Abstract
Because of the shortage of agalsidase-beta in 2009, many patients with Fabry disease were treated with lower doses or were switched to agalsidase-alfa. This observational study assessed end-organ damage and clinical symptoms during dose reduction or switch to agalsidase-alfa. A total of 105 adult patients with Fabry disease who had received agalsidase-beta (1.0 mg/kg body weight) for ≥1 year were nonrandomly assigned to continue this treatment regimen (regular-dose group, n=38), receive a reduced dose of 0.3–0.5 mg/kg (dose-reduction group, n=29), or switch to 0.2 mg/kg agalsidase-alfa (switch group) and were followed prospectively for 1 year. We assessed clinical events (death, myocardial infarction, severe arrhythmia, stroke, progression to ESRD); changes in cardiac, renal, and neurologic function; and Fabry-related symptoms (neuropathic pain, hypohidrosis, diarrhea, and disease severity scores). Organ function and Fabry-related symptoms remained stable in the regular-dose group. In contrast, estimated GFR decreased by about 3 ml/min per 1.73 m2 (P=0.01) in the dose-reduction group, and the median albumin-to-creatinine ratio increased from 114 (0–606) mg/g to 216 (0–2062) mg/g (P=0.03) in the switch group. Furthermore, mean Mainz Severity Score Index scores and frequencies of pain attacks, chronic pain, gastrointestinal pain, and diarrhea increased significantly in the dose-reduction and switch groups. In conclusion, patients receiving regular agalsidase-beta dose had a stable disease course, but dose reduction led to worsening of renal function and symptoms. Switching to agalsidase-alfa is safe, but microalbuminuria may progress and Fabry-related symptoms may deteriorate.
Fabry disease (FD) is an X-linked rare progressive multisystemic disorder resulting from lysosomal enzyme α-galactosidase A deficiency.1 α-Galactosidase A deficiency leads to cellular accumulation of globotriaosylceramide (GL-3/Gb3) in different tissues.2 Renal failure, cardiomyopathy, and peripheral and central nervous system alterations are the main causes of morbidity and reduced life expectancy.3 In Europe, two different compounds of enzyme replacement therapy (ERT) are available for treatment: agalsidase-alfa (Replagal [Shire], 0.2 mg/kg body weight intravenously every other week) and agalsidase-beta (Fabrazyme [Genzyme], 1.0 mg/kg intravenously every other week). Randomized controlled trials and clinical studies showed the safety and efficacy of both ERT compounds in patients with FD,4–9 and >4000 patients worldwide currently receive ERT. An extended shortage in agalsidase-beta supply (since June 2009) resulted in a change of treatment regimen in many patients: Either the agalsidase-beta dose was reduced (0.3–0.5 mg/kg every other week) or the patient was switched to agalsidase-alfa. Very limited clinical data are available for patients who were switched from one compound to another.10–13 Recently, an observational study in a small Japanese group of 11 patients analyzed the clinical outcome after switch from agalsidase-beta to agalsidase-alfa.10 However, the long-term effect of a dose reduction of agalsidase-beta or a switch to agalsidase-alfa on disease progression in a large, well characterized patient cohort remains elusive. The current study assessed clinical stability and safety during ERT dose reduction (agalsidase-beta, 0.3 or 0.5 mg/kg every other week) or switch to agalsidase-alfa (0.2 mg/kg) by investigating clinical events, end-organ damage, and disease-related clinical symptoms.
Results
Patients
Data completeness for general FD data and cardiac, renal, and neurologic assessment is shown in Figure 1. A comprehensive diagnostic work-up, as detailed in Table 1, was performed in all patients at each visit; this included medical history and cardiac, renal, and neurologic evaluation (for further information, see Concise Methods). The characteristics of all patients with FD and the three subgroups at the baseline visit are presented in Table 2. In total, 105 patients with FD were included in the study. The mean age±SD was 45.3±12.8 years, and 40.9% of patients were female. On average, patients had been receiving agalsidase-beta, 1.0 mg/kg every other week, for 39±29 months at baseline. Overall, 22.6% of patients were receiving dialysis (15%) or had undergone kidney transplantation (7.6%), 6.7% had had a stroke or a transient ischemic attack, and 61% already had fibrosis in the left ventricle (late enhancement positive).
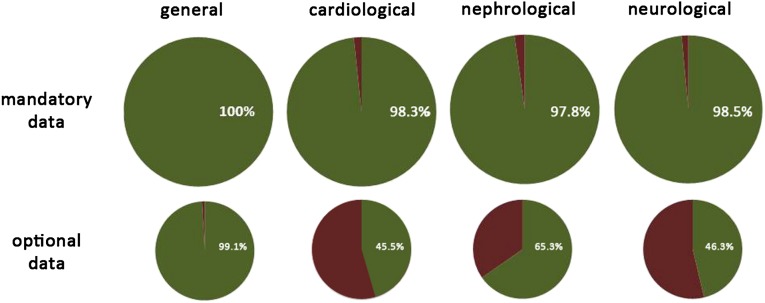
Figure 1.
Overview of data completeness for general FD data and cardiac, renal, and neurologic assessment. The upper row shows the mandatory data and the lower row the optional data (green, data available; red, data not available).
Table 1.
Clinical work-up performed at each visit
| Organ/Domain | Investigation/Measure | Patients Receiving Work-Up (%) |
|---|---|---|
| Medical history (mandatory) | Date of birth, date of medical check-up, time of ERT start, enzyme compound, dose of ERT, frequency of ERT, changes in ERT dose, switch of enzyme compound, infusion rate, premedication, symptoms (diarrhea, abdominal pain, hypohidrosis, cornea verticillata, tinnitus, hearing loss, FD crisis, fatigue, chronic neuropathic pain, pain attacks, TIA, stroke, dyspnea, stress dyspnea, NYHA class, myocardial infarction, severe arrhythmia, pacemaker implantation), onset of symptoms, family history, current medication, dose of current medication | 100 |
| Physical examination (mandatory) | Routine examination, height, weight, BP, heart rate, angiokeratoma | 99.1 |
| Cardiology | ||
| Mandatory | Electrocardiography, echocardiography (LVEDD, LVESD, LVSd, LVPWd, EF, diastolic function) | 98.3 |
| Optional | Cardiac MRI (standard, late enhancement imaging) | 45.5 |
| Neurology | ||
| Mandatory | Examination, history, polyneuropathy tests | 98.5 |
| Optional | Pain severity scores (CES-D, GCPS), quantitative sensoneurologic testing, biopsies (IEFND) | 46.3 |
| Nephrology | ||
| Mandatory | Albuminuria (albumin-to-creatinine ratio), eGFR (CKD-EPI), kidney transplantation, urine analysis | 97.8 |
| Optional | Cystatin C–based GFR | 65.3 |
| Laboratory | ||
| Mandatory | Enzyme activity, FD mutation, complete setting (including BNP, hemoglobin, electrolytes, cell count, cholesterol) | 100 |
| Disease severity scores (mandatory) | DS3, MSSI | 100 |
| Other disciplines (optional) | Otorhinolaryngology, dermatology, ophthalmology | 60.1 |
TIA, transient ischemic attack; NYHA, New York Heart Association; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVSd, left ventricular septum in diastole; LVPWd, left ventricular posterior wall in diastole; EF, ejection fraction; MRI, magnetic resonance imaging; CES-D, Center for Epidemiologic Studies-Depression scale; IEFND, intraepidermal nerve fiber density; CKD-EPI, CKD–Epidemiology Collaboration; BNP, brain natriuretic peptide; DS3, Disease Severity.
Table 2.
Baseline data for all patients receiving agalsidase-beta, 1.0 mg/kg every other week
| Variable | Agalsidase-Beta Regular- Dose Group (ERT, 1.0 mg/kg) | Agalsidase-Beta Dose-Reduction Group (ERT, 0.5 mg/kg) | Agalsidase-Alfa Switch Group (ERT, 0.2 mg/kg) | Total |
|---|---|---|---|---|
| Patients (n) | 38 | 29 | 38 | 105 |
| Women | 12 (31.6) | 10 (34.5) | 21 (55.3)a,b | 43 (40.9) |
| Age (yr) | 46.1±12.4 | 45.1±13.7 | 44.7±12.9 | 45.3±12.8 |
| Weight (kg) | 71.9±14.2 | 73.9±13.3 | 75.2±14.7 | 73.7±14.0 |
| Height (cm) | 174.9±8.4 | 173.7±8.9 | 170.5±10.8 | 173.0±9.5 |
| Heart rate (beats/min) | 65.7±11.1 | 63.9±10.1 | 66.9±10.4 | 65.6±10.5 |
| Systolic BP (mmHg) | 119.1±11.5 | 120.7±13.5 | 120.3±12.2 | 119.9±12.3 |
| Diastolic BP (mmHg) | 79.6±7.5 | 81.1±10.6 | 80.9±8.3 | 80.5±8.8 |
| Nonclassic mutation | 1 (2.6) | 0 | 1 (2.6) | 2 (1.9) |
| α-Galactosidase A activity (nmol/mg per ml)c | 0.06±0.07 | 0.06±0.09b | 0.12±0.12a,b | 0.08±0.09 |
| α-Galactosidase A activity (nmol/mg per ml) in menc | 0.03±0.01 | 0.03±0.01 | 0.02±0.01 | 0.03±0.01 |
| Patients receiving RAAS blocker | 13 (34) | 17 (58) | 9 (24) | 39 (37) |
| Patients receiving analgesic drugs | 7 (18) | 8 (28) | 8 (21) | 23 (22) |
| Duration of ERT (mo) | 31±24 | 45±25d | 41±33 | 39±29 |
| Clinical presentation | ||||
| Angiokeratoma | 21 (55) | 15 (52) | 15 (41) | 51 (49) |
| Edema | 11 (29) | 4 (14) | 8 (21) | 23 (22) |
| Gastrointestinal pain | 8 (21) | 6 (21) | 5 (13) | 19 (18) |
| Diarrhea (d/mo) | 1.5±5.1 | 2.0±5.7 | 0.8±1.7 | 1.4±4.4 |
| Hypohidrosis | 25 (66) | 22 (76) | 18 (47)b | 65 (62) |
| Cornea verticillata | 11 (29) | 12 (43) | 19 (50)a | 54 (40) |
| Tinnitus | 13 (34) | 7 (24) | 10 (26) | 30 (29) |
| Hypacusis | 8 (21) | 11 (38) | 6 (16) | 25 (24) |
| Paresthesia | 23 (61) | 18 (64) | 22 (58) | 63 (60) |
| Pain attacks | 6 (16) | 6 (21) | 3 (8.1) | 15 (14) |
| Chronic pain | 15 (40) | 15 (52) | 9 (24)b | 39 (37) |
| Pain crises | 8 (21) | 5 (17) | 7 (18) | 20 (19) |
| TIA/stroke | 4 (11) | 1 (3.4) | 2 (5.3) | 7 (6.7) |
| Fatigue | 6 (16) | 8 (28) | 11 (29) | 25 (24) |
| Disease severity scores | ||||
| DS3 | 13.5±8.3 | 12.2±8.4 | 14.6±8.4 | 13.5±8.3 |
| MSSI | 20.5±11.6 | 21.8±10.5 | 18.1±9.8 | 19.9±10.6 |
| Cardiac measures | ||||
| Dyspnea | 1 (2.6) | 0 | 0 | 1 (1) |
| NYHA class | ||||
| I | 19 (50) | 16 (55) | 24 (63) | 59 (56) |
| II | 12 (32) | 7 (24) | 8 (23) | 27 (27) |
| III | 6 (16) | 6 (21) | 3 (9) | 15 (15) |
| IV | 1 (2.6) | 0 | 0 | 1 (1) |
| LV diastolic diameter (mm) | 48.1±7.9a | 49.5±5.1 | 50.2±5.5 | 47.8±6.5 |
| LV systolic diameter (mm) | 30.7±6.6 | 31.2±6.7 | 33.0±7.7 | 30.0±6.4 |
| Septal diameter (mm) | 12.9±3.1 | 12.8±3.6 | 12.5±3.2 | 12.8±3.0 |
| Posterior wall diameter (mm) | 12.5±2.1 | 11.2±2.8d | 12.8±2.4 | 12.2±2.5 |
| Ejection fraction (%) | 63.1±8.3 | 61.3±7.1 | 61.5±7.4 | 62.0±7.6 |
| Myocardial mass (g/m2) | 95.7±35.8 | 91.5±34.7 | 82.8±26.9a,b | 90.3±32.6 |
| Diastolic function | ||||
| Normal | 18 (56) | 15 (60) | 15 (48) | 48 (46) |
| Relaxation abnormality | 9 (28) | 5 (20) | 9 (29) | 23 (22) |
| Pseudo-normal | 4 (13) | 4 (16) | 7 (23) | 15 (14) |
| Restriction | 1 (3.1) | 1 (3.4) | 0 | 2 (1.9) |
| ECG abnormalities | 10 (28) | 9 (31) | 7 (21) | 26 (26) |
| Pacemaker | 5 (13) | 4 (14) | 1 (2.8) | 10 (9.7) |
| Heart transplant | 0 | 0 | 0 | 0 |
| MRI late enhancement | 9 (60) | 11 (61) | 8 (62) | 28 (61) |
| Renal measures | ||||
| Albumin-to-creatinine-ratio (mg/g creatinine)c | 173 (0–375) | 273 (0–1010) | 114 (0–606) | 205 (0–1010) |
| Cystatin C GFR (ml/min per 1.73m2)c | 104 (18–158) | 109 (65–132) | 101 (52–151) | 103 (18–158) |
| eGFR (CKD-EPI) (ml/min per 1.73m2)c | 99 (63–119) | 98 (86–114) | 98 (66–112) | 99 (63–119) |
| Creatinine (mg/dl)c | 0.89 (0.50–3.40) | 0.80 (0.60–1.20) | 0.87 (0.60–2.30) | 0.90 (0.50–3.40) |
| Hemoglobin (mg/dl) | 13.8±1.2 | 13.6±1.2 | 13.4±1.3 | 13.6±1.2 |
| Dialysis | 6 (16) | 5 (17) | 5 (13) | 16 (15) |
| Kidney transplant | 1 (2.6) | 5 (17) | 3 (8) | 8 (7.6) |
| Neurologic measures | ||||
| CES-D score | 18.6±10.1 | 15.3±12.4 | 15.0±8.7 | 16.2±10.5 |
| GCPS 2 (maximum pain) | 4.3±3.7 | 4.1±2.6 | 4.0±3.3 | 4.1±3.1 |
| GCPS 5 (impairment) | 2.2±2.8 | 2.0±2.9 | 2.2±3.2 | 2.1±2.9 |
| NPSI sum score | 0.18±0.25 | 0.19±0.25 | 0.13±0.11 | 0.17±0.21 |
| Sural nerve | ||||
| SNAP (µV) | 16.2±8.5 | 18.4±11.0 | 17.4±12.9 | 17.3±10.6 |
| NCV (m/s) | 43.1±4.1 | 44.6±5.9 | 42.9±8.0 | 43.5±6.0 |
| CDT | −16.2±7.1 | −16.6±7.7 | −11.5±8.2 | −14.9±7.8 |
Categorical data are presented as number (percentage) of patients. Data expressed with a plus/minus sign are the mean±SD. RAAS, renin-angiotensin-aldosterone system; TIA, transient ischemic attack; DS3, Disease Severity; NYHA, New York Heart Association; LV, left ventricular; ECG, electrocardiographic; MRI, magnetic resonance imaging; CKD-EPI, CKD–Epidemiology Collaboration; CES-D, Center for Epidemiologic Studies-Depression scale; SNAP, sensory nerve action potential; NCV, nerve conduction velocity; CDT, cold detection threshold.
P<0.05 for agalsidase-beta, 1 mg/kg, versus agalsidase-alfa, 0.2 mg/kg, every other week.
P<0.05 for agalsidase-beta, 0.5 mg/kg, versus agalsidase-alfa, 0.2 mg/kg, every other week.
Expressed as median (range).
P<0.05 for agalsidase-beta, 1 mg/kg versus 0.5 mg/kg every other week.
Owing to the observational design, patients were not randomly assigned. The long-term treatment strategy was chosen by a team of physicians specializing in FD and by the patient. This strategy resulted in inhomogeneous treatment groups in which more severely affected patients with FD were in the stable agalsidase-beta groups and more mildly affected patients were in the agalsidase-alfa group. More specifically, compared with the agalsidase-beta regular-dose group (n=38), patients in the switch group (n=38) were more often female (55.3% versus 31.6%), had higher α-galactosidase A activity (0.12±0.12 nmol/mg per ml versus 0.06±0.07 nmol/mg per ml), had a lower myocardial mass (82.8±26.9 g/m2 versus 95.7±35.8 g/m2), and experienced chronic neuropathic pain less often (24% versus 40%). Patients in the dose-reduction group (n=29) had been receiving ERT for a longer time than the agalsidase-beta regular-dose group (45±25 months versus 31±24 months) (Table 2).
Outcome for Clinical Events
During a median follow-up of 12 (range, 8–16) months (agalsidase-beta regular-dose group: 11 [range, 8–15] months; dose-reduction group: 12 [range, 8–16] months; and switch group: 12 [range, 8–16] months), no patient developed a clinical event. In one patient with CKD stage 4 (estimated GFR [eGFR] at retrospective visit, 22 ml/min per 1.73 m2) from the agalsidase-beta regular-dose group, kidney replacement therapy was initiated 4 weeks after the baseline visit.
Outcome for Change in Organ Function or Structure
The changes in cardiac, renal, and neurologic measures are shown in Tables 3–6. In all three groups, the predefined measures for organ function were stable between the retrospective visit and the baseline visit on agalsidase-beta 1 mg/kg every other week. In the agalsidase-beta regular-dose group (1 mg/kg every other week), cardiac, renal, and neurologic organ function did not change significantly (Tables 3–6).
Table 3.
Changes in cardiac measures
| Measure | 1-Year Retrospective Visit | P Value | Baseline Visit | P Value | 1-Year Follow-Up Visit |
|---|---|---|---|---|---|
| Agalsidase-beta regular-dose group | |||||
| Dyspnea, n (%) | 1 (2.6) | 1.00 | 1 (2.6) | 1.00 | 1 (2.6) |
| LV diastolic diameter (mm) | 47.1±6.4 | 0.54 | 48.1±7.9 | 0.16 | 47.3±7.3 |
| LV systolic diameter (mm) | 30.6±5.0 | 0.71 | 30.7±6.6 | 0.44 | 31.7±7.2 |
| Septal diameter (mm) | 12.8±2.6 | 0.99 | 12.9±3.1 | 0.08 | 13.1±3.3 |
| Posterior wall (mm) | 12.2±2.3 | 0.89 | 12.5±2.1 | 0.27 | 12.8±2.9 |
| Ejection fraction (%) | 64.5±6.6 | 0.86 | 63.1±8.3 | 0.27 | 59.1±8.9 |
| Myocardial mass (g/m2) | 107.2±38.6 | 0.77 | 95.7±35.8 | 0.94 | 86.3±24.9 |
| ECG abnormalities, n (%) | 10 (26) | 1.00 | 10 (26) | 1.00 | 9 (24) |
| Pacemaker, n (%) | 4 (11) | 1.00 | 5 (13) | 1.00 | 5 (13) |
| Heart transplant (n) | 0 | — | 0 | — | 0 |
| MRI late enhancement, n (%) | 9 (60) | 1.00 | 9 (60) | 1.00 | 11 (69) |
| Agalsidase-beta dose-reduction group | |||||
| Dyspnea, n (%) | 0 | — | 0 | — | 0 |
| LV diastolic diameter (mm) | 48.1±7.9 | 0.07 | 49.5±5.1 | 0.35 | 50.2±5.5 |
| LV systolic diameter (mm) | 30.7±6.6 | 0.71 | 31.2±6.7 | 0.19 | 33.0±7.7 |
| Septal diameter (mm) | 12.9±3.1 | 0.12 | 12.8±3.6 | 0.41 | 12.5±3.2 |
| Posterior wall diameter (mm) | 12.5±2.1 | 0.08 | 11.2±2.8 | 0.52 | 11.0±3.0 |
| Ejection fraction (%) | 63.1±8.3 | 0.48 | 61.3±7.1 | 0.10 | 56.4±9.8 |
| Myocardial mass (g/m2) | 95.7±35.8 | 0.63 | 91.5±34.7 | 0.69 | 87.1±21.8 |
| ECG abnormalities, n (%) | 9 (31) | 1.00 | 9 (31) | 0.38 | 12 (41) |
| Pacemaker, n (%) | 2 (7) | 0.50 | 4 (14) | 1.00 | 4 (14) |
| Heart transplant, n (%) | 0 | — | 0 | — | 0 |
| MRI late enhancement, n (%) | 7 (54) | 0.33 | 11 (61) | 1.00 | 11 (61) |
| Agalsidase-alfa switch group | |||||
| Dyspnea, n (%) | 0 | — | 0 | — | 0 |
| LV diastolic diameter (mm) | 47.4±5.9 | 0.16 | 50.2±5.5 | 0.23 | 47.2±7.2 |
| LV systolic diameter (mm) | 29.2±5.4 | 0.16 | 33.0±7.7 | 0.17 | 30.0±4.9 |
| Septal diameter (mm) | 13.2±2.8 | 0.17 | 12.5±3.2 | 0.05 | 12.3±2.5 |
| Posterior wall diameter (mm) | 12.7±2.2 | 0.75 | 12.8±2.4 | 0.13 | 12.2±2.9 |
| Ejection fraction (%) | 64.6±6.6 | 0.13 | 61.5±7.4 | 0.48 | 61.1±8.2 |
| Myocardial mass (g/m2) | 78.6±16.3 | 0.52 | 82.8±26.9 | 0.26 | 80.3±14.7 |
| ECG abnormalities, n (%) | 4 (11) | 0.25 | 7 (18) | 1.00 | 7 (18) |
| Pacemaker, n (%) | 1 (2.6) | 1.00 | 1 (2.6) | 1.00 | 3 (8) |
| Heart transplant, n (%) | 0 | — | 0 | — | 0 |
| MRI late enhancement, n (%) | 7 (50) | 0.50 | 8 (62) | 1.00 | 9 (64) |
Categorical data are presented as number (percentage) of patients. Otherwise, data are presented as mean±SD. LV, left ventricular; ECG, electrocardiographic; MRI, magnetic resonance imaging.
Table 6.
Renal measures by sexes
| Measure | 1-Year Retrospective Visit | P Value | Baseline Visit | P Value | 1-Year Follow-Up Visit |
|---|---|---|---|---|---|
| Agalsidase-beta regular-dose group | |||||
| Men | |||||
| Hyperfiltration (n) | 1 | 1 | 3 | ||
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | 99 (64–120) | 0.55 | 101 (63–119) | 0.69 | 101 (58–120) |
| Albumin-to-creatinine-ratio (mg/g creatinine) | 47 (6–142) | 0.38 | 61 (0–158) | 0.33 | 66 (5–231) |
| Women | |||||
| Hyperfiltration (n) | 1 | 1 | 1 | ||
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | 93 (72–118) | 0.61 | 91 (71–117) | 0.13 | 99 (75–119) |
| Albumin-to-creatinine-ratio (mg/g creatinine) | 183 (4–369) | 0.24 | 319 (0–375) | 0.21 | 212 (97–283) |
| Agalsidase-beta dose-reduction group | |||||
| Men | |||||
| Hyperfiltration (n) | 3 | 2 | 2 | ||
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | 94 (61–116) | 0.49 | 102 (86–112) | 0.02 | 99 (82–114) |
| Albumin-to-creatinine-ratio (mg/g creatinine) | 437 (0–1807) | 0.18 | 272 (0–963) | 0.95 | 175 (0–1420) |
| Women | |||||
| Hyperfiltration (n) | 1 | 1 | 1 | ||
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | 94 (84–112) | 0.16 | 95 (86–114) | 0.05 | 92 (82–110) |
| Albumin-to-creatinine-ratio (mg/g creatinine) | 50 (23–854) | 0.78 | 273 (10–1010) | 0.17 | 293 (20–1222) |
| Agalsidase-alfa switch group | |||||
| Men | |||||
| Hyperfiltration (n) | 2 | 0 | 2 | ||
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | 103 (79–123) | 0.70 | 99 (71–112) | 0.19 | 97 (64–101) |
| Albumin-to-creatinine-ratio (mg/g creatinine) | 131 (0–830) | 0.87 | 152 (0–606) | 0.04 | 199 (0–1131) |
| Women | |||||
| Hyperfiltration (n) | 0 | 0 | 0 | ||
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | 98 (68–112) | 0.25 | 99 (66–112) | 0.31 | 93 (68–116) |
| Albumin-to-creatinine-ratio (mg/g creatinine) | 40 (10–1292) | 0.28 | 79 (15–344) | 0.03 | 209 (3–2062) |
Categorical data are presented as numbers of patients. Otherwise data are presented as median (range). For eGFR analyses, all patients with hyperfiltration (125–175 ml/min per 1.73 m2) and undergoing dialysis were excluded. CKD-EPI, CKD–Epidemiology Collaboration.
Table 5.
Changes in neurologic measures
| Measure | 1-Year Retrospective Visit | P Value | Baseline Visit | P Value | 1-Year Follow-Up Visit |
|---|---|---|---|---|---|
| Agalsidase-beta regular-dose group | |||||
| CES-D score | 19.8±9.8 | 0.92 | 18.6±10.1 | 0.45 | 16.1±11.6 |
| GCPS 2 (maximum pain) | 5.1±3.8 | 0.43 | 4.3±3.7 | 0.74 | 4.2±3.6 |
| GCPS 5 (impairment) | 2.7±2.6 | 0.37 | 2.2±2.8 | 0.45 | 2.3±3.3 |
| NPSI sum score | 0.18±0.21 | 0.74 | 0.18±0.25 | 0.86 | 0.21±0.25 |
| Sural nerve | |||||
| SNAP (n=11) (µV) | 13.8±6.5 | 0.38 | 16.2±8.5 | 0.86 | 15.1±7.2 |
| NCV (m/s) | 41.0±2.9 | 0.44 | 43.1±4.1 | 0.65 | 43.2±5.1 |
| CDT | −15.7±6.2 | 0.20 | −16.2±7.1 | 0.57 | −13.4±7.8 |
| Agalsidase-beta dose-reduction group | |||||
| CES-D score | 15.0±9.7 | 0.05 | 15.3±12.4 | 0.91 | 20.8±10.6 |
| GCPS 2 (maximum pain) | 5.1±2.9 | 0.49 | 4.1±2.6 | 0.67 | 5.3±2.8 |
| GCPS 5 (impairment) | 1.5±1.9 | 0.08 | 2.0±2.9 | 0.85 | 3.6±2.6 |
| NPSI sum score | 0.20±0.18 | 0.54 | 0.19±0.25 | 0.25 | 0.40±0.24 |
| Sural nerve | |||||
| SNAP (n=12) (µV) | 15.5±9.1 | 0.49 | 18.4±11.0 | 0.64 | 15.5±13.8 |
| NCV (m/s) | 45.0±4.3 | 0.72 | 44.6±5.9 | 0.19 | 46.2±5.4 |
| CDT | −14.7±7.8 | 0.90 | −16.6±7.7 | 0.12 | −14.9±8.6 |
| Agalsidase-alfa switch group | |||||
| CES-D score | 17.4±12.6 | 0.25 | 15.0±8.7 | 0.23 | 17.7±12.6 |
| GCPS 2 (maximum pain) | 6.9±2.5 | 0.08 | 4.0±3.3 | 0.59 | 4.4±3.0 |
| GCPS 5 (impairment) | 4.4±3.2 | 0.20 | 2.2±3.2 | 0.46 | 2.4±3.3 |
| NPSI sum score | 0.22±0.02 | 0.10 | 0.13±0.12 | 0.19 | 0.21±0.19 |
| Sural nerve | |||||
| SNAP (n=15) (µV) | 19.2±12.8 | 0.74 | 17.4±12.9 | 0.95 | 16.5±8.3 |
| NCV (m/s) | 46.2±6.4 | 0.07 | 42.8±8.1 | 0.20 | 45.9±6.3 |
| CDT | −10.5±6.2 | 0.32 | −11.5±8.2 | 0.68 | −10.4±6.5 |
Data are presented as mean±SD. CES-D, Center for Epidemiologic Studies-Depression Scale; SNAP, sensory nerve action potential; NCV, nerve conduction velocity; CDT, cold detection threshold.
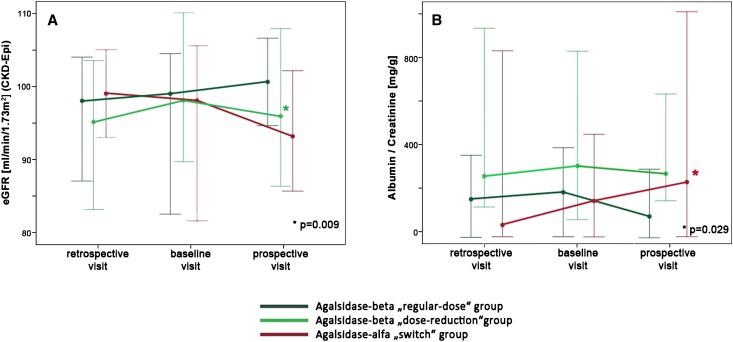
However, renal function determined by eGFR decreased significantly in the agalsidase-beta dose-reduction group by about 3 ml/min per 1.73 m2 between baseline and follow-up visit (P=0.01) (Figure 2A, Table 4). The GFR decrease remained significant in men and women when those groups were analyzed separately (P=0.02 and P=0.05, respectively) (Table 6). In the switch group, microalbuminuria values were higher, with an albumin-to-creatinine ratio of 114 [range, 0–606] mg/g at baseline and 216 [range, 0–2062] mg/g at follow-up (P=0.03) (Figure 2B, Table 4). When the sexes were analyzed separately, the albumin-to-creatinine ratio increased significantly in both women and men (P=0.03 and P=0.04, respectively) (Table 6).
Figure 2.
Decrease in renal function under agalsidase-beta dose reduction and switch to agalsidase-alfa. Change in renal function quantified by (A) eGFR (calculated with the CKD–Epidemiology Collaboration formula) and (B) albuminuria (albumin-to-creatinine ratio) from spot urine for the three visits. Appropriate values are given as medians (95% confidence intervals). Asterisks mark significant changes between baseline and prospective visits. P values are given in the figures.
Table 4.
Changes in renal measures
| Measure | 1-Year Retrospective Visit | P Value | Baseline Visit | P Value | 1-Year Follow-Up Visit |
|---|---|---|---|---|---|
| Agalsidase-beta regular-dose group | |||||
| Albumin-to-creatinine ratio (mg/g creatinine) (n=35) | 155 (4–369) | 0.80 | 173 (0–375) | 0.46 | 97 (5–283) |
| Cystatin C GFR (ml/min per 1.73 m2) (n=22) | 91 (21–125) | 0.44 | 104 (18–158) | 0.98 | 101 (49–151) |
| eGFR (CKD-EPI) (ml/min/1.73 m2) (n=37) | 98 (64–120) | 0.25 | 99 (63–119) | 0.49 | 101 (58–120) |
| Creatinine (mg/dl) (n=37) | 0.91 (0.70–3.10) | 0.32 | 0.89 (0.50–3.40) | 0.24 | 0.83 (0.60–1.30) |
| Hemoglobin (mg/dl) (n=33) | 13.7±1.4 | 0.81 | 13.8±1.2 | 0.76 | 13.6±1.5 |
| Dialysis, n (%) | 6 (16) | 1.00 | 6 (16) | 1.00 | 7 (18) |
| Kidney transplant, n (%) | 1 (2.6) | 1.00 | 1 (2.6) | 1.00 | 1 (2.6) |
| Agalsidase-beta dose-reduction group | |||||
| Albumin-to-creatinine ratio (mg/g creatinine) (n=32) | 231 (0–1807) | 0.58 | 273 (0–1010) | 0.27 | 239 (0–1420) |
| Cystatin-C GFR (ml/min per 1.73 m2) (n=13) | 96 (35–132) | 0.33 | 109 (65–132) | 0.02 | 92 (69–135) |
| eGFR (CKD-EPI) (ml/min per 1.73 m2) (n=27) | 94 (61–116) | 0.34 | 98 (86–114) | 0.01 | 95 (82–114) |
| Creatinine (mg/dl) (n=26) | 0.85 (0.70–2.70) | 0.57 | 0.80 (0.60–1.20) | 0.60 | 0.80 (0.70–1.00) |
| Hemoglobin (mg/dl) (n=25) | 13.3±1.4 | 0.27 | 13.6±1.2 | 0.50 | 13.5±1.2 |
| Dialysis, n (%) | 5 (17) | 1.00 | 5 (17) | 1.00 | 5 (17) |
| Kidney transplant, n (%) | 4 (14) | 1.00 | 5 (17) | 1.00 | 5 (17) |
| Agalsidase-alfa switch group | |||||
| Albumin-to-creatinine ratio (mg/g creatinine) (n=36) | 40 (0–1292) | 0.37 | 114 (0–606) | 0.03 | 216 (0–2062) |
| Cystatin-C GFR (ml/min per 1.73m2) (n=23) | 108 (53–130) | 0.47 | 101 (52–151) | 0.09 | 96 (64–141) |
| eGFR (CKD-EPI) (n=37) (ml/min per 1.73 m2) | 99 (68–123) | 0.53 | 99 (66–112) | 0.05 | 95 (64–116) |
| Creatinine (mg/dl) (n=37) | 0.88 (0.50–1.40) | 0.06 | 0.87 (0.60–2.30) | 0.18 | 0.83 (0.60–1.20) |
| Hemoglobin (mg/dl) (n=36) | 13.6±1.3 | 0.09 | 13.4±1.3 | 0.24 | 13.6±1.1 |
| Dialysis, n (%) | 5 (13) | 1.00 | 5 (13) | 1.00 | 5 (13) |
| Kidney transplant, n (%) | 3 (8) | 1.00 | 3 (8) | 1.00 | 3 (8) |
Categorical data are presented as number (percentage) of patients. Otherwise data are presented as median (range) or mean±SD. For eGFR analyses, all patients with hyperfiltration (125–175 ml/min per 1.73 m2) and undergoing dialysis were excluded. CKD-EPI, CKD–Epidemiology Collaboration.
Outcome for Change of FD-Related Symptoms
The changes for FD-related symptoms are shown in Table 7. Again, all predefined measures remained stable between the retrospective visit and the baseline visit on agalsidase-beta (1 mg/kg every other week). These measures did not change in the agalsidase-beta regular-dose group between baseline and follow-up visit. However, patients in the dose-reduction group more often reported pain attacks (38% versus 21%; P=0.03) as well as pain crises (28% versus 17%; P=0.03) and worsened for the Disease Severity (14.3±7.4 versus 12.2±8.4; P=0.02) and Mainz Severity Score Index (MSSI) scores (23.9±11.3 versus 21.8±10.5; P=0.01). In parallel, patients in the switch group more often developed pain attacks (24% versus 8%; P=0.03), chronic pain (38% versus 24%; P=0.04), gastrointestinal pain (29% versus 13%; P=0.03), diarrhea (2.0±5.1 versus 0.8±1.7; P=0.05), and worsened MSSI score (19.9±9.6 versus 18.1±9.8; P=0.004) (Table 7).
Table 7.
Changes in FD-related symptoms
| Measure | 1-Year Retrospective Visit | P Value | Baseline Visit | P Value | 1-Year Follow-Up Visit |
|---|---|---|---|---|---|
| Agalsidase-beta regular-dose group | |||||
| Angiokeratoma | 20 (53) | 1.00 | 21 (55) | 0.50 | 23 (61) |
| Edema | 14 (37) | 0.51 | 11 (29) | 0.63 | 13 (34) |
| Gastrointestinal pain | 6 (16) | 0.63 | 8 (21) | 1.00 | 9 (24) |
| Diarrhea (d/mo) | 1.5±5.1 | 1.000 | 1.5±5.1 | 0.06 | 3.6±8.2 |
| Hypohidrosis | 27 (71) | 0.50 | 25 (66) | 1.00 | 25 (66) |
| Cornea verticillata | 12 (32) | 1.00 | 11 (29) | 1.00 | 11 (29) |
| Tinnitus | 8 (21) | 0.06 | 13 (34) | 1.00 | 14 (37) |
| Hypoacusis | 8 (21) | 1.00 | 8 (21) | 0.25 | 11 (29) |
| Paresthesia | 27 (71) | 0.22 | 23 (61) | 0.13 | 28 (73) |
| Pain attacks | 9 (24) | 0.45 | 6 (16) | 1.00 | 6 (16) |
| Chronic pain | 13 (34) | 0.69 | 15 (40) | 1.00 | 16 (42) |
| Pain crises | 10 (26) | 0.50 | 8 (21) | 1.00 | 7 (18) |
| TIA | 1 (2.6) | — | 1 (2.6) | 1.00 | 0 |
| Stroke | 1 (2.6) | — | 0 | — | 0 |
| Fatigue | 6 (16) | 1.00 | 6 (16) | 0.38 | 9 (24) |
| DS3 score | 11.3±8.1 | 0.08 | 13.5±8.3 | 0.18 | 14.8±8.3 |
| MSSI score | 19.3±10.9 | 0.11 | 20.5±11.3 | 0.05 | 22.4±11.3 |
| Agalsidase-beta dose-reduction group | |||||
| Angiokeratoma | 15 (52) | 1.00 | 15 (52) | 0.50 | 17 (59) |
| Edema | 5 (17) | 1.00 | 4 (14) | 0.50 | 6 (21) |
| Gastrointestinal pain | 5 (17) | 0.50 | 6 (21) | 0.50 | 8 (28) |
| Diarrhea (d/mo) | 2.3±6.7 | 0.35 | 2.0±5.7 | 0.20 | 2.3±5.8 |
| Hypohidrosis | 20 (69) | 0.50 | 22 (76) | 1.00 | 22 (76) |
| Cornea verticillata | 11 (38) | 1.00 | 12 (41) | 1.00 | 12 (41) |
| Tinnitus | 8 (28) | 1.00 | 7 (24) | 1.00 | 8 (28) |
| Hypoacusis | 11 (38) | 1.00 | 11 (38) | 1.00 | 12 (41) |
| Paresthesia | 18 (62) | 1.00 | 18 (62) | 0.45 | 22 (76) |
| Pain attacks | 7 (24) | 1.00 | 6 (21) | 0.03 | 11 (38) |
| Chronic pain | 17 (59) | 0.50 | 15 (52) | 0.50 | 17 (59) |
| Pain crises | 5 (17) | 1.00 | 5 (17) | 0.03 | 8 (28) |
| TIA | 0 | — | 0 | — | 0 |
| Stroke | 0 | — | 0 | — | 0 |
| Fatigue | 9 (31) | 1.00 | 8 (28) | 0.22 | 12 (41) |
| DS3 score | 11.9±9.4 | 0.71 | 12.2±8.4 | 0.02 | 14.3±7.4 |
| MSSI score | 21.1±11.8 | 0.40 | 21.8±10.5 | 0.01 | 23.9±11.3 |
| Agalsidase-alfa switch group | |||||
| Angiokeratoma | 16 (42) | 1.00 | 16 (42) | 0.25 | 19 (50) |
| Edema | 8 (21) | 1.00 | 8 (21) | 0.13 | 12 (32) |
| Gastrointestinal pain | 5 (13) | 1.00 | 5 (13) | 0.03 | 11 (29) |
| Diarrhea (d/mo) | 0.8±1.8 | 0.92 | 0.8±1.7 | 0.05 | 2.0±5.1 |
| Hypohidrosis | 17 (45) | 1.00 | 18 (47) | 0.63 | 16 (42) |
| Cornea verticillata | 19 (50) | 1.00 | 19 (50) | 1.00 | 20 (53) |
| Tinnitus | 6 (16) | 0.13 | 10 (26) | 1.00 | 10 (26) |
| Hypoacusis | 6 (16) | 1.00 | 6 (16) | 0.25 | 9 (23) |
| Paresthesia | 21 (55) | 1.00 | 22 (58) | 0.69 | 20 (53) |
| Pain attacks | 6 (16) | 0.25 | 3 (8) | 0.03 | 9 (24) |
| Chronic pain | 11 (29) | 0.63 | 9 (24) | 0.04 | 14 (38) |
| Pain crises | 5 (13) | 0.50 | 7 (18) | 1.00 | 6 (16) |
| TIA | 0 | — | 0 | — | 2 (5.2) |
| Stroke | 0 | — | 0 | — | 0 |
| Fatigue | 9 (24) | 0.63 | 11 (19) | 0.38 | 14 (37) |
| DS3 score | 13.5±7.9 | 0.18 | 14.6±8.4 | 0.43 | 15.3±8.3 |
| MSSI score | 18.1±10.3 | 0.93 | 18.1±9.8 | 0.004 | 19.9±9.6 |
Categorical data are presented as number (percentage) of patients. Otherwise data are presented as mean±SD. DS3, Disease Severity; TIA, transient ischemic attack.
Additional General Measures
In general, the switch to agalsidase-alfa was well tolerated. Three patients (7.9%) in the switch group developed mild infusion reactions, such as skin rash, shivering, and mild dyspnea. In these patients, premedication with steroids and histamine antagonists was administered before infusion.
Treatment for the renin-angiotensin-aldosterone system and analgesics (i.e., ibuprofen, tramadol, or pregabalin) at baseline are presented in Table 2. During follow-up, the number of patients receiving medication and drug doses was unchanged. For both types of medications, no significant differences among the three groups and among the three visits were observed.
Discussion
During the phase of agalsidase-beta supply shortage, patients were treated with a reduced dose of agalsidase-beta or were switched to a regular dose of agalsidase-alfa. In our observational study, we systematically followed a large Fabry population during this change of treatment conditions. The main findings were as follows: (1) patients receiving a regular agalsidase-beta dose showed a stable clinical disease course with respect to organ function and FD-related symptoms; (2) patients undergoing a reduction in agalsidase-beta dose showed a mild, but significant, decrease in renal function, and the number of patients experiencing pain attacks or pain crises increased; and (3) after switching to agalsidase-alfa, patients had stable cardiac organ function but a significant increase in microalbuminuria, and they developed more FD-related symptoms, such as pain aggravation and gastrointestinal symptoms.
ERT in FD
Randomized controlled trials and clinical studies with both compounds (agalsidase-beta and agalsidase-alfa) have shown that ERT is safe and efficient for the treatment of patients with FD.4,5,8,14,15 Because adverse effects and infusion reactions are fairly rare, ERT compound and dosage usually remain unchanged over longer periods. In 2009, a viral contamination of Genzyme Corporation’s production facilities and subsequent ongoing manufacturing problems caused an acute and prolonged shortage of agalsidase-beta to approximately 30% of the prior global supply.13 The clinical consequence of this shortage was that patients received a reduced agalsidase-beta dosage or were switched to a regular dose of agalsidase-alfa. Because agalsidase-beta is produced by Chinese hamster ovary cells and agalsidase-alfa by a genetically engineered human cell system, there are minor biochemical differences between the two compounds. Thus, in theory, a compound switch may have a clinical effect on organ function and symptoms. So far, only scarce clinical data on ERT switch or dosage change are available. In a small group of 11 Japanese patients, the switch from agalsidase-beta to agalsidase-alfa was safe and did not lead to disease progression.10 In a recent observational study, 35 patients initially treated with agalsidase-beta were followed up after dose reduction or switch to agalsidase-alfa.13 In that study, clinical event rate, renal function, and pain did not change during follow-up. However, health-related quality of life decreased in women, and lysoGb3 as a biochemical disease marker increased in the patients who were receiving a reduced dose of agalsidase-beta or were switched to agalsidase-alfa.13
Our data on 105 patients corroborate the findings of the two smaller studies because no clinical event occurred after dose reduction or compound switch. However, renal function declined and FD-related symptoms increased after dose reduction and after switch to agalsidase-alfa. In this respect, a higher frequency of pain attacks, pain crises, and gastrointestinal symptoms was observed and caused an increase in the MSSI score. It remains conjectural whether the change in therapy leads quite rapidly to an increase of lysoGb3 (biochemical response)13 and subsequently within 1 year to a recurrence of clinical symptoms (clinical response).
Because the group of patients who were followed-up after dose reduction of agalsidase-beta was relatively large, we could document a significant decrease in renal function for the first time, a finding that is, of course, clinically important. Even after exclusion of patients with hyperfiltration, our separate analyses of men and women confirmed a mild but significant eGFR decline in both sexes. Indeed, already a relatively short treatment period of only 1 year of dose reduction of agalsidase-beta seems to have led to the combined disease progression in organ function and clinical symptoms.
The switch to agalsidase-alfa led to a significant progression of albuminuria (nearly a 2.0-fold increase) and a borderline significant reduction of eGFR. Our separate analyses of men and women confirmed a significant increase in albuminuria in both sexes. Even if we had not performed renal biopsies with structural glomerular analyses in our study, it is known that albuminuria may indicate podocytes injury. Podocytes are highly differentiated cells essential for maintaining glomerular filtration function. It is reported that patients with FD and proteinuria of ≥1 g/24 hours had poorer renal function at baseline and follow-up (faster eGFR decline) than patients with protein excretion of 500–1000 mg/24 hours or with proteinuria<500 mg/24 hours, even while receiving ERT.16,17 West et al. reported that the negative influence of proteinuria probably extends to as low as 0.3 g/d.18 Therefore, it is assumed that the prognostic effect of microalbuminuria in patients with FD is relatively large and that increased microalbuminuria is also associated with a poor renal prognosis.17 In patients with microalbuminuria, podocyte injury may play a pivotal role in the development and progression of FD-associated nephropathy. As shown by Najafian and colleagues, podocyte GL-3 inclusion volume density as well as foot process width correlated with rates of urinary protein excretion.19 Even if our observational study period was too short to detect the effect of microalbuminuria on clinical outcome, it is known that the increase in microalbuminuria is indeed an important risk factor for renal and cardiovascular events, such as myocardial infarction and stroke, because it indicates a vascular dysfunction in the entire organism in several populations without FD.20–22 Therefore, additional studies are warranted in patients with FD to validate this clinical relevant relationship.
Clinical Effect
The supply restriction for agalsidase-beta caused distress and concern in both patients and physicians. However, this extreme situation allowed for learning more about dose reduction and compound switch in FD treatment. Because of the observational study design, it is not possible to qualify the regular agalsidase-beta dose regimen as the optimal treatment for all patients with FD. However, whenever agalsidase-beta is chosen, a dose reduction should be avoided. In addition, a switch from agalsidase-beta to agalsidase-alfa is safe in general, but clinicians should be aware that microalbuminuria and FD-related symptoms may worsen. It has been reported that both enzymes can induce neutralizing antibodies,23,24 which also indicates that a frequent ERT switch should be avoided.
Limitations
Part of the clinical response may be influenced by the development of antibodies against agalsidase-alfa and agalsidase-beta, which were not measured. Because of the multicenter approach, we could not systematically assess the antibody status. However, the initial aim was to conduct a comprehensive clinical assessment in a relatively large patient group, which was assured by the implementation of a harmonized data extraction mode in all participating centers. The 1-year follow-up period was relatively short. However, even during this short period, clinically relevant changes could be observed. Further long-term follow-up studies of patients who were switched as well as those who were re-switched to the former ERT are warranted.
Owing to the observational design, patients were not randomly assigned. Thus, we could not directly compare the progression of organ function and FD-related symptoms among the three groups (or between agalsidase-beta and agalsidase-alfa).
Another limitation is that although we used validated tools to assess neuropathic pain (Neuropathic Pain Symptom Inventory [NPSI], Graded Chronic Pain Scale [GCPS] questionnaires), the questionnaire scores may not fully reflect distinct pain phenotype in patients with FD (inducible pain attacks, pain crises, chronic pain), because an FD-specific pain questionnaire is not yet available. Furthermore, we cannot rule out a possible effect of analgesic drugs, although the quantity of analgesics taken did not change significantly during follow-up.
For eGFR analyses, we excluded patients with hyperfiltration and those undergoing dialysis. The threshold for glomerular hyperfiltration was adapted to that reported in recent studies.25 A limitation of this definition is that it does not consider the age-related decline of GFR, and eGFR does not exactly correlate with the measured GFR.
During the 1-year follow-up period, patients receiving a regular agalsidase-beta dose showed a stable clinical disease course, whereas patients with an agalsidase-beta dose reduction exhibited mild renal deterioration and an increase in FD-related symptoms. In addition, a switch to agalsidase-alfa is safe, but patients may experience deterioration of albuminuria and several clinically relevant symptoms, thus affecting quality of daily life. Further medium- and long-term follow-up studies are required to better characterize the differences between ERT regimens in FD.
Concise Methods
Study Design
In this prospective observational study, a cohort of 105 patients (62 men and 43 women) with genetically confirmed FD from three German Fabry Centers in Berlin (n=13), Muenster (n=42), and Wuerzburg (n=50) were consecutively recruited. Patients had been receiving a stable treatment with agalsidase-beta, 1.0 mg/kg every other week, for at least 1 year and reported to the Fabry centers for their regular clinical follow-up. The routine clinical assessment included cardiac, renal, and neurologic measures. The documentation of assessments follows clinical practice of the German Fabry Expert Centers for a rare multisystemic disorder. Inclusion criteria were the following: (1) adult patients (≥18 years of age) with genetically determined FD; (2) at least 1 year of treatment with the regular dose of agalsidase-beta, 1.0 mg/kg every other week; (3) ≥90% completeness of the mandatory predefined organ and symptomatic data (see below); and (4) informed consent for examinations and participation in the study.
All investigations were performed after approval of the ethical committees of the participating centers (project number 2011–347-f), and patients provided written informed consent for molecular analysis and publication. Patients who fulfilled the inclusion criteria were given written and verbal information on the study and gave written informed consent.
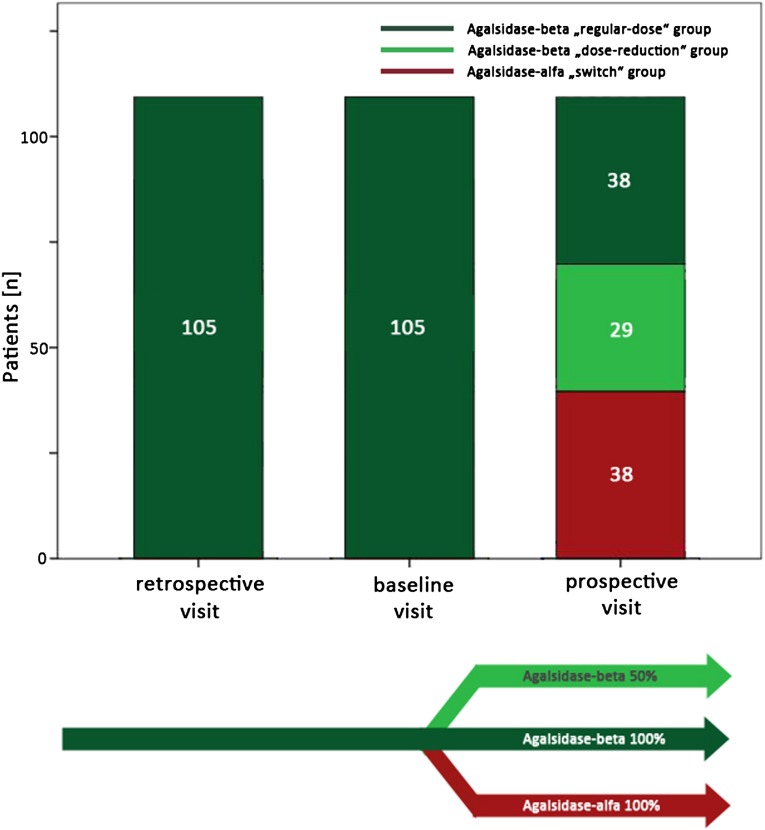
Patients changing therapy more often than twice were excluded (n=12). The baseline visit was defined as the last visit at which patients were still treated with the regular dose of agalsidase-beta, 1.0 mg/kg every other week. After this visit, the treating physicians determined the long-term therapy strategy in accordance with the patient. The following options were proposed: (1) continue receiving the regular dose of agalsidase-beta of 1.0 mg/kg every other week (regular-dose group) (this was possible because in Germany it was negotiated with the supplier [Genzyme Corp] that around 40 of all patients treated with agalsidase-beta may continue with the regular dose); (2) change to a reduced dose of agalsidase-beta of 0.3 or 0.5 mg/kg (dose-reduction group) (this cumulative dose reduction was achieved by extending the treatment interval to 4 weeks and keeping the regular dose, or by an absolute reduction in the dose, keeping the infusion intervals at 2 weeks); or (3) switch to agalsidase-alfa at a standard dose of 0.2 mg/kg every other week (switch group).
The regular-dose group should not be considered a control group but does help illuminate the results obtained in the two core groups (the dose-reduction group and the switch group). The chosen long-term treatment was initiated, and patients were invited for a follow-up visit after 1 year of stable treatment with the implemented strategy. The visit preceding the baseline visit by 1 year was termed the retrospective visit. Thus, in the final data set, all data from three consecutive visits were included (retrospective-visit plus baseline visit plus follow-up visit) (Figure 3).
Figure 3.
Overview of the study design. After the baseline visit, the treatment strategy for the following year was chosen by an FD specialist physician team and the patient: (1) continue the regular dose of agalsidase-beta of 1.0 mg/kg every other week; (2) reduce the agalsidase-beta dose to 0.3 or 0.5 mg/kg every other week; or (3) switch to a regular dose of agalsidase-alfa at 0.2 mg/kg every other week.
Clinical Work-Up
A comprehensive diagnostic work-up as detailed in Table 1 was performed in all patients at each visit, including medical history and cardiac, renal, and neurologic evaluation. Cardiac assessment included echocardiography, cardiac magnetic resonance imaging, and electrocardiography. Renal function was quantified by the eGFR using the CKD–Epidemiology Collaboration equation26 and albuminuria (albumin-to-creatinine ratio) from spot urine. Microalbuminuria is defined as an albumin-to-creatinine ratio between 30 and 300 mg albumin per g creatinine. For eGFR analyses, patients with hyperfiltration (125–175 ml/min per 1.73 m2) were excluded (regular-dose group, n=2; dose-reduction group, n=4; switch group, n=2). In addition, for eGFR analyses, all patients receiving dialysis were excluded. All patients underwent neurologic examination and a clinical interview focusing on a history of strokes or transient ischemic attacks and pain. The following pain phenotypes were assessed: (1) pain attacks: episodic severe, mostly acral pain triggered by such factors as fever, physical exertion, or heat; (2) chronic pain: persistent pain; (3) pain crises: periodically recurring extreme pain triggered by fever, physical activity, or stress, mostly spreading from the hands and feet over the entire body. The Results section presents the numbers of patients with these pain types. In addition, pain questionnaires were applied: the German versions of the GCPS with a 4-week recall27 and the NPSI with a 24-hour recall.28 From the GCPS, we used question 2 (maximum pain intensity) and question 5 (degree of impairment of daily activities due to pain). The NPSI investigates pain intensity and characteristics, resulting in a sum score between 0 (no pain at all) and 1 (maximum pain). To assess depressive symptoms, the German version of the Center for Epidemiologic Studies-Depression Scale was applied. Antidromic nerve conduction studies of the right sural nerve were performed in all patients using surface electrodes to screen for polyneuropathy. In addition, the cold detection threshold at the left dorsal foot was determined with a thermode (Somedic, Hörby, Sweden) and following a standardized protocol for quantitative sensory testing.29
Outcome Measures
To quantify the clinical outcome, three groups of FD-related progression parameters were analyzed.
Clinical Events
These included (1) death; (2) symptomatic cardiac arrhythmia requiring implantation of an implantable cardioverter-defibrillator or pacemaker, myocardial infarction, coronary artery bypass graft, or percutaneous transluminal coronary angioplasty; (3) progression of renal disease to CKD stage 5 (eGFR<15 ml/min per 1.73 m2 [with ≥30% decrease of eGFR]) necessitating kidney transplantation or dialysis); and (4) stroke or transient ischemic attack.
Change in Organ Function or Structure
These changes were categorized as cardiac, renal, and neurologic. Cardiac changes involved end-systolic and end-diastolic left ventricular diameter, end-diastolic septal and posterior wall thickness, left ventricular mass, late enhancement on magnetic resonance imaging, ejection fraction, and electrocardiographic T-negativations. Renal changes included eGFR and albumin-to-creatinine ratio in spot urine. Neurologic changes were determined on the basis of clinical examination, interview regarding stroke or stroke-like symptoms, neurography of the sural nerve, quantitative sensory testing for cold detection threshold assessment.
Change in FD-Related Symptoms
These changes consisted of gastrointestinal pain (abdominal pain, tenesmus, or cramping more than once a week), diarrhea (more than three loose bowels or >250 g of stool weight per day), hypohidrosis or anhidrosis (loss of ability to sweat), tinnitus, neuropathic pain in terms of pain attacks, chronic pain or pain crises and using the GCPS and the NPSI scores, fatigue (defined by the Fukuda criteria),30 the Disease Severity score,31 the MSSI score,32 and depressive symptoms (using the Center for Epidemiologic Studies Depression Scale score).
Statistical Analyses
Data are presented as the mean±SD, median (range), or number (percentage), where appropriate. Baseline and follow-up values were compared using a paired t test after checking for normal distribution. Otherwise, the Wilcoxon rank-sum test was performed. For categorical data, the chi-square or Fisher exact tests were used. All differences were tested two sided. P values<0.05 were considered to represent statistically significant differences. Data were analyzed using SPSS software, version 18.0 (SPSS, Inc., Chicago, IL).
Disclosures
F.W., J.K., M.N., C.W., T.D., S.C.K., and E.B. have received speaker honoraria from Genzyme and Shire Corporation. F.W. and C.W. are members of the Fabry Registry European Board of Advisors and have received travel assistance and speaker honoraria. Research grants were given to the institutions (Wuerzburg and Muenster) by Genzyme and Shire Corporations. N.Ü. has received travel assistance and speaker honoraria from Genzyme Corporation and travel assistance from Shire Corporation. M.L. has received travel assistance and speaker honoraria from Genzyme Corporation. A.K. and C.S. have received speaker honoraria from Genzyme Corporation. S.S. declares advisory board activities for Genzyme.
Acknowledgments
We thank Barbara Broll, Irina Turkin, Anne Huster, Samira Schiwek, and Jutta Beilker for expert technical assistance.
This study was funded by Genzyme Europe B.V. The funding agency had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The researchers were independent of the funding agency. E.B. was supported by a Heisenberg professorship from the Deutsche Forschungsgemeinschaft (Br1589/8-2).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Fabry Disease: Dose Matters,” on pages 653–655.
References
- 1.Desnick R, Ionnou Y, Eng C: Fabry disease: alpha galactosidase A deficiency. In: The metabolic and molecular bases of inherited disease, edited by Scriver C, Beaudet A, Sly W, Valle D, New York, McGraw-Hill, 1995, pp 2741–2784 [Google Scholar]
- 2.Zarate YA, Hopkin RJ: Fabry’s disease. Lancet 372: 1427–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR: Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 138: 338–346, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ, Fabry Disease Clinical Trial Study Group : Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann Intern Med 146: 77–86, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ, International Collaborative Fabry Disease Study Group : Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry’s disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Hughes DA, Elliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, Mehta AB: Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: A randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart 94: 153–158, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassmann G, Schiffmann R, Barbey F, Ries M, Clarke JT, Fabry Outcome Survey investigators : Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: An analysis of registry data. Lancet 374: 1986–1996, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Schiffmann R, Kopp JB, Austin HA, 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Wraith JE, Tylki-Szymanska A, Guffon N, Lien YH, Tsimaratos M, Vellodi A, Germain DP: Safety and efficacy of enzyme replacement therapy with agalsidase beta: An international, open-label study in pediatric patients with Fabry disease. J Pediatr 152: 563–570, e1, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi K, Yamamoto H: Clinical observation of patients with Fabry disease after switching from agalsidase beta (Fabrazyme) to agalsidase alfa (Replagal). Genet Med 14: 779–786, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linthorst GE, Germain DP, Hollak CE, Hughes D, Rolfs A, Wanner C, Mehta A, European Medicines Agency : Expert opinion on temporary treatment recommendations for Fabry disease during the shortage of enzyme replacement therapy (ERT). Mol Genet Metab 102: 99–102, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Pisani A, Spinelli L, Visciano B, Capuano I, Sabbatini M, Riccio E, Messalli G, Imbriaco M: Effects of switching from agalsidase Beta to agalsidase alfa in 10 patients with anderson-fabry disease. JIMD Rep 9: 41–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smid BE, Rombach SM, Aerts JM, Kuiper S, Mirzaian M, Overkleeft HS, Poorthuis BJ, Hollak CE, Groener JE, Linthorst GE: Consequences of a global enzyme shortage of agalsidase beta in adult Dutch Fabry patients. Orphanet J Rare Dis 6: 69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM: Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: A prospective strain rate imaging study. Circulation 108: 1299–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Störk S, Voelker W, Ertl G, Wanner C, Strotmann J: Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: Evidence for a better outcome with early treatment. Circulation 119: 524–529, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Feriozzi S, Torras J, Cybulla M, Nicholls K, Sunder-Plassmann G, West M, FOS Investigators : The effectiveness of long-term agalsidase alfa therapy in the treatment of Fabry nephropathy. Clin J Am Soc Nephrol 7: 60–69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, Lee P, Loew T, Vedder AC, Abichandani R, Wilcox WR, Guffon N: Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 18: 1547–1557, 2007 [DOI] [PubMed] [Google Scholar]
- 18.West M, Nicholls K, Mehta A, Clarke JTR, Steiner R, Beck M, Barshop BA, Rhead W, Mensah R, Ries M, Schiffmann R: Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol 20: 1132–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najafian B, Svarstad E, Bostad L, Gubler MC, Tøndel C, Whitley C, Mauer M: Progressive podocyte injury and globotriaosylceramide (GL-3) accumulation in young patients with Fabry disease. Kidney Int 79: 663–670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gansevoort RT, de Jong PE: The case for using albuminuria in staging chronic kidney disease. J Am Soc Nephrol 20: 465–468, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke 27: 2033–2039, 1996 [DOI] [PubMed] [Google Scholar]
- 22.de Zeeuw D, Parving HH, Henning RH: Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol 17: 2100–2105, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Linthorst GE, Hollak CE, Donker-Koopman WE, Strijland A, Aerts JM: Enzyme therapy for Fabry disease: Neutralizing antibodies toward agalsidase alpha and beta. Kidney Int 66: 1589–1595, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka A, Takeda T, Hoshina T, Fukai K, Yamano T: Enzyme replacement therapy in a patient with Fabry disease and the development of IgE antibodies against agalsidase beta but not agalsidase alpha. J Inherit Metab Dis 33[Suppl 3]: S249–S252, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Korff M, Ormel J, Keefe FJ, Dworkin SF: Grading the severity of chronic pain. Pain 50: 133–149, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Sommer C, Richter H, Rogausch JP, Frettlöh J, Lungenhausen M, Maier C: A modified score to identify and discriminate neuropathic pain: A study on the German version of the Neuropathic Pain Symptom Inventory (NPSI). BMC Neurol 11: 104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 123: 231–243, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, International Chronic Fatigue Syndrome Study Group : The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med 121: 953–959, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Giannini EH, Mehta AB, Hilz MJ, Beck M, Bichet DG, Brady RO, West M, Germain DP, Wanner C, Waldek S, Clarke JT, Mengel E, Strotmann JM, Warnock DG, Linhart A: A validated disease severity scoring system for Fabry disease. Mol Genet Metab 99: 283–290, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A, Gal A, Beck M: The Mainz Severity Score Index: A new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 65: 299–307, 2004 [DOI] [PubMed] [Google Scholar]