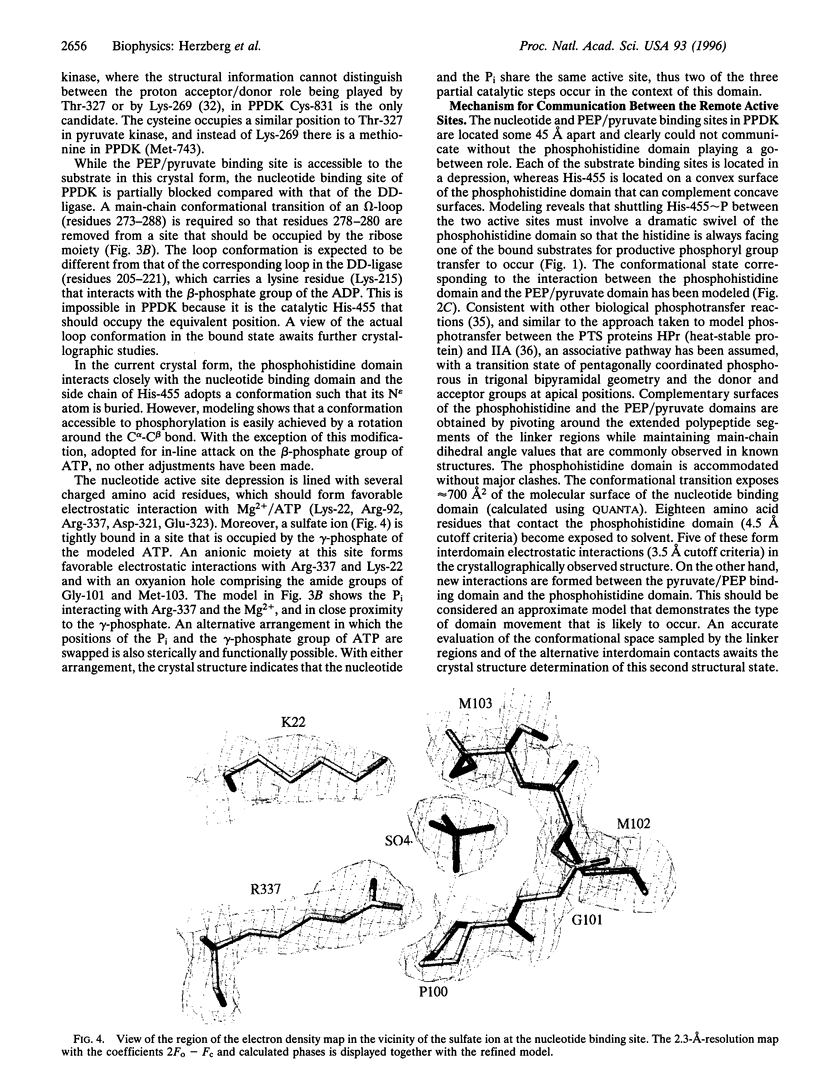
The crystal structure of pyruvate phosphate dikinase, a histidyl multiphosphotransfer enzyme that synthesizes adenosine triphosphate, reveals a three-domain molecule in which the phosphohistidine domain is flanked by the nucleotide and the phosphoenolpyruvate/pyruvate domains, with the two substrate binding sites approximately 45 angstroms apart. The modes of substrate binding have been deduced by analogy to D-Ala-D-Ala ligase and to pyruvate kinase. Coupling between the two remote active sites is facilitated by two conformational states of the phosphohistidine domain. While the crystal structure represents the state of interaction with the nucleotide, the second state is achieved by swiveling around two flexible peptide linkers. This dramatic conformational transition brings the phosphocarrier residue in close proximity to phosphoenolpyruvate/pyruvate. The swiveling-domain paradigm provides an effective mechanism for communication in complex multidomain/multiactive site proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Carroll L. J., Xu Y., Thrall S. H., Martin B. M., Dunaway-Mariano D. Substrate binding domains in pyruvate phosphate dikinase. Biochemistry. 1994 Feb 8;33(5):1134–1142. doi: 10.1021/bi00171a012. [DOI] [PubMed] [Google Scholar]
- Cook A. G., Knowles J. R. Phosphoenolpyruvate synthetase and pyruvate, orthophosphate dikinase: stereochemical consequences at both the beta-phospho and gamma-phospho groups of ATP. Biochemistry. 1985 Jan 1;24(1):51–58. doi: 10.1021/bi00322a009. [DOI] [PubMed] [Google Scholar]
- Evans C. T., Goss N. H., Wood H. G. Pyruvate phosphate dikinase: affinity labeling of the adenosine 5'-triphosphate--adenosine 5'-monophosphate site. Biochemistry. 1980 Dec 9;19(25):5809–5814. doi: 10.1021/bi00566a023. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Wood H. G. The mechanism of the pyruvate, phosphate dikinase reaction. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1448–1453. doi: 10.1073/pnas.61.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Moews P. C., Shi Y., Walsh C. T., Knox J. R. A common fold for peptide synthetases cleaving ATP to ADP: glutathione synthetase and D-alanine:d-alanine ligase of Escherichia coli. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1172–1176. doi: 10.1073/pnas.92.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Moews P. C., Walsh C. T., Knox J. R. Vancomycin resistance: structure of D-alanine:D-alanine ligase at 2.3 A resolution. Science. 1994 Oct 21;266(5184):439–443. doi: 10.1126/science.7939684. [DOI] [PubMed] [Google Scholar]
- Goss N. H., Evans C. T., Wood H. G. Pyruvate phosphate dikinase: sequence of the histidyl peptide, the pyrophosphoryl and phosphoryl carrier. Biochemistry. 1980 Dec 9;19(25):5805–5809. doi: 10.1021/bi00566a022. [DOI] [PubMed] [Google Scholar]
- Herzberg O. An atomic model for protein-protein phosphoryl group transfer. J Biol Chem. 1992 Dec 5;267(34):24819–24823. [PubMed] [Google Scholar]
- Holm L., Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993 Sep 5;233(1):123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- Hyde C. C., Ahmed S. A., Padlan E. A., Miles E. W., Davies D. R. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988 Nov 25;263(33):17857–17871. [PubMed] [Google Scholar]
- Jenkins C. L., Hatch M. D. Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase. Arch Biochem Biophys. 1985 May 15;239(1):53–62. doi: 10.1016/0003-9861(85)90811-2. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Larsen T. M., Laughlin L. T., Holden H. M., Rayment I., Reed G. H. Structure of rabbit muscle pyruvate kinase complexed with Mn2+, K+, and pyruvate. Biochemistry. 1994 May 24;33(20):6301–6309. doi: 10.1021/bi00186a033. [DOI] [PubMed] [Google Scholar]
- Mehl A., Xu Y., Dunaway-Mariano D. Energetics of pyruvate phosphate dikinase catalysis. Biochemistry. 1994 Feb 8;33(5):1093–1102. doi: 10.1021/bi00171a007. [DOI] [PubMed] [Google Scholar]
- Milner Y., Michaels G., Wood H. G. Pyruvate, phosphate dikinase of Bacteroides symbiosus. Catalysis of partial reactions and formation of phosphoryl and pyrophosphoryl forms of the enzyme. J Biol Chem. 1978 Feb 10;253(3):878–883. [PubMed] [Google Scholar]
- Murzin A. G., Brenner S. E., Hubbard T., Chothia C. SCOP: a structural classification of proteins database for the investigation of sequences and structures. J Mol Biol. 1995 Apr 7;247(4):536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- Pocalyko D. J., Carroll L. J., Martin B. M., Babbitt P. C., Dunaway-Mariano D. Analysis of sequence homologies in plant and bacterial pyruvate phosphate dikinase, enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system and other PEP-utilizing enzymes. Identification of potential catalytic and regulatory motifs. Biochemistry. 1990 Dec 4;29(48):10757–10765. doi: 10.1021/bi00500a006. [DOI] [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal. Proc Natl Acad Sci U S A. 1989 May;86(10):3639–3643. doi: 10.1073/pnas.86.10.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Spronk A. M., Yoshida H., Wood H. G. Isolation of 3-phosphohistidine from phosphorylated pyruvate, phosphate dikinase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4415–4419. doi: 10.1073/pnas.73.12.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall S. H., Dunaway-Mariano D. Kinetic evidence for separate site catalysis by pyruvate phosphate dikinase. Biochemistry. 1994 Feb 8;33(5):1103–1107. doi: 10.1021/bi00171a008. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Wang H. C., Ciskanik L., Dunaway-Mariano D., von der Saal W., Villafranca J. J. Investigations of the partial reactions catalyzed by pyruvate phosphate dikinase. Biochemistry. 1988 Jan 26;27(2):625–633. doi: 10.1021/bi00402a020. [DOI] [PubMed] [Google Scholar]
- Weigel N., Kukuruzinska M. A., Nakazawa A., Waygood E. B., Roseman S. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14477–14491. [PubMed] [Google Scholar]
- Wolodko W. T., Fraser M. E., James M. N., Bridger W. A. The crystal structure of succinyl-CoA synthetase from Escherichia coli at 2.5-A resolution. J Biol Chem. 1994 Apr 8;269(14):10883–10890. doi: 10.2210/pdb1scu/pdb. [DOI] [PubMed] [Google Scholar]
- Wood H. G., O'brien W. E., Micheales G. Properties of carboxytransphosphorylase; pyruvate, phosphate dikinase; pyrophosphate-phosphofructikinase and pyrophosphate-acetate kinase and their roles in the metabolism of inorganic pyrophosphate. Adv Enzymol Relat Areas Mol Biol. 1977;45:85–155. doi: 10.1002/9780470122907.ch2. [DOI] [PubMed] [Google Scholar]
- Xu Y., McGuire M., Dunaway-Mariano D., Martin B. M. Separate site catalysis by pyruvate phosphate dikinase as revealed by deletion mutants. Biochemistry. 1995 Feb 21;34(7):2195–2202. doi: 10.1021/bi00007a013. [DOI] [PubMed] [Google Scholar]
- Xu Y., Yankie L., Shen L., Jung Y. S., Mariano P. S., Dunaway-Mariano D., Martin B. M. Location of the catalytic site for phosphoenolpyruvate formation within the primary structure of Clostridium symbiosum pyruvate phosphate dikinase. 1. Identification of an essential cysteine by chemical modification with [1-14C]bromopyruvate and site-directed mutagenesis. Biochemistry. 1995 Feb 21;34(7):2181–2187. doi: 10.1021/bi00007a011. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kato H., Hata Y., Nishioka T., Kimura A., Oda J., Katsube Y. Three-dimensional structure of the glutathione synthetase from Escherichia coli B at 2.0 A resolution. J Mol Biol. 1993 Feb 20;229(4):1083–1100. doi: 10.1006/jmbi.1993.1106. [DOI] [PubMed] [Google Scholar]
- Yankie L., Xu Y., Dunaway-Mariano D. Location of the catalytic site for phosphoenolpyruvate formation within the primary structure of Clostridium symbiosum pyruvate phosphate dikinase. 2. Site-directed mutagenesis of an essential arginine contained within an apparent P-loop. Biochemistry. 1995 Feb 21;34(7):2188–2194. doi: 10.1021/bi00007a012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Wood H. G. Crystalline pyruvate, phosphate dikinase from Bacteroides symbiosus. Modification of essential histidyl residues and bromopyruvate inactivation. J Biol Chem. 1978 Nov 10;253(21):7650–7655. [PubMed] [Google Scholar]