Abstract
Marked hypophosphatemia is common after major hepatic resection, but the pathophysiologic mechanism remains unknown. We used a partial hepatectomy (PH) rat model to investigate the molecular basis of hypophosphatemia. PH rats exhibited hypophosphatemia and hyperphosphaturia. In renal and intestinal brush-border membrane vesicles isolated from PH rats, Na+-dependent phosphate (Pi) uptake decreased by 50%–60%. PH rats also exhibited significantly decreased levels of renal and intestinal Na+-dependent Pi transporter proteins (NaPi-IIa [NaPi-4], NaPi-IIb, and NaPi-IIc). Parathyroid hormone was elevated at 6 hours after PH. Hyperphosphaturia persisted, however, even after thyroparathyroidectomy in PH rats. Moreover, DNA microarray data revealed elevated levels of nicotinamide phosphoribosyltransferase (Nampt) mRNA in the kidney after PH, and Nampt protein levels and total NAD concentration increased significantly in the proximal tubules. PH rats also exhibited markedly increased levels of the Nampt substrate, urinary nicotinamide (NAM), and NAM catabolites. In vitro analyses using opossum kidney cells revealed that NAM alone did not affect endogenous NaPi-4 levels. However, in cells overexpressing Nampt, the addition of NAM led to a marked decrease in cell surface expression of NaPi-4 that was blocked by treatment with FK866, a specific Nampt inhibitor. Furthermore, FK866-treated mice showed elevated renal Pi reabsorption and hypophosphaturia. These findings indicate that hepatectomy-induced hypophosphatemia is due to abnormal NAM metabolism, including Nampt activation in renal proximal tubular cells.
Inorganic phosphate (Pi) absorption in the renal proximal tubules and small intestine is important for Pi homeostasis.1 The Na+-dependent Pi (Na/Pi) transport system includes type IIa and type IIc Na/Pi transporters, which are localized in the apical membrane of the proximal tubular cells, and type IIb Na/Pi transporters, which are localized in the apical membrane of the intestinal epithelial cells.1,2 Pi (re)absorption is regulated by the dietary Pi content, parathyroid hormone (PTH), and the active metabolite of vitamin D, 1α, 25-dihydroxyvitamin D3 [1,25(OH)2D3].3 Other phosphaturic hormones, termed phosphatonins, also control renal Pi handling.4 The discovery that fibroblast growth factor (FGF) 23, the first identified phosphatonin,5 originated from osteocytes established the concept of the bone-kidney axis.6,7
The incidence of liver transplantation has steadily increased and the incidence of partial hepatectomy (PH) has also consequently increased.8 Hypophosphatemia frequently occurs after liver resection.9–11 Acute hypophosphatemia causes septicemia and is associated with a poor prognosis.11,12 Acute hypophosphatemia is of considerable clinical relevance because many hepatectomized patients develop marked hypophosphatemia and, thus, large doses of Pi replacement are required to maintain metabolic homeostasis.13 Urinary Pi excretion is markedly increased in many patients. After hepatectomy, hypophosphatemia is associated with hyperphosphaturia.13
For many years, the increased metabolic demand of the regenerating liver was considered the underlying pathologic mechanism of hypophosphatemia. The magnitude of Pi uptake by the recovering liver, however, cannot explain the severity of the resulting hypophosphatemia.11 Hepatectomy-induced hypophosphatemia is associated with an increased renal fractional excretion index for Pi unrelated to intact FGF23, FGF7, or secreted frizzled-related protein 4 as a phosphaturic factor,14 indicating that other factors have a role in the pathogenesis of hypophosphatemia.
Nicotinamide (NAM) inhibits intestinal and renal Na/Pi transport activity in normal rats.15–17 Administration of NAM to rats produces a specific dose-dependent inhibition of Na/Pi transport across the renal brush-border membrane (BBM) and an increase in urinary Pi excretion.16,17 NAM suppresses hyperphosphatemia in hemodialysis patients.18 Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the first rate-limiting step in converting NAM to NAD,19,20 which is essential for cellular metabolism, energy production, and DNA repair.20–22 Nampt exists in two known forms: intracellular Nampt (iNampt) and secreted extracellular Nampt (eNampt).23 eNampt also generates an intermediate product, nicotinamide mononucleotide (NMN).23
Our findings indicate that the acceleration of NAM metabolism through Nampt function in the kidney is involved in the hepatectomy-induced hypophosphatemia in rodent models. This study also suggests that NAM metabolism through the liver-kidney axis is important in Pi homeostasis.
Results
Biochemical Analyses of PH Rats
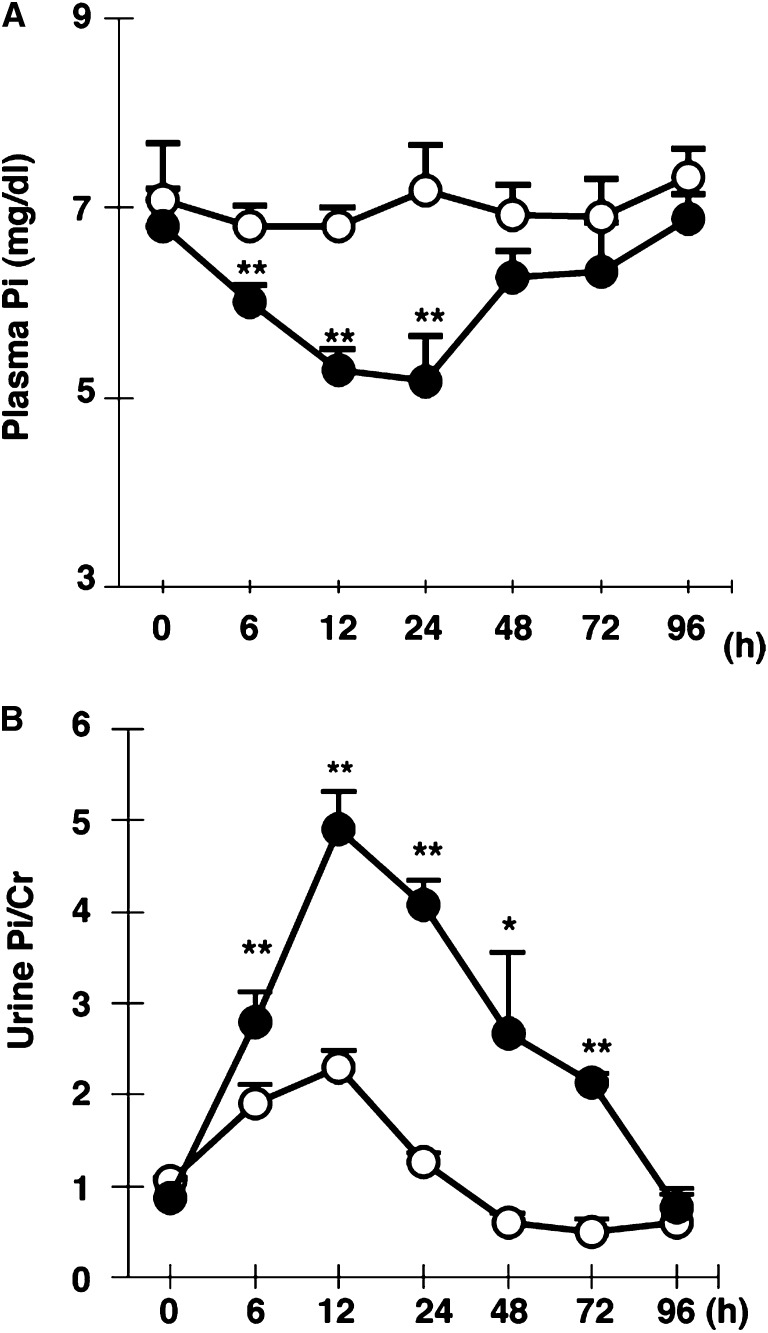
In the first set of experiments, we investigated the plasma parameters in PH rats. Plasma Pi levels were significantly reduced at 6, 12, and 24 hours after PH (Figure 1A). Hypophosphatemia in PH rats was due to elevated urinary Pi excretion (Figure 1B). The recovery of urinary Pi excretion was corrected based on the plasma Pi levels (Figure 1). In the biochemical data for PH rats, plasma pH, PCO2, HCO3, Na+, K+, and Ca2+ levels were not changed at 6, 12, and 24 hours after PH (Table 1). At 6 hours after PH, the plasma-intact PTH (iPTH) levels were approximately 2.7-fold higher than that in sham controls (Table 1). In contrast, serum FGF23 levels were significantly decreased at 6, 12, and 24 hours after PH (Table 1). At 24 hours after PH, urine cAMP levels were not changed (Table 1). Except for iPTH, we did not detect any candidate factors for hyperphosphaturia in the biochemical data for PH rats. The changes in the biochemical parameters in PH rats were similar to those in human PH patients.14
Figure 1.
Renal Pi excretion after 70% PH. Plasma Pi (A) and urinary Pi/urinary creatinine (B) in sham rats versus PH rats (open and closed circles, respectively). Blood and urine are collected at 6, 12, 24, 48, 72, and 96 hours. Metabolic cages are used for urine collection from sham and PH rats. Data are presented as the mean±SEM (n=5–7/group). *P<0.05; **P<0.01. Cr, creatinine.
Table 1.
Blood and urine biochemical markers in partial hepatectomized rat
| Marker | After 6 h | After 12 h | After 24 h | |||
|---|---|---|---|---|---|---|
| Sham | PH | Sham | PH | Sham | PH | |
| Blood | ||||||
| pH | 7.43±0.02 | 7.41±0.02 | 7.46±0.01 | 7.45±0.01 | 7.43±0.01 | 7.49±0.01 |
| PCO2 (mmHg) | 48.6±1.9 | 50.0±5.2 | 45.4±2.4 | 41.7±2.5 | 49.0±1.5 | 40.0±2.6 |
| HCO3 (nmol/L) | 31.6±0.7 | 30.3±2.3 | 31.3±1.4 | 28.3±1.6 | 31.7±1.3 | 29.6±1.5 |
| Na+ (nmol/L) | 145.0±0.5 | 144.0±1.5 | 145.6 ±0.8 | 141.7 ±1.2 | 145.7±0.3 | 140.3±2.9 |
| K+ (nmol/L) | 6.6±0.2 | 6.8±0.4 | 5.9±0.2 | 6.8±0.4 | 6.6±0.2 | 5.4±0.5 |
| Ca2+ (nmol/L) | 1.13±0.03 | 1.13±0.05 | 1.21±0.01 | 1.09±0.05 | 1.25±0.02 | 1.17±0.02 |
| Plasma | ||||||
| Pi (mg/dl) | 6.2±0.23 | 5.6±0.18a | 6.4±0.21 | 5.3±0.21a | 6.6±0.2 | 4.4±0.7a |
| Ca (mg/dl) | 8.6±0.31 | 7.8±0.36 | 8.2±0.24 | 7.9±0.37 | 9.0±0.31 | 8.5±0.41 |
| iPTH (pg/ml) | 92.8±9.0 | 253.4± 66.8b | 88.9±33.4 | 114.3±21.8 | 81.0±28.8 | 75.6±26.4 |
| 1,25(OH)2D3 (pg/ml) | 414.7± 28.6 | 552.8± 26.9b | 534.7±81.1 | 706.0±39.6 | 348.8±86.9 | 473.8±85.8 |
| Serum | ||||||
| FGF23 (mg/dl) | 160±5.0 | 150±3.0b | 161±10 | 90±6.0a | 162±10 | 97±6.0a |
| Urine | ||||||
| Pi/Cr | 1.9±0.20 | 2.8±0.33a | 2.3±0.19 | 4.9±0.41a | 2.8±0.41 | 4.6±0.66a |
| Ca/Cr | 0.078±0.030 | 0.076±0.038 | 0.051±0.012 | 0.049±0.006 | 0.037±0.006 | 0.047±0.005 |
| cAMP/Cr (nmol/mg) | – | – | – | – | 262.0±37.1 | 337.5±29.6 |
All data are presented as the mean±SEM (n=12). Blood and urine were collected at the age of 9–10 weeks. Cr, creatinine.
P<0.01.
P<0.05.
Renal Na/Pi Transport Activity in PH Rats
We then investigated renal and intestinal Na/Pi transport activities at 12 hours after PH. Renal and intestinal Na/Pi transport activities were significantly reduced in PH rats compared with sham-operated rats (Figure 2, A and B). Western blotting analysis also showed a marked reduction in NaPi-II transporter protein levels (NaPi-IIa, NaPi-IIc in the kidney, and NaPi-IIb in the jejunum; Figure 2, C and D). Immunohistochemical analysis provided similar findings (Figure 2E). Moreover, the NaPi-IIa and NaPi-IIc protein expression levels recovered at 96 hours after PH (data not shown).
Figure 2.
Protein abundance of renal NaPi-IIa, NaPi-IIc, and intestinal NaPi-IIb in PH rats. (A) Renal Na/Pi transport activity in sham and PH rats. Na/Pi transport activity is determined by 32P uptake in kidney BBMVs. (B) Intestinal Na/Pi transport activity in sham and PH rats. Na/Pi transport activity is determined by 32P uptake in jejunum BBMVs. (C) Immunoblotting analysis of NaPi-IIa and NaPi-IIc proteins in renal BBMVs from sham and PH rats. BBMVs are prepared 12 hours postsurgically. All membranes are reprobed for actin. Actin is used as an internal control. (D) Immunoblotting analysis of NaPi-IIb in jejunum BBMVs from sham and PH rats. BBMVs are isolated 12 hours after PH. All membranes are reprobed for actin. Actin is used as an internal control. (E) Immunohistochemical analysis of renal NaPi-IIa and NaPi-IIc in PH rats. Representative tubules with prominent staining are indicated by the arrowhead. Data are presented as the mean±SEM (n=5–7/group). *P<0.05; **P<0.01.
Effect of PH on Pi Excretion in Thyroparathyroidectomized Rats
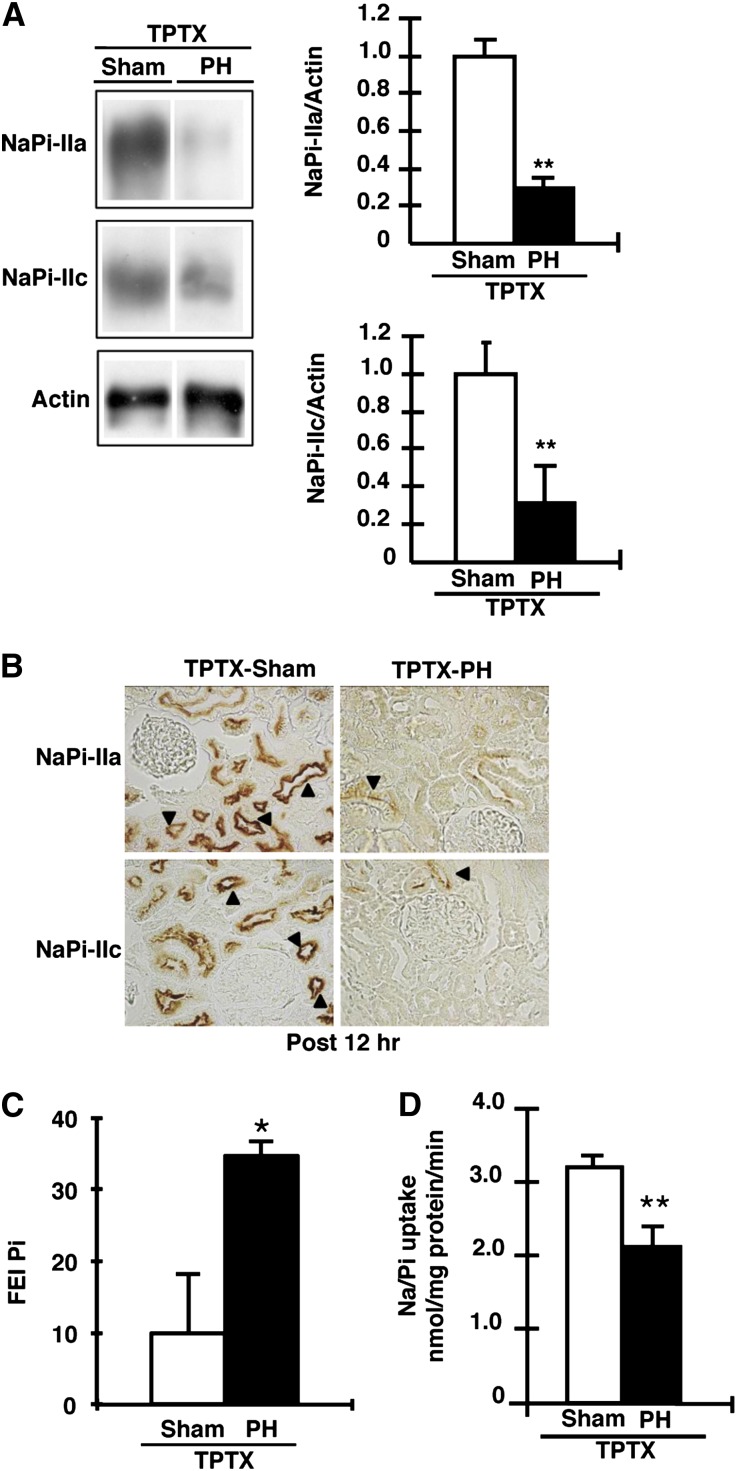
To further investigate the cause of hyperphosphaturia, we studied the effect of PTH on Pi homeostasis in PH rats. Using thyroparathyroidectomized (TPTX) rats, we analyzed the effects of PH on renal NaPi-II transporter protein levels (Figure 3, A and B). In the TPTX rats, urinary Pi excretion was markedly reduced because the renal Pi transport activity and NaPi-IIa/NaPi-IIc protein levels were significantly increased compared with those in sham-operated animals (data not shown), as previously described.24
Figure 3.
Effect of PH on Pi excretion in TPTX rats. TPTX rats with 70% PH are analyzed 12 hours after PH. (A) Immunoblotting of NaPi-IIa and NaPi-IIc proteins in renal BBMVs from sham and TPTX-PH rats. BBMVs are prepared at 12 hours after PH. The open column shows sham operation (TPTX-Sham) rats, whereas the filled column shows TPTX-PH rats. (B) Immunohistochemical detection of NaPi-IIa and NaPi-IIc in the kidney sections of TPTX-sham and TPTX-PH rats. Representative tubules with prominent staining are indicated by the arrowhead. (C) The fractional excretion index for Pi (FEI Pi) is calculated as follows: urine Pi/(urine creatinine×plasma Pi). (D) Renal Na/Pi transport activity in TPTX-sham and TPTX-PH rats. Na/Pi transport activity is determined by 32P uptake in renal BBMVs. Data are presented as the mean±SEM (n=4–6/group). *P<0.05; **P<0.01. Original magnification, ×400 in B.
After PH in the TPTX or sham-operated TPTX rats, fractional excretion index levels for Pi remained increased in TPTX rats at 12 hours compared with those in sham-operated TPTX rats (Figure 3C). Furthermore, in TPTX rats, the renal Na/Pi transport activity was also significantly decreased at 12 hours after PH (Figure 3D).
Expression of Nampt in PH Rats
We then analyzed the gene expression profiles in the kidney cortex after PH. Total RNA from the kidney cortex was measured in PH and sham rats at 12 hours after PH. cRNA synthesis and microarray analysis were performed using the Affymetrix Rat Gene 1.0 ST array (Affymetrix, Santa Clara, CA). Expression levels of most of the genes did not differ between PH and sham rats (data not shown). Nampt cDNA levels, however, were increased 2.54-fold in the kidney of PH rats compared with sham rats.
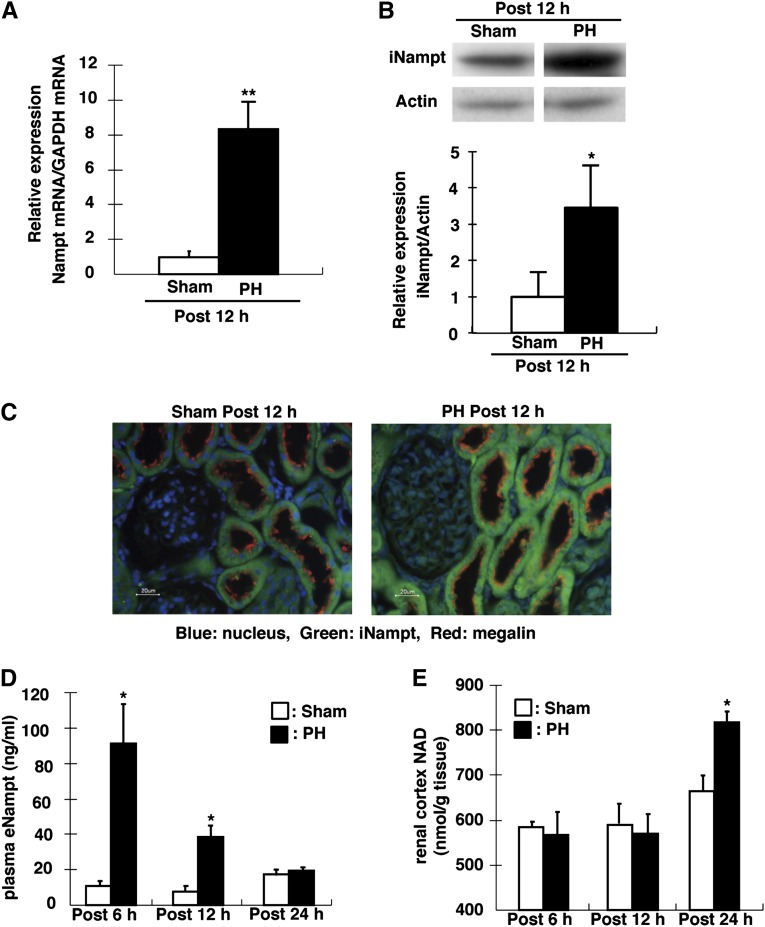
We used quantitative PCR to analyze Nampt mRNA expression in PH rats compared with sham rats. At 12 hours after PH, the Nampt mRNA (approximately 8-fold) and protein (iNampt; approximately 3.5-fold) levels in the kidney cortex were increased (Figure 4, A and B). Immunohistochemical analyses revealed similar findings, and indicated that Nampt (iNampt) was expressed in the proximal tubules (Figure 4C). Plasma extracellular Nampt (eNampt) levels were markedly increased at 6 and 12 hours after PH (Figure 4D). NAD levels were significantly increased in the kidney cortex at 24 hours, but not at 6 or 12 hours, after PH (Figure 4E).
Figure 4.
Expression of iNampt in proximal tubules and serum eNampt in PH rats. (A) Nampt mRNA levels in the kidney of sham and PH rats are assessed by quantitative PCR. GAPDH is used as the internal control. The relative intensity of Nampt expression from the kidney of sham rats is defined as 1.0. (B) iNampt protein levels in the kidney of sham and PH rats are assessed by Western blotting analysis. Each lane is loaded with 20 µg of total lysate. Actin is used an internal control. Expression levels in sham rats are defined as 1.0. (C) Nampt localization in the kidney cortex is analyzed in the sham and PH rats. Nampt (green) is observed in cytosol of proximal tubules, and megalin (red) is expressed in BBM of proximal tubules. DAPI (blue) is used to stain the nucleus. (D) Serum eNampt is measured at 6, 12, and 24 hours after PH. The open column shows sham rats, whereas the filled closed column shows PH rats. (E) Total NAD of the kidney cortex is measured at 6, 12, and 24 hours after PH. The open column shows sham rats, whereas the filled column shows PH rats. Data are presented as the mean±SEM (n=4–6/group). *P<0.05; **P<0.01. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Urinary Excretion of NAM and Its Catabolites in PH Rats
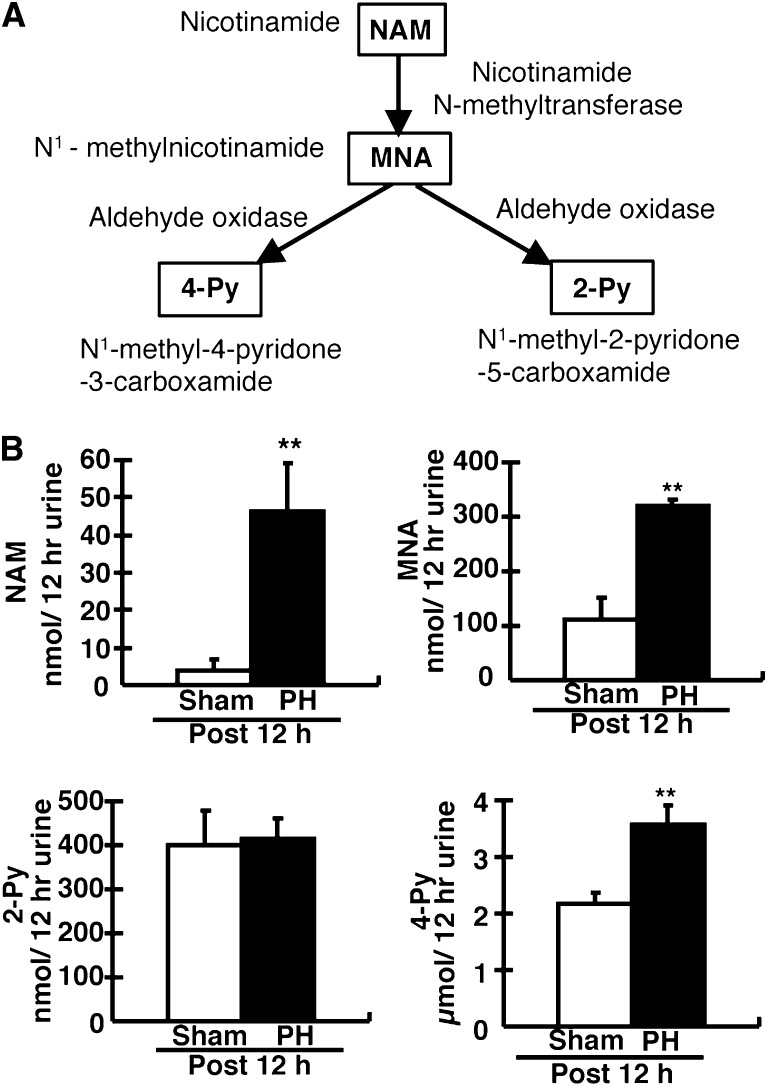
To further investigate NAM metabolism in PH rats, we analyzed urinary NAM catabolites. N1-Methylnicotinamide (MNA), N1-methyl-2-pyridone-5-carboxamide (2-Py), and N1-methyl-4-pyridine-3-carboxamide (4-Py) are excreted in the urine (Figure 5A). 4-Py is the major catabolite of NAM in rats (Figure 5A).25 MNA and 4-Py levels were increased in PH rats compared with sham rats (Figure 5B). Urinary NAM levels were markedly increased (approximately 10-fold) in PH rats at 12 hours (Figure 5B). In vivo, administration of NAM to rats markedly increased urine NAM, MNA, 2-Py, and 4-Py levels approximately 100-fold (Supplemental Figures 1 and 2). The pattern of excreted NAM and its catabolites was similar between PH rats and NAM-treated rats.
Figure 5.
Analysis of NAM and its catabolites in PH rats. (A) A schematic of the mammalian NAM catabolic pathway in liver. Excess NAM is converted into MNA, 2-Py, and 4-Py. These catabolites are excreted into the urine. (B) The values represent the total amount of NAM and its catabolites in urine for 12 hours after PH. Male 9- to 10-week-old rats are used. Data are presented as the mean±SEM (n=5–7/group). **P<0.01.
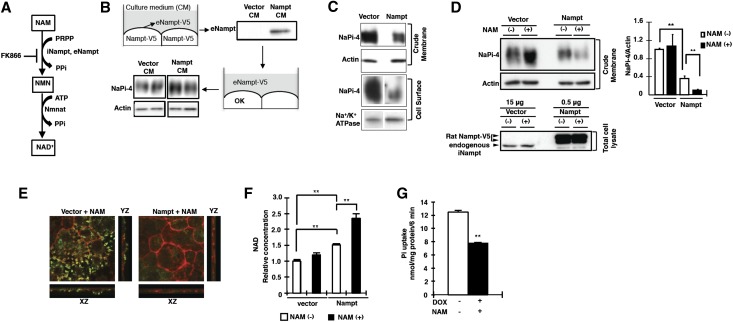
Effect of Nampt Expression on NaPi-4 Levels in Opossum Kidney Cells
We first examined the possibility that the elevation of plasma eNampt leads to a decrease in NaPi-IIa protein in the proximal tubular cells. As shown in Figure 6A, eNampt converts NAM to NMN. Conditioned medium containing eNampt was prepared as described in the legend for Figure 6. The addition of medium containing eNampt to the opossum kidney (OK) cell culture medium induced no observable changes in the NaPi-4 (endogenous NaPi-IIa in OK cells) levels in the cell homogenate and cell surface fractions for 24 hours (Figure 6B).
Figure 6.
Effect of Nampt on NaPi-4 expression levels in OK cells. (A) A schematic of mammalian NAD metabolism. Metabolites are shown in boxes. Nicotinamide is processed to NAD+ by two enzymes: Nampt and Nmnat. Nampt is a catalytic enzyme that is the rate-limiting step in this process. FK866 is a specific Nampt inhibitor. (B) OK cells are transfected with Nampt, and the resulting conditioned medium is collected. The conditioned medium is immunoblotted for eNampt detection (upper). OK cells are incubated with 10% conditioned medium for 24 hours. Crude membrane of OK cells is immunoblotted for opossum NaPi-IIa (NaPi-4) detection (lower). (C) OK cells are transfected with Nampt as shown and crude membrane fractions are collected 48 hours later. Crude membranes or cell surface proteins are immunoblotted for NaPi-4 detection. Actin or Na+/K+ ATPase is used as an internal control. (D) OK cells are treated with NAM for 4 hours before collecting crude membrane and total cell lysate fractions. The crude membrane fraction is immunoblotted for NaPi-4 detection. Total OK cell lysate proteins (15 µg or 0.5 µg/lane) are immunoblotted for rat Nampt-V5 and endogenous Nampt detection. Band density is normalized against that of actin and is expressed as relative intensity to that of the control [nontreatment: vector (NAM (–)] level. (E) OK cells transfected with Nampt are stained with antibodies against phalloidin (actin marker) and NaPi-4, and then processed for confocal microscopy. NaPi-4 is shown in green, actin is shown in red, and the overlay of both signals is yellow. The xz cross-section is indicated in the lower panels. The yz cross-section is indicated in the right panels. (F) NAM is treated with cells for 4 hours before analysis of NAD concentration. Analysis of NAD concentration in Nampt-overexpressing OK cells treated with NAM. (G) Pi uptake assays are performed in OK cells transfected with inducible Nampt. OKpTet/Nampt cells are treated with doxycycline (1 µg/ml) or without doxycycline (−). NAM (10 mM) is added to OKpTet/Nampt cells. Data are presented as the mean±SEM (n=6/group). **P<0.01. PPi, pyrophosphate; CM, conditioned medium; DOX, doxycycline.
We further investigated whether the elevation of Nampt (iNampt) protein stimulates the downregulation of NaPi-4 protein in OK cells. Using specific Nampt antibodies, we showed that the antibodies reacted with 52- to 54-kD protein bands, suggesting that endogenous Nampt protein is present in OK cells (Figure 6D). In addition, the Nampt-positive protein bands of OK cells overexpressing V5-tagged Nampt were 56–60 kD. Using an avidin-biotin labeling system for cell surface proteins, we investigated the effect of Nampt overexpression on NaPi-4 protein levels in OK cells (Figure 6C). NaPi-4 protein levels in the cell surface membrane fraction were significantly decreased compared with controls (Figure 6C). In addition, administration of NAM to rats markedly reduced the NaPi-IIa protein levels in the apical membrane of the renal tubular cells (Supplemental Figure 3). In contrast, NaPi-4 levels on the control OK cell surface were not affected by incubation with NAM, whereas NaPi-4 levels on Nampt-overexpressing OK cells were markedly decreased (Figure 6D). The reduction of NaPi-4 immunoreactive signals was confirmed by immunocytochemical analysis (Figure 6E). In addition, cellular NAD levels, the final product of the Nampt enzyme reaction, were significantly increased in Nampt-overexpressing OK cells treated with NAM (Figure 6F). To further analyze the association between Nampt and NaPi-4 function, we made a stable cell line with doxycycline-inducible Nampt. In the stable cell line, Pi uptake was significantly decreased after inducing the Nampt protein (Figure 6G). These findings suggest that Nampt overexpression reduced NaPi-4 protein levels and Na/Pi transport activity in OK cells, and incubation with NAM further stimulated the NaPi-4 protein reduction.
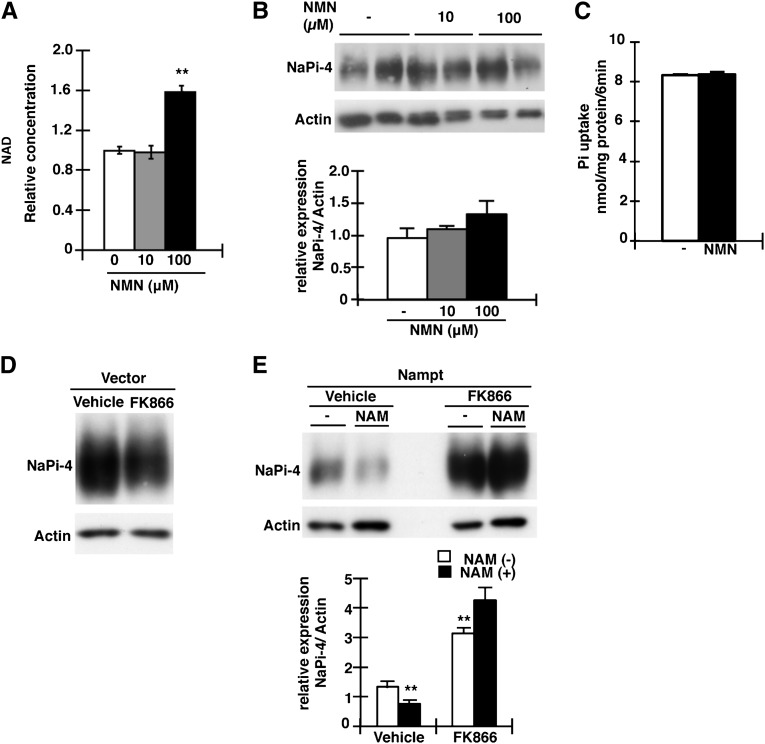
Effect of Nampt-Specific Inhibitor FK866 and NMN on NaPi-4 Expression
We investigated whether the increase in cellular NAD levels affects the amounts of NaPi-4 protein in OK cells. The addition of NMN, a product of the Nampt enzyme reaction (Figure 6A), to the incubation medium significantly increased the levels of cellular NAD (Figure 7A), but the NaPi-4 protein levels remained unchanged, suggesting that the Nampt-independent elevation of NAD is not involved in the downregulation of NaPi-4 and Pi uptake in OK cells (Figure 7, B and C). To further examine whether Nampt enzyme activity is a key factor for NaPi-4 suppression in OK cells, we used the Nampt-specific inhibitor FK866. In control vector-transfected OK cells, FK866 did not affect the NaPi-4 protein levels (Figure 7D). Nampt overexpression significantly decreased NaPi-4 protein (see Figure 6D), whereas the addition of FK866 completely blocked the NaPi-4 protein reduction (Figure 7E). Interestingly, in the Nampt-expressing cells, the addition of FK866 markedly increased NaPi-4 protein levels (Figure 7E). Moreover, in the Nampt-expressing cells, FK866 protected against further reduction of NaPi-4 caused by NAM (Figure 7E).
Figure 7.
Effects of NMN and FK866 on NaPi-4 expression in OK cells. (A) Analysis of NAD concentrations in NMN-treated OK cells. OK cells are treated by NMN (0, 10, 100 µM) for 4 hours. (B) Crude membranes are immunoblotted using anti–NaPi-4. NMN is added to OK cells for 4 hours before collecting the crude membrane fraction. (C) Pi uptake assays are performed in the NMN-treated (100 µM) OK cells. The experiments are repeated three times. (D) Crude membranes are Western blotted and immunoprobed using anti–NaPi-4. OK cells are treated with FK866 (10 µM) for 12 hours before collecting the crude membrane fraction. (E) OK cells transfected with Nampt or empty vector. Crude membranes are immunoblotted using NaPi-4 antibody. FK866 (10 µM) or vehicle-treated OK cells for 12 hours before collecting crude membrane. NAM (10 mM) is added to OK cells for 4 hours before collecting crude membrane. Actin expression is used as an internal control. Data are presented as the mean±SEM (n=6/group). **P<0.01.
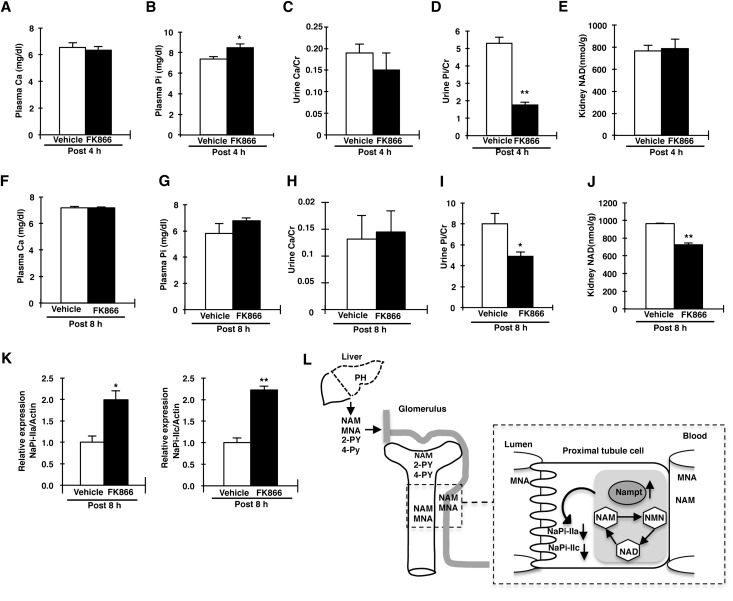
Effect of the Nampt-Specific Inhibitor FK866 on Renal Pi Reabsorption
We investigated the effect of FK866 on Pi homeostasis in normal mice, because of the limited amounts of FK866 available (Figure 8). Plasma Ca and urine Ca/urine creatinine levels did not differ at 4 hours after FK866 injection (Figure 8, A and C). Urine Pi/urine creatinine levels were also markedly decreased in FK866-treated mice compared with control mice (Figure 8D). Plasma Pi levels were increased in FK866-treated mice compared with control mice (Figure 8B). At 8 hours after injecting FK866, plasma Pi, Ca, and urine Ca/urine creatinine levels did not differ between vehicle and FK866-treated mice (Figure 8, F–H). Urine Pi/urine creatinine levels were significantly decreased in FK866-injected mice compared with vehicle-injected mice (Figure 8I). NAD levels in the kidney cortex were significantly decreased compared with control mice at 8 hours, but not 4 hours (Figure 8, E and J). Western blotting analysis showed that NaPi-IIa and NaPi-IIc protein levels were significantly increased in FK866-treated mice (Figure 8K), suggesting that FK866 administration influenced Pi reabsorption in normal mouse kidney. We investigated the effect of FK866 on Pi excretion in PH mice. The PH mice also showed hyperphosphaturia and hypophosphatemia (Supplemental Figure 4). At 8 hours after FK866 injection into PH mice, urinary Pi excretion was significantly decreased. These results suggested that FK866 prevented hepatectomy-induced hypophosphatemia.
Figure 8.
FK866 affects renal Pi reabsorption. C57BL6/J mice (aged 10 weeks) are treated with FK866 (70 mg/kg body weight) by intraperitoneal injection. The concentration of FK866 is selected according to a previous report.31 The open column shows the vehicle injection, whereas the filled column shows the FK866 injection. (A–E) Plasma Ca concentration (A), plasma Pi concentration (B), urine Ca/urine creatinine (Cr) (C), urine Pi/urine creatinine (D), and NAD concentration in the kidney (E) at 4 hours after the administration of FK866 or vehicle (n=8/group). (F–J) Plasma Ca concentration (F), plasma Pi concentration (G), urine Ca/urine creatinine (H), urine Pi/urine creatinine (I), and NAD concentration in the kidney (J) at 8 hours after the administration of FK866 or vehicle (n=8/group). (K) Immunoblotting of NaPi-IIa and NaPi-IIc proteins in kidney BBMVs from FK866-injected mice. BBMVs are prepared at 8 hours after FK866 treatment. All membranes are reprobed for actin. Actin is used as an internal control. (L) Proposed mechanism of hepatectomy-hypophosphatemia through the liver-kidney axis. After PH, NAM and its catabolites MNA, 2-Py, and 4-Py in the liver are released into the blood. MNA is excreted by the kidney, whereas NAM is reabsorbed by the renal proximal tubules. MNA is oxidized in the liver and produces 2-Py and 4-Py, catabolites that are both excreted into the urine. After PH, Nampt protein levels are increased in the renal proximal tubular cells. The increased NAM influx and Nampt activity in the proximal tubular cells leads to the downregulation of the NaPi-IIa and NaPi-IIc protein levels. Data are presented as the mean±SEM (n=8/group). *P<0.05; **P<0.01.
Discussion
This study indicated that posthepatectomy hypophosphaturia is not associated with known phosphaturic factors such as FGF23 and PTH. After PH in rats, large amounts of NAM and its catabolites (MNA and 4-Py) were excreted into the urine compared with sham rats. The liver stores NAD and is a major organ for NAM metabolism.26 NAM, 2-Py, and 4-Py, catabolic products in the liver, are excreted from the kidney.27 In regenerating liver, competition occurs for the synthesis of nucleic acid precursors (DNA, RNA), at the expense of NAD synthesis.28 Therefore, after PH, the NAD content continues to decrease in the remaining liver for 24 hours and DNA synthesis increases in the remaining liver.28 In addition, the accumulation of cellular NAM inhibits DNA synthesis29; therefore, the excess NAM after PH is expected to be excreted by the kidney.
In this study, we found that high amounts of NAM (approximately 40 nmol/12 h urine) and its catabolites were excreted in the urine of PH rats (Figure 5B). A pharmacologic dose of NAM inhibits renal and intestinal Na/Pi transport activity in normal rats.15,16 In those animals, cellular NAD levels are increased in inverse proportion to Na/Pi transport activity.16 Indeed, we showed that NaPi-II protein levels were markedly decreased and NAD concentrations were increased at kidney in PH animals, suggesting that the effects were similar to those in NAM-treated animals. NAM concentrations in the urine of PH rats, however, were approximately 10 times lower than those in the rats administered a pharmacologic dose of NAM (1 g/kg body weight; Supplemental Figure 1). Because a low dose of NAM (0.1 g/kg body weight) did not result in a significant reduction of renal NaPi-IIa transporter protein levels, even if the urinary NAM concentration was increased, we hypothesized that other factors were involved in the downregulation of NaPi-IIa protein in the proximal tubules.
On the basis of DNA microarray analysis, the elevated Nampt protein in the kidney was a candidate mechanism of the hyperphosphaturia in PH animals. Nampt initiates the major NAD biosynthesis pathway by converting NAM and 5-phosphoribosyl 1-pyrophosphate (PRPP) to NMN19,20 In addition, Nampt is the rate-limiting enzyme in NAD synthesis and thus has a key role in regulating sirtuin activity.20,22,30 In OK cells, the Nampt enzyme reaction is an important determinant for the reduction of NaPi-4 protein levels. Moreover, NAM itself did not affect the NaPi-4 levels in OK cells, whereas Nampt expression with its substrate (NAM) markedly decreased NaPi-4 protein levels. In addition, the downregulation of NaPi-4 protein by Nampt and NAM was completely blocked by FK866.31,32 Incubation with NMN, the product of Nampt enzymatic action, did not affect NaPi-4 protein levels in OK cells. Therefore, Nampt activity, in addition to extracellular NAM, is an important factor for NaPi-4 reduction in OK cells.
Furthermore, FK866 administration to normal mice significantly increased NaPi-IIa and NaPi-IIc protein levels and suppressed the urinary Pi excretion. The findings of these studies indicate that, in addition to NAM, Nampt activity is involved in Pi reabsorption. Dousa previously demonstrated that NAD inhibits renal Na/Pi transport mainly in response to metabolic stimuli, and suggested that NAD acts indirectly by first being converted to cyclic ADP-ribose, a potent stimulator of intracellular Ca2+ mobilization.33 According to this hypothesis, the increase in the cellular NAD levels is an important factor for renal Na/Pi transport activity.33 Although many studies have assessed the association between NAD and Na/Pi transport activity, the data are controversial.34,35 In this study, we showed that the reduction in NaPi-II transporters occurs earlier than the increase in the renal NAD concentration after PH. Our in vitro study also demonstrated that NaPi-4 levels were not altered by the increase in NAD levels after incubation with NMN. Therefore, the increase in cellular NAD levels is not a trigger for renal NaPi-II downregulation. In contrast, after a large NAM influx into the renal proximal tubular cells, the Nampt enzyme reaction consumes a large amount of PRPP. We speculate that the reduced cellular PRPP levels are an important determinant for NaPi-II transporters.
In a previous report, we demonstrated that the administration of NAM into rats decreases Na/Pi activity in the rat small intestine.15 In this study, intestinal NaPi-IIb protein levels were also decreased after PH, suggesting that Nampt function through NAM in the small intestine is involved in hepatectomy-induced hypophosphatemia. Further studies are necessary to clarify the mechanisms underlying the downregulation of intestinal NaPi-IIb after PH.
Nampt also mediates many inflammatory processes.21 eNampt elevation is observed in several inflammatory diseases such as rheumatoid arthritis, acute lung injury, colitis, and sepsis.21 Indeed, plasma eNampt levels are increased 7- to 8-fold after PH. Therefore, we investigated whether the elevation of plasma eNampt levels causes PH-induced hypophosphatemia. The results of studies both in vitro (OK cells; Figure 6B) and in vivo (injection of eNampt naked DNA into mice; data not shown) indicated that eNampt did not stimulate NaPi-II transporter downregulation. On the basis of these findings, we suggest that the increase in eNampt observed after PH is not involved in PH-induced hypophosphatemia.
Finally, postsurgical hypophosphatemia is an established complication that is particularly severe and extremely common after hepatic surgery. The present data suggest that the cause of hypophosphatemia and hyperphosphaturia observed after 70% hepatectomy is caused by abnormal NAM metabolism in the liver and kidney (Figure 8L). The increased Pi excretion is not due simply to an increase in the Nampt protein levels. Rather, NAM must be secreted from the liver after hepatectomy. A better understanding of the involvement of NAM and Nampt in Pi metabolism will allow for improved management of Pi homeostasis disorders, including postoperative hypophosphatemia. In addition, studies of the NAM metabolism pathway of the liver-kidney axis may contribute to a better understanding of Pi homeostasis.
Concise Methods
Animal Experiments
Male Wistar rats and C57BL6/J mice (aged 8–10 weeks) were purchased from CLEA Japan, Inc. (Tokyo, Japan). TPTX rats were prepared by Japan SLC, Inc. (Shizuoka, Japan).24,36 Rats and mice were maintained under pathogen-free conditions and handled in accordance with the guidelines for animal experimentation of the Tokushima University School of Medicine.
Hepatic Resection Experiments
PH of 8- to 10-week-old male Wistar rats and C57BL6/J mice was performed by surgical excision of the median and left lateral lobes, which comprise approximately 70% of the total liver weight.37 Sham-operated rats and mice were used as controls.
Biochemical Analyses
Heparinized mixed arterial-venous blood was collected and analyzed immediately for pH, blood gases, and electrolytes using an OPTI CCA TS blood gas analyzer (Sysmex Corporation, Kobe, Japan). Plasma and urinary Pi and Ca concentrations were determined using the Phospha-C test or the Calcium-E test (both from Wako Pure Chemical Industries Ltd., Osaka, Japan), respectively.38 Urine creatinine, serum intact FGF23, serum eNampt, iPTH, and plasma 1,25(OH)2D3 were determined using the creatinine-Wako test (Wako), FGF-23 ELISA kit (Kainos Laboratories, Inc., Tokyo, Japan), Nampt (Visfatin/PBEF) (mouse/rat) Dual ELISA Kit (Adipogen Corporation, San Diego, CA), rat iPTH ELISA kit (Immutopics, San Clemente, CA), and the radioreceptor assay (SRL, Inc., Tokyo, Japan), respectively.38 Metabolic cages were used to collect urine samples each hour.39
Gene Expression Studies
Total RNA was isolated from kidney cortex using TRIzol Reagent (Invitrogen, San Diego, CA). For quantitative RT-PCR, total RNA (1 µg) was reverse transcribed with oligo(dT) using Superscript II (Invitrogen, Tokyo, Japan), and samples were analyzed using the ABI PRISM 7500 Real-Time PCR System (Applied Biosystems, Tokyo, Japan). The reaction mixture comprised 10 µl SYBR Premix Ex Taq (Perfect Real Time; Takara, Shiga, Japan) with specific primers. The following primers were used: 5′-CTGCACCACCAACTGCTTAGC-3′ and 5′-CATCCACAGTCTTCTGGGTG-3′ for glyceraldehyde 3-phosphate dehydrogenase, and 5′-TCTGGCCCGAGATGAATG-3′ and 5′-GGGTGGGTATTGTTTATAGTGAGTAAC-3′ for Nampt. The amount of target mRNA was normalized to that of glyceraldehyde 3-phosphate dehydrogenase.
Preparation of BBM Vesicles and Transport Assay
BBM vesicles (BBMVs) were prepared from kidney and jejunum using the Ca2+ precipitation method, and used for immunoblot analysis as previously described.40,41 Levels of leucine aminopeptidase, Na+-K+-ATPase, and cytochrome c oxidase were measured to assess membrane purity. Uptake of 32P into the BBMVs was measured by the rapid filtration technique.40,41
Immunoblot Analyses
Immunoblot analyses were performed using the following primary antibodies. Affinity-purified anti–NaPi-IIa,40 anti–NaPi-IIc,41 anti–NaPi-IIb (for rat BBMVs),42 and anti–NaPi-4,43 were described previously. Anti–NaPi-IIb (for mouse BBMVs; Alpha Diagnostics, San Antonio, TX)44 and anti-Nampt (for rat kidney total lysate or total cell lysate of OK cells; AdipoGen, Incheon, Korea) were used following the manufacturers’ instructions. Mouse anti-actin mAb (Chemicon, Temecula, CA) was used as an internal control. Membranes were exposed to standard X-ray film and the densitometric quantification was performed using ImageJ software (National Institutes of Health, Bethesda, MD). All experiments were repeated at least five times.
NAM and Its Metabolite Analyses
The concentration of tissue and plasma NAD (NAD+ and NADH) was measured by the colorimetric method.45 The amount of NAM, urinary 2-Py, 4-Py, and MNA were determined using high-pressure liquid chromatography as previously described.46
Immunohistochemical Analyses
Immunohistochemical analyses of rat kidney sections were performed as described previously with minor modifications.47 Specimens were embedded in paraffin and subjected to immunohistochemistry for affinity-purified NaPi-IIa antibodies or affinity-purified NaPi-IIc antibodies. Sections were then treated with Envision (+) rabbit peroxidase (Dako, Carpinteria, CA) for 30 minutes at room temperature. Immunoreactivity was detected by incubating the sections with 0.8 mM diaminobenzidine. Nampt and megalin were detected by indirect fluorescence immunohistochemistry using anti-Nampt antibody (GeneTex, Inc., San Antonio, TX) and anti-megalin antibody that was kindly provided by Dr. Saito (Niigata University, Niigata, Japan). Alexa Fluor 568 anti-rabbit or mouse IgG or Alexa Fluor 488 anti-mouse IgG (both Molecular Probes, Eugene, OR) was used as the secondary antibody. We used 4′,6-diamidino-2-phenylindole Fluoromount G (Southern Biotech, Birmingham, UK) to stain cell nuclei.
Cell Culture and Transfection
OK 3B2 cells were kindly provided by Dr. J. Biber (Institute of Physiology, University of Zurich, Zurich, Switzerland) and were maintained in DMEM-F12 (Sigma-Aldrich, Tokyo, Japan) with 10% FBS (Invitrogen) and penicillin/streptomycin (Invitrogen). The cells were grown at 37°C in a humidified environment (5% CO2 and 95% air).43 Before transfection, the cells were plated in six-well dishes. Subconfluent cultures were transfected with 4 µl LipofectAmine2000 (Invitrogen) and 4 µg plasmids according to the manufacturer’s instructions. All subsequent experiments were performed with confluent cells 48 hours after transfection.
At 32 hours after transfection, culture medium was changed to DMEM-F12 with 0.1% BSA. Transfected cells were treated with 10 mM NAM for 4 hours or 10 µM FK866 for 12 hours before collecting cells. Preparation of total cell lysates and crude membranes was performed as previously described.43,48 Biotinylated cell surface proteins were isolated using the Pierce cell surface protein isolation kit (Pierce, Rockford, IL) according to the manufacturer’s instructions and as previously reported with some modifications.43,48
Immunostaining of OK Cells
OK cells were immunostained as previously described.43 OK cells, grown to subconfluence on glass coverslips, and were transfected with constructs expressing rat Nampt cDNA fused with C terminus His or V5 tags. The cells were fixed with 3% paraformaldehyde and permeabilized with 0.05% saponin in PBS. The cells were treated with anti-opossum NaPi-IIa (NaPi-4) affinity-purified antibody,43 followed by Alexa Fluor 488 anti-rabbit IgG or -mouse IgG combined with Alexa Fluor 568-phalloidin (Molecular Probes) to detect actin filaments. Fluorescent images were obtained with a TCS-SL confocal laser-scanning microscope equipped with a ×63 oil-immersion objective (Leica, Wetzlar, Germany).43
Expression of Inducible Nampt in OK Cells
We established an inducible OK cell line in which rat Nampt was stably expressed only after induction with tetracycline/doxycycline. A commercial “Tet-on” kit (Clontech, Palo Alto, CA) was used to express rat Nampt under the control of a doxycycline-inducible promoter in OK cells.43 The Nampt-inducible cell line, OKpTet/Nampt cells, exhibited low background expression of Nampt without doxycycline, and high doxycycline-induced Nampt expression.
Na/Pi Uptake in OK Cells
Na/Pi transport measurement was performed according to a previously described procedure.43,48 Briefly, OKpTet/Nampt cell, were grown in 12-well dishes in the presence or absence of doxycycline. The amount (in nanomoles) of inorganic 32P taken up into the cells during 6 minutes per milligram of total protein was calculated. The experiments were performed in triplicate.
Statistical Analyses
Data are presented as the mean±SEM. Statistical analysis was performed using unpaired the t test or ANOVA followed by Dunnett’s test. P values<0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Masanori Yasugi, Ai Niimi, Megumi Kiyota, Atsuko Takata, Ryoma Sugeoi, Ayako Yoshimi, Natsuki Oka, Miyuki Fujita, and Tatsuya Kamatani for technical assistance, and to Hideaki Horikawa (The University of Tokushima Graduate School) for support in microarray analysis, and to Dr. Masayuki Shono (The University of Tokushima Graduate School) for support in immunocytochemical analysis.
This study was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grants-in-Aid for Scientific Research on Innovative Areas 23136511 and 25136715; Grants 23591218 and 20590976 to S.T. and 23390226 and 23650480 to K.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013060569/-/DCSupplemental.
References
- 1.Murer H, Hernando N, Forster I, Biber J: Proximal tubular phosphate reabsorption: Molecular mechanisms. Physiol Rev 80: 1373–1409, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto K, Haito-Sugino S, Kuwahara S, Ohi A, Nomura K, Ito M, Kuwahata M, Kido S, Tatsumi S, Kaneko I, Segawa H: Sodium-dependent phosphate cotransporters: Lessons from gene knockout and mutation studies. J Pharm Sci 100: 3719–3730, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Berndt T, Kumar R: Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 69: 341–359, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Quarles LD: Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118: 3820–3828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98: 6500–6505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S, Quarles LD: How fibroblast growth factor 23 works. J Am Soc Nephrol 18: 1637–1647, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Jüppner H, Wolf M, Salusky IB: FGF-23: More than a regulator of renal phosphate handling? J Bone Miner Res 25: 2091–2097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White SA, Al-Mukhtar A, Lodge JP, Pollard SG: Progress in living donor liver transplantation. Transplant Proc 36: 2720–2726, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Buell JF, Berger AC, Plotkin JS, Kuo PC, Johnson LB: The clinical implications of hypophosphatemia following major hepatic resection or cryosurgery. Arch Surg 133: 757–761, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Pomposelli JJ, Pomfret EA, Burns DL, Lally A, Sorcini A, Gordon FD, Lewis WD, Jenkins R: Life-threatening hypophosphatemia after right hepatic lobectomy for live donor adult liver transplantation. Liver Transpl 7: 637–642, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Datta HK, Malik M, Neely RD: Hepatic surgery-related hypophosphatemia. Clin Chim Acta 380: 13–23, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Giovannini I, Chiarla C, Nuzzo G: Pathophysiologic and clinical correlates of hypophosphatemia and the relationship with sepsis and outcome in postoperative patients after hepatectomy. Shock 18: 111–115, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Salem RR, Tray K: Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg 241: 343–348, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nafidi O, Lapointe RW, Lepage R, Kumar R, D’Amour P: Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg 249: 824–827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katai K, Tanaka H, Tatsumi S, Fukunaga Y, Genjida K, Morita K, Kuboyama N, Suzuki T, Akiba T, Miyamoto K, Takeda E: Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant 14: 1195–1201, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Kempson SA, Colon-Otero G, Ou SY, Turner ST, Dousa TP: Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest 67: 1347–1360, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu KI, Bacon RA, Al-Mahrouq HA, Kempson SA: Nicotinamide as a rapid-acting inhibitor of renal brush-border phosphate transport. Am J Physiol 255: F15–F21, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Tanaka A, Nakamura T, Fukuwatari T, Shibata K, Shimada N, Ebihara I, Koide H: Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int 65: 1099–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Martin PR, Shea RJ, Mulks MH: Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J Bacteriol 183: 1168–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revollo JR, Grimm AA, Imai S: The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279: 50754–50763, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Zhang LQ, Heruth DP, Ye SQ: Nicotinamide phosphoribosyltransferase in human diseases. J Bioanal Biomed 3: 13–25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai S: The NAD World: A new systemic regulatory network for metabolism and aging—Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys 53: 65–74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai S: From heterochromatin islands to the NAD World: A hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim Biophys Acta 1790: 997–1004, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K: Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol 292: F395–F403, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Shibata K, Kakehi H, Matsuo H: Niacin catabolism in rodents. J Nutr Sci Vitaminol (Tokyo) 36: 87–98, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Collins PB, Chaykin S: The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem 247: 778–783, 1972 [PubMed] [Google Scholar]
- 27.Bender DA, Olufunwa R: Utilization of tryptophan, nicotinamide and nicotinic acid as precursors for nicotinamide nucleotide synthesis in isolated rat liver cells. Br J Nutr 59: 279–287, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Ferris GM, Clark JB: Nicotinamide nucleotide synthesis in regenerating rat liver. Biochem J 121: 655–662, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris GM, Clark JB: The control of nucleic acid and nicotinamide nucleotide synthesis in regenerating rat liver. Biochem J 128: 869–877, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai S: Dissecting systemic control of metabolism and aging in the NAD World: The importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett 585: 1657–1662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittelli M, Formentini L, Faraco G, Lapucci A, Rapizzi E, Cialdai F, Romano G, Moneti G, Moroni F, Chiarugi A: Inhibition of nicotinamide phosphoribosyltransferase: Cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem 285: 34106–34114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasmann M, Schemainda I: FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res 63: 7436–7442, 2003 [PubMed] [Google Scholar]
- 33.Dousa TP: Modulation of renal Na-Pi cotransport by hormones acting via genomic mechanism and by metabolic factors. Kidney Int 49: 997–1004, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Tenenhouse HS, Chu YL: Hydrolysis of nicotinamide-adenine dinucleotide by purified renal brush-border membranes. Mechanism of NAD+ inhibition of brush-border membrane phosphate-transport activity. Biochem J 204: 635–638, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell PI, al-Mahrouq HA, Abraham MI, Kempson SA: Specific inhibition of rat renal Na+/phosphate cotransport by picolinamide. J Pharmacol Exp Ther 251: 188–192, 1989 [PubMed] [Google Scholar]
- 36.Takahashi F, Morita K, Katai K, Segawa H, Fujioka A, Kouda T, Tatsumi S, Nii T, Taketani Y, Haga H, Hisano S, Fukui Y, Miyamoto KI, Takeda E: Effects of dietary Pi on the renal Na+-dependent Pi transporter NaPi-2 in thyroparathyroidectomized rats. Biochem J 333: 175–181, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins GM, Anderson RM: Experimental pathology of the liver: I. Restoration of the liver in the white rat following partial remova. Arch Pathol (Chic) 12: 186–202, 1931 [Google Scholar]
- 38.Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K: Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J 390: 325–331, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K: Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol 20: 104–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katai K, Segawa H, Haga H, Morita K, Arai H, Tatsumi S, Taketani Y, Miyamoto K, Hisano S, Fukui Y, Takeda E: Acute regulation by dietary phosphate of the sodium-dependent phosphate transporter (NaP(i)-2) in rat kidney. J Biochem 121: 50–55, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K: Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277: 19665–19672, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K: Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol 287: F39–F47, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Ito M, Sakurai A, Hayashi K, Ohi A, Kangawa N, Nishiyama T, Sugino S, Uehata Y, Kamahara A, Sakata M, Tatsumi S, Kuwahata M, Taketani Y, Segawa H, Miyamoto K: An apical expression signal of the renal type IIc Na+-dependent phosphate cotransporter in renal epithelial cells. Am J Physiol Renal Physiol 299: F243–F254, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Ohi A, Hanabusa E, Ueda O, Segawa H, Horiba N, Kaneko I, Kuwahara S, Mukai T, Sasaki S, Tominaga R, Furutani J, Aranami F, Ohtomo S, Oikawa Y, Kawase Y, Wada NA, Tachibe T, Kakefuda M, Tateishi H, Matsumoto K, Tatsumi S, Kido S, Fukushima N, Jishage K, Miyamoto K: Inorganic phosphate homeostasis in sodium-dependent phosphate cotransporter Npt2b⁺/⁻ mice. Am J Physiol Renal Physiol 301: F1105–F1113, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Fukuwatari T, Morikawa Y, Hayakawa F, Sugimoto E, Shibata K: Influence of adenine-induced renal failure on tryptophan-niacin metabolism in rats. Biosci Biotechnol Biochem 65: 2154–2161, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Shibata K, Kawada T, Iwai K: Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J Chromatogr A 424: 23–28, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K: Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol 292: F769–F779, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Haito-Sugino S, Ito M, Ohi A, Shiozaki Y, Kangawa N, Nishiyama T, Aranami F, Sasaki S, Mori A, Kido S, Tatsumi S, Segawa H, Miyamoto K: Processing and stability of type IIc sodium-dependent phosphate cotransporter mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria. Am J Physiol Cell Physiol 302: C1316–C1330, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.