Abstract
The human gut harbors >100 trillion microbial cells, which influence the nutrition, metabolism, physiology, and immune function of the host. Here, we review the quantitative and qualitative changes in gut microbiota of patients with CKD that lead to disturbance of this symbiotic relationship, how this may contribute to the progression of CKD, and targeted interventions to re-establish symbiosis. Endotoxin derived from gut bacteria incites a powerful inflammatory response in the host organism. Furthermore, protein fermentation by gut microbiota generates myriad toxic metabolites, including p-cresol and indoxyl sulfate. Disruption of gut barrier function in CKD allows translocation of endotoxin and bacterial metabolites to the systemic circulation, which contributes to uremic toxicity, inflammation, progression of CKD, and associated cardiovascular disease. Several targeted interventions that aim to re-establish intestinal symbiosis, neutralize bacterial endotoxins, or adsorb gut-derived uremic toxins have been developed. Indeed, animal and human studies suggest that prebiotics and probiotics may have therapeutic roles in maintaining a metabolically-balanced gut microbiota and reducing progression of CKD and uremia-associated complications. We propose that further research should focus on using this highly efficient metabolic machinery to alleviate uremic symptoms.
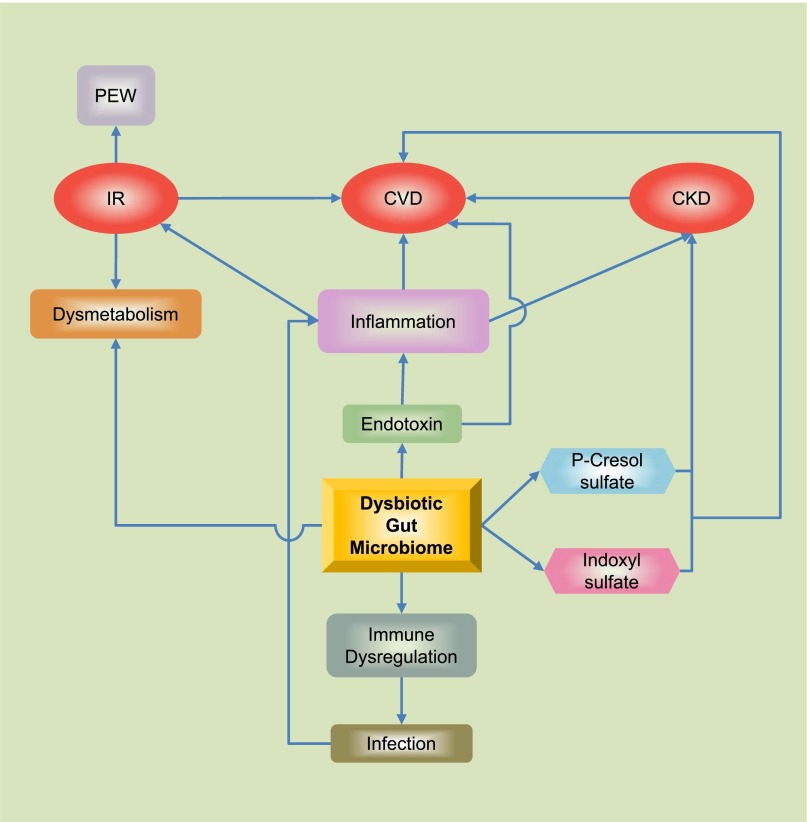
The gut microbiota has coevolved with humans for a mutually beneficial coexistence and plays an important role in health and disease.1 Normal gut microbiota influences the well-being of the host by contributing to its nutrition, metabolism, physiology, and immune function.2,3 Disturbance of normal gut microbiota (dysbiosis) has been implicated in the pathogenesis of diverse illnesses, such as obesity,4 type 2 diabetes,5 inflammatory bowel disease,6 and cardiovascular disease.7,8 Quantitative and qualitative alterations in gut microbiota are noted in patients with CKD and ESRD.9–11 Preliminary evidence indicates that toxic products generated by a dysbiotic gut microbiome may contribute to progression to CKD and CKD-related complications (Figure 1).12,13
Figure 1.
The human gut is host to >100 trillion bacteria with an enteric reservoir of >1 g of endotoxin. Alterations in gut microbiota and impaired intestinal barrier function in patients with CKD/ESRD have been linked to endotoxemia and accumulation of gut-derived uremic toxins leading to insulin resistance, protein energy wasting, immune dysregaulation, and atheroscleroisis. CVD, cardiovascular disease; IR, insulin resistance; PEW, protein energy wasting.
Gut Microbiota: An Endogenous Organ
The human gut harbors a complex community of >100 trillion microbial cells that constitute the gut microbiota. The combined microbial genome of the gut microbiota is known as the gut microbiome. In general, the adult gut is dominated by two bacterial phyla, Firmicutes and Bacteroidetes; other phyla, including Actinobacteria, Proteobacteria, Verrucomicrobia, Cyanobacteria, Fusobacteria, Spirochaetes, and TM7, are present in smaller proportions.14,15 Each species of bacteria colonizes a specific niche, leading to different bacterial composition along the intestinal tract (Table 1). Gut microbiota performs a multitude of functions and can be considered a metabolically active endogenous “organ” in itself. Under physiologic conditions, it participates in certain complementary metabolic activities that have not been fully evolved in the human host, such as breakdown of undigestible plant polysaccharides,3 synthesis of certain vitamins,16 biotransformation of conjugated bile acids,17 and degradation of dietary oxalates.18 Importantly, postnatal colonization of the intestine educates our immune system and reduces allergic responses to food and environmental antigens.19
Table 1.
Distribution and composition of the microbiota along the intestinal tract
| Gastrointestinal Tract | Normal | CKD/ESRD |
|
|---|---|---|---|
| Phyla, Families, and Genera of Dominant Bacterial Species | Microbial Number (cells/g) | Alterations from Normal Microbiota | |
| Stomach | Lactobacillus | 101 | |
| Helicobacter | |||
| Duodenum | Staphylococcus | 103 | Human studies: increased counts10 (106–107) |
| Streptococcus | |||
| Lactococcus | |||
| Jejunum | Enterococcus | 104 | Human studies: increased counts10 (106–107) |
| Streptococcus | |||
| Lactobacillus | |||
| Ileum | Enterobacteriaceae | 107 | |
| Bacteroides | |||
| Clostridium | |||
| Segmented filamentous bacteria | |||
| Colon | Firmicutes | 1012 | Experimental animal studies: increased Proteobacteria and Enterobacteriaceae, increased Escherichia, Enterobacter, Acinetobacter, Proteus, and Proteus spp,82 and decreased Lactobacillus and Bifidobacterium spp.82 Decreased Lactobacillaceae and Prevotellaceae11 Human studies: overgrowth of aerobic bacteria (about 100 times)9 Decreased Bifidobacteria and higher Clostridium perfringens9 Lower species richness11 |
| Bacteroidetes | |||
| Actinobacteria | |||
| Proteobacteria | |||
| Clostridium | |||
| Lactobacillaceae | |||
| Prevotellaceae | |||
| Fusobacteria | |||
| TM7 | |||
The utility of human gut microbiota in the diagnosis, treatment, and prevention of disease requires a clear understanding of its composition, dynamics, and stability within an individual. A recent study aimed at characterizing the long-term stability of the human gut microbiota used low-error amplicon sequencing of fecal samples from 37 healthy adults collected over a period of 296 weeks.20 The results revealed that on average, the microbiota was remarkably stable over time within an individual and between family members but not between unrelated individuals. These findings further emphasize the importance of the early gut colonizers, such as those acquired from parents and siblings, and their potential life-long effect on our health and disease.
Microbiota-Host Signaling
Mammalian gut microbiota forms a complex ecosystem that requires proper interaction with its host for symbiotic benefits. One of the best examples of the microbiota-host signaling is the host immunomodulation by Bacteroides fragilis polysaccharide A molecule, which directs the maturation of the developing immune system by mediating establishment of a proper T-helper cell (TH1/TH2) balance.21 The gut microbiota can also sense host-produced molecules. For instance, norepinephrine released in response to stress could increase the growth and production of virulence-associated factors of Gram-negative bacteria.22 Finally, different members of the gut microbiota also communicate for establishment or maintenance of homeostasis in the intestinal ecosystem. When germ-free mice were colonized with Bacteroides thetaiotaomicron and Methanobrevibacter smithii, the latter directed B. thetaiotaomicron to focus on fermentation of dietary fructans to acetate, whereas B. thetaiotaomicron–derived formate was used by M. smithii for methanogenesis.23
Intestinal Epithelial Barrier
In addition to allowing absorption of nutrients, the intestinal epithelium also functions as a barrier to prevent systemic translocation of antigens and pathogens (Figure 2A). The intestinal epithelium is a single layer of columnar epithelial cells that separates the intestinal lumen from the underlying lamina propria.24 These epithelial cells are bound together by tight junctions, making a multifunctional complex that forms a seal between adjacent epithelial cells.25 Commensal gut microbes maintain functional integrity of gut by several mechanisms, including restoration of tight junction protein structure,26 induction of epithelial heat-shock proteins,27,28 upregulation of mucin genes,29 competition with pathogenic bacteria for binding to intestinal epithelial cells,30 and secretion of antimicrobial peptides.31 Probiotic bacteria enhance intestinal epithelial barrier function in murine models of colitis and in patients with Crohn disease.32,33 Treating human epithelial cell monolayers with metabolites secreted by Bifidobacterium infantis causes an increase in tight junction proteins ZO-1 and occludin while reducing claudin-2, thus demonstrating the ability of bacteria and bacterial products to modify ion permeability and selectivity of tight junction.26 In germ-free mice, colonization with B. thetaiotaomicron resulted in modulation in expression of genes involved in several important intestinal functions.34,35
Figure 2.
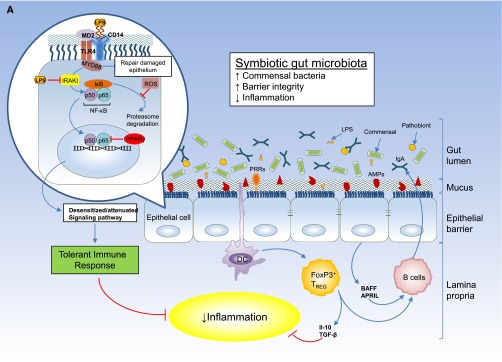
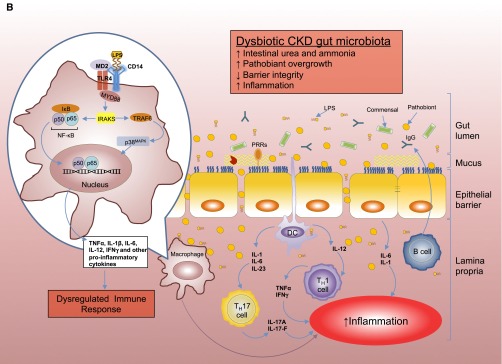
(A) Intestinal epithelial barrier and inflammatory responses in symbiotic and dysbiotic gut microbiota. A symbiotic gut microbiota leads to development of a functional barrier, with normal amounts of mucus, pattern recognition receptors (PRRs), antimicrobial peptides (AMPs), and secreted IgA, which in turn contain the microbiota in the intestinal lumen and away from the intestinal epithelial cells. As a result, the intestinal immune system becomes largely tolerant to the resident commensals. Similar to immune cells, the signaling cascades that occur downstream of TLRs (enlarged on the left) are used by epithelial cells to detect microbes through PRRs, such as the TLR4. Briefly, upon LPS ligation, the MYD88 is recruited, which activates the NF-κB pathway and leads to production of antimicrobial proteins and proinflammatory cytokines. In a symbiotic gut, epithelial cells are desensitized by continuous exposure to LPS168 or are attenuated by (1) LPS-mediated downregulation of the IL-1 receptor–associated kinase 1 (IRAK1), which is the proximal activator of the NF-κB cascade;168 (2) LPS-mediated induction of peroxisome proliferator-activated receptor-γ (PPArγ), which can divert NF-κB from the nucleus;169 or (3) commensal bacteria-derived reactive oxygen species (ROS)–mediated inhibition of polyubiquitylation and degradation of the aortic inhibitor of κB.170 (T bars indicate the checkpoints that are controlled by the microbiota.) Exposure to LPS induces epithelial cells to secrete TGF-β, B-cell–activating factor of the TNF family (BAFF), and a proliferation-inducing ligand (APRIL), all promoting the development of tolerogenic immune cell responses to the microbiota. CD103+ dendritic cells (DCs) support the development of regulatory T (Treg) cells secreting IL-10 and TGF-β, and together they stimulate the production of commensal-specific IgA.171 (B) Increased intestinal concentration of uremic toxins associated with the progression of CKD leads to microbial dysbiosis and overgrowth of pathobionts. Pathobiont overgrowth leads to the loss of barrier integrity and the breach in the epithelia barrier. Translocation of bacteria and bacterial components triggers the intestinal immune system to direct a potentially harmful proinflammatory response to clear invading bacteria by secreting IL-1 and -6 from intestinal epithelial cells, promoting a TH1 and TH17 response by DCs and macrophages and producing higher levels of commensal-specific IgG by B cells. In this context, LPS binding to its receptor complex on macrophages (enlarged on the left) results in enhanced production of inflammatory cytokines including IFN-β, IFN-γ, IL-1β, IL-6, TNFα, and IL-12, the production of which has been shown to require activation of p38MAPK.172 Subclinical endotoxemia is a potential cause of inflammation in CKD.90–92 Dysregulated immune response and chronic production of proinflammatory cytokines lead to systemic inflammation, which could further accelerate the progression of CKD and development of cardiovascular disease. IκB, inhibitor of NF-κB.
Commensal bacteria also play an important role in maintaining the intestinal epithelial barrier by suppressing intestinal inflammation. Toll-like receptors (TLRs) comprise a family of pattern-recognition receptors that detect conserved molecular products of microorganisms, such as LPS and lipoteichoic acid, recognized by TLR4 and TLR2, respectively.36 TLR2 stimulation effectively preserved tight junction-associated barrier assembly against stress-induced damage through promotion of phosphatidylinositol 3-kinase/protein kinase B–mediated cell survival via myeloid differentiation factor 88 (MyD88).37 Microbiota signaling through mucosal TLRs was also shown to be required for maintenance of intestinal epithelial homeostasis and repair following intestinal injury.38
Gut Microbiota in Obesity and Insulin Resistance
Data from the US Renal Data System shows an epidemic of obesity among the ESRD population.39 Insulin resistance is common in patients with CKD40 and is in part due to a high prevalence of shared risk factors, such as obesity and sedentary lifestyle. Recent findings suggest that our gut microbiota might be involved in the development of obesity and related disorders, such as insulin resistance.41–44 Weight gain is associated with an increase in the capacity of the microbiota to extract nutrients from the diet and in inducing metabolic changes in the host, such as increased fatty acid oxidation in muscle and increased triglyceride storage in the liver.43,45 Germ-free mice ingesting a high-fat diet do not gain weight or develop adiposity; however, reconstitution of germ-free mice gut with microbiota from lean mice or from genetically or diet-induced obese mice causes weight gain.46 Gut microbiota composition is significantly different in genetically obese mice and obese patients compared with lean controls.4,41 A high-fat (Western) diet modifies the gut microbiota by reducing the relative abundance of Bacteroidetes and increasing the relative abundance of Firmicutes.41 An increase of genes involved in the import and processing of sugars in the gut metagenome was also found in mice fed with Western diet.44
The role of the gut microbiota in type 1 and 2 diabetes has been researched in mouse models. The development of type 1 diabetes in MyD88-deficient nonobese diabetic (NOD) mice depended on the presence or absence of the gut microbiota, and nearly all germ-free MyD88-deficient NOD mice developed diabetes, whereas colonization of these germ-free MyD88-deficient NOD mice with a defined gut microbiota (representing bacterial phyla normally present in human gut) attenuated type 1 diabetes.47 Another study compared the fecal microbiota profile in lean control, obese diabetic, and obese nondiabetic participants and noted that diabetes was associated with a reduction of Faecalibacterium prausnitzii species.48 A case-control study of type 2 patients with diabetes found decreased Bacteroides vulgatus and Bifidobacterium genus in the diabetic group compared with a healthy control group.49 Thus, altered gut microbiota could play an important role in the development of obesity, insulin resistance, and diabetes.
Intestinal Dysbiosis in CKD/ESRD
Gut Microbiome in CKD/ESRD
Uremic patients show greatly increased counts of both aerobic (approximately 106 bacteria/ml) and anaerobic (approximately 107 bacteria/ml) organisms in the duodenum and jejunum, normally not colonized heavily by bacteria in healthy persons (Table 1).10 Lower intestinal microbial flora has also been shown to be altered in patients with CKD, most notably with decreases in both Lactobacillaceae and Prevotellaceae families.11 Hida et al.9 studied the colonic composition of microbiota in healthy controls and hemodialysis patients. Analysis of the fecal microbiota revealed a disturbed composition of the microbiota characterized by an overgrowth of aerobic bacteria. Although this study did not show a significant difference in the total number of bacteria, the number of aerobic bacteria, such as Enterobacteria and Enterococci species, was approximately 100 times higher in hemodialysis patients. Of the anaerobic bacteria, hemodialysis patients had significantly lower numbers of Bifidobacteria and higher Clostridium perfringens.9 Patients with ESRD were also at a high risk of Clostridium difficile–associated diarrheas.50 Vaziri et al.11 showed significant differences in the abundance of 190 microbial operational taxonomic units (OTUs) between the patients with ESRD and the normal control individuals. To isolate the effect of renal failure, the investigators also examined the gut microbiota in nephrectomized rats.11 The study revealed substantially lower species richness as measured by the number of operational taxonomic units in the nephrectomized rats compared with the controls.
The intestinal dysbiosis may be due to iatrogenic causes or uremia per se.51,52 Loss of kidney function leads to secretion of urea into the gastrointestinal tract. Subsequent hydrolysis of urea by urease expressed by some gut microbes, results in the formation of large quantities of ammonia, which could affect the growth of commensal bacteria.51,52 Other contributing factors include decreased consumption of dietary fiber,53–55 frequent use of antibiotics,56,57 slow colonic transit,58,59 metabolic acidosis,60,61 intestinal wall edema,62–64 and possibly oral iron intake.65,66
There is high prevalence of insufficiency or deficiency in vitamin K among patients with CKD and ESRD.67,68 Pioneering work of Almquist and Stokstad has recognized the biosynthesis of vitamin K by intestinal bacteria as an important source in animals and humans.69 Investigators have shown that certain strains, such as B. fragilis, Bifidobacteria species, Clostridia species, and Streptococcus faecalis, are involved in the biosynthesis of vitamin K.70 The lower part of the intestinal tract, where the bacterial density is highest, is most likely site for the absorption of the vitamin. Consistent with these findings, the intestinal flora has been associated with symptomatic vitamin K deficiency and severe hemorrhage.71–73
Gut Barrier Function in CKD
The gastrointestinal system is at the interphase between the blood and the potentially toxic contents of the gut.74 Histologic changes, including reduction of villous height, elongation of the crypts, and infiltration of lamina propria with inflammatory cells are noted in CKD (Figure 2B).52 Uremia increases intestinal permeability, both in uremic rats and in patients with CKD.75,76 The disruption of colonic epithelial tight junction could subsequently lead to translocation of bacteria and endotoxin across the intestinal wall.77–79 Studies in uremic rats have shown marked azotemia, systemic oxidative stress, and marked depletion of the key protein constituents of the epithelial tight junction (claudin-1, occludin, and ZO1) in the stomach, jejunum, and ileum,80 as well as penetration of bacteria across the intestinal wall and localization in the mesenteric lymph nodes.52 Hemodialysis-induced systemic circulatory stress and recurrent regional ischemia may also damage the mechanical barrier of the gut.81 In addition, factors that promote intestinal dysbiosis may also contribute to the leaky gut in CKD. Gut microbiome dysbiosis is associated with bacterial translocation, thereby contributing to microinflammation in experimental uremia82 as well as in patients with ESRD.83
Endotoxin as a Cause of Inflammation in CKD
Endotoxin, the hydrophobic anchor of LPS, is a phospholipid that constitutes the outer membranes of most Gram-negative bacteria. It is continuously produced in the gut and is transported into intestinal capillaries through a TLR4-dependent mechanism.84 Endotoxin circulates in the plasma of healthy humans at low concentrations (between 1 and 200 pg/ml).85,86 It is taken up by liver and mononuclear phagocyte cells and eventually cleared.87 Endotoxin provokes an array of host responses by binding to the 55-kD glycosyl-phosphatidyl-inositol–anchored myeloid differentiation antigen, CD14.88 LPS-binding protein is a key modulator of cellular response to endotoxin.89 Endotoxin stimulates cells of the immune system, particularly macrophages and the endothelial cells, to become activated and to synthesize and secrete a variety of effector molecules that cause an inflammatory response. Recent evidence indicates that subclinical endotoxemia is a potential cause for inflammation in patients with CKD.90–92
Endotoxin and Atherosclerosis
The association between bacteria and atherosclerosis has been known for more than two decades.93 Recently, focus has shifted from bacteria to its product, endotoxin, for its role in the development of atherosclerosis.85,94 Endotoxin is a key factor in initiation and progression of atherosclerosis through mediation of endothelial cell injury, promotion of recruitment of monocytes, transformation of macrophages to foam cells, and procoagulant activity.95,96 Furthermore, vascular smooth muscle cells exhibit profound responsiveness to even very low levels of endotoxin.97,98 The Bruneck study showed that elevated endotoxin level is a strong risk factor for the development of atherosclerosis in the general population.85 Elevated plasma level of sCD14 is noted in patients with unstable angina and is related to increased aortic stiffness and carotid plaque formation.99,100 Szeto et al.79 showed that circulating endotoxemia in patients undergoing peritoneal dialysis is related to systemic inflammation and features of atherosclerosis. Using two separate cohorts, we demonstrated that sCD14 is associated with mortality in patients with ESRD.92,101
Gut-Derived Uremic Toxins
Certain intestinal bacteria can generate uremic toxins that are absorbed into the blood and are normally cleared by the kidney. Protein fermentation by gut microbiota results in the generation of different metabolites, including phenols102 and indoles.103 Aronov et al.104 compared plasma from hemodialysis patients with and without colon and confirmed the colonic origin of indoxyl sulfate and p-cresol. These are prototype members of a large group of protein-bound uremic toxins that are resistant to clearance by dialysis.105 P-cresol, a 108-Da protein-bound solute, is a colonic fermentation product of the amino acid tyrosine and phenylalanine.106 Most of the p-cresol generated by the intestinal flora is conjugated to p-cresyl sulfate in the intestinal wall and to p-cresyl glucuronide in the liver.107 Intestinal bacteria also have tryptophanase that converts tryptophan to indole, which is subsequently absorbed and metabolized to indoxyl sulfate in the liver.106
Concentrations of indoxyl sulfate and p-cresyl sulfate in the serum are negatively correlated with the level of kidney function.12 A prospective, observational study performed in 268 patients with CKD indicated that baseline levels of indoxyl sulfate and p-cresyl sulfate were predictors of CKD progression.13 Animal studies suggest that these uremic toxins may damage renal tubular cells.108 In uremic rats, administration of indoxyl sulfate mediates the renal expression of genes related to tubulointerstitial fibrosis, such as TGF-β1, tissue inhibitor of metalloproteinases, and pro-α 1, accompanied by a significant decline in renal function and worsening of glomerular sclerosis.109 Indoxyl sulfate also induces nephrotoxicity via organic anion transporter–mediated uptake in the basolateral membrane of renal proximal tubular cells,110,111 where it activates NF-κB and plasminogen activator inhibitor type 1 expression.110,112
Barreto et al.113 showed that an elevated level of indoxyl sulfate is associated with vascular stiffness, aortic calcification, and higher cardiovascular mortality. Indoxyl sulfate is a potential vascular toxin that induces oxidative stress in endothelial cells,114 increases shedding of endothelial microparticles,115 impairs endothelial cell repair mechanism,116 and increases vascular smooth muscle cell proliferation.117 Bammens et al.118 reported that free serum levels of p-cresol is associated with mortality in hemodialysis patients. In vitro evidence indicates that p-cresol inhibits cytokine-stimulated expression of endothelial adhesion molecules—intercellular adhesion molecule 1 and vascular cell adhesion molecule 119—and induces increase in endothelial permeability.120 Thus, gut-derived uremic toxins contribute to progression of CKD as well as cardiovascular disease.
Targeted Interventions to Treat Intestinal Dysbiosis
Recent advances in our understanding of the gut microbiome’s physiologic functions and pathologic consequences of dysbiosis have led to exploration of various ways of reestablishing symbiosis. Most therapies targeting the colonic microenvironment in CKD aim to modulate gut microbiota, block LPS or attenuate inflammation, or target adsorption of uremic toxin end products of microbial fermentation. Some of these approaches are briefly discussed below (reviewed in Table 2).
Table 2.
Effect of probiotics and prebiotics on uremic toxins, inflammation, and atherosclerosis
| Reference | Patient Type/Model (number) | Intervention | Comments |
|---|---|---|---|
| Uremic toxins | |||
| Simenhoff et al.10 | HD patients (8) | L. acidophilus | ↓ Serum dimethylamine ↓ Nitrosodimethylamine |
| Prakash et al.142 | Uremic rats | Genetically engineered E. coli | ↓ Plasma urea |
| Ranganathan et al.173 | Nephrectomized rats | Various combinations of probiotics | ↑ Lifespan |
| ↓ BUN | |||
| Ranganathan et al.174 | Patients with CKD (13) | S. thermophilus, L. acidophilus, and B. longum | ↓ BUN ↓ Uric acid concentration |
| Ranganathan et al.175 | Patients with CKD (246) | S. thermophilus, L. acidophilus, and B. longum | ↓ BUN |
| Meijers et al.128 | HD patients (22) | Oligofructose-enriched inulin | ↓ Serum p-cresyl sulfate and generation rate |
| de Preter et al.176 | Healthy persons (50) | Oligofructose-enriched inulin | ↓ Urinary excretion of p-cresol |
| Nakabayashi et al.177 | HD patients (7) | Galacto-oligosaccharides, L. casei, and B. breve | ↓ Serum p-cresyl sulfate |
| Swanson et al.178 | Healthy persons (68) | Fructooligosaccharides and/or L. acidophilus | ↓ Fecal protein catabolites (beneficial) with fructooligosaccharides ↑ Fecal protein catabolites (harmful) with L. acidophilus |
| Atherosclerosis | |||
| Chen et al.141 | ApoE−/− mice | L. acidophilus | ↓ Atherosclerotic burden |
| Uchida et al.179 | Rabbits on a high cholesterol diet | Exopolysaccharide | ↓ Atherosclerotic lesions |
| Oxman et al.180 | Sprague-Dawley rats | L. bulgaricus-51 | ↓ Reperfusion tachyarrhythmia ↑ Functional recovery of the ischemic rat hearts ↓ Norepinephrine release |
| Naruszewicz et al.181 | Healthy persons (36) | L. plantarum 299v | ↓ Systolic BP and fibrinogen ↓ F2-Isoprostanes and IL-6 ↓ Monocyte adhesion to endothelial cells |
| Inflammation | |||
| Neyrinck et al.182 | Mice | High-molecular-weight arabinoxylane | ↑ Bifidobacteria |
| ↓ Inflammation | |||
| Cani et al.124 | Mice | Oligofructose | ↓ Endotoxemia and proinflammatory cytokines |
| Dewulf et al.183 | Obese women (30) | Inulin/oligofructose | ↓ Endotoxemia |
| Andreasen et al.184 | Patients with T2DM (45) | L. acidophilus NCFM75 | Preserved insulin sensitivity ↓ Inflammation |
| Schiffrin et al.185 | Elderly persons (74) | Oligosaccharides | ↓TNF-α and IL-6 mRNAs |
| ↓Serum sCD14 | |||
| Andreasen et al.184 | Patients with T2DM (45) | L. acidophilus | Preserved insulin sensitivity |
| Did not affect systemic inflammation | |||
| Anderson et al.186 | Elective surgical patients (137) | Combination of probiotics | No measurable effect on bacterial translocation or systemic inflammation |
| Kotzampassi et al.187 | Trauma patients | Probiotics along with and inulin, oat bran, pectin, and resistant starch | ↓ Rate of systemic inflammatory response, syndrome, infections, severe sepsis, and mortality |
HD, hemodialysis; T2DM, type 2 diabetes mellitus.
Modulation of Gut Microbiota
Prebiotics
A prebiotic is a nondigestible (by the host) food ingredient that has a beneficial effect through its selective stimulation of the growth or activity of one or a limited number of bacteria in the colon.121,122 The candidate prebiotics include inulin, fructo-oligosaccharides, galacto-oligosaccharides, soya-oligosaccharides, xylo-oligosaccharides, and pyrodextrins. Prebiotics promote the growth of Bifidobacteria and Lactobacilli species at the expense of other groups of bacteria in the gut, such as Bacteroides species, Clostridia species, and enterobacteria.123 Preliminary evidence indicates that prebiotic oligofructose-enriched inulin (p-inulin) promotes growth of Bifidobacteria species, mediates weight loss, reduces inflammation, and improves metabolic function.124–126 High dietary fiber intake is associated with lower risk of inflammation and reduced mortality in patients with CKD.127 Meijers et al.128 reported that serum concentrations of p-cresol and indoxyl sulfate are reduced by the oral intake of p-inulin in hemodialysis patients.
One of the mechanisms by which p-inulin mediates weight loss may be by enhancing satiety due to bacterial fermentation and increased production of short-chain fatty acids in the gut lumen.126 Short-chain fatty acids stimulate secretion of glucagon-like peptide 1 (GLP-1)129 and peptide YY (PYY).130 GLP-1 has antiobesity and antidiabetic actions by such mechanisms as inhibiting food intake, stimulating insulin secretion, and inducing β-cell proliferation.131 PYY colocalizes with GLP-1 in the intestinal L cells and is also considered an anorexigenic peptide.133 Plasma concentrations of GLP-1133 and PYY134 are reduced in obese individuals, and oligofructose supplementation in rats resulted in reductions in energy intake and increased plasma GLP-1 and PYY concentrations.135
Probiotics
Probiotics are defined by the United Nations’ Food and Agriculture Organization and the World Health Organization as “live microorganisms” that when administered in adequate amounts confer a health benefit on the host.136 Probiotics consist of living bacteria, such as Bifidobacteria species, lactobacilli, and streptococci,137 that can alter gut microbiota and affect the inflammatory state.138,139 Treatment with Bacillus pasteurii and Sporlac slowed the progression of kidney disease and prolonged the life span of fifth/sixth nephrectomized Sprague-Dawley rats.140 Hemodialysis patients treated with oral Lactobacillus acidophilus showed decreased serum dimethylamine, a potential uremic toxin.10 In another study, treatment with L. acidophilus ATCC-4356 reduced the atherosclerotic burden in ApoE−/− mice.141 This was accompanied by an inhibition of translocation of NF-κB p65 from cytoplasm to nucleus, suppression of degradation of aortic inhibitor of κB α, and improvements in gut microbiota distribution. Prakash et al.142 reduced BUN in uremic rats by orally administering microencapsulated, genetically engineered live cells that contained living urease-producing Escherichia coli–DH5.
Acarbose
Acarbose is an inhibitor of α-glucosidase enzymes in the intestinal brush-border that blocks the hydrolysis of poly- and oligosaccharides to glucose and other monosaccharides. The undigested oligosaccharides that enter the colon act as fermentable carbohydrates. Evenepoel et al.143 showed that treatment with acarbose reduces the colonic generation of p-cresol in healthy persons.
Gut Microbiome Transplantation
Manichanh et al.144 examined the long-term effects of exogenous microbiota transplantation alone and combined with antibiotic pretreatment in a rat model. A short intake of antibiotics produced profound long-term effects on the rat intestinal microbiome, with reduced gut microbial diversity. Transplantation of a rich pool of exogenous bacteria led to an increase in bacterial diversity and changing the microbiome of the recipients to resemble that of the donor. Human fecal transplantation has demonstrated efficacy against Clostridium difficile colitis.145
Essential Oils
The potential of essential oils as agents to treat dysbiosis was examined in an in vitro study.146 Results indicated that Carum carvi, Lavandula angustifolia, Trachyspermum copticum, and Citrus aurantium var. amara essential oils displayed the greatest degree of selectivity, inhibiting the growth of potential pathogens at concentrations that had no effect on the beneficial bacteria examined.146 More research is needed, however, to evaluate tolerability and safety concerns and to verify the selective action of these agents.
Blocking of LPS/Attenuation of Inflammation
Sevelamer
Sevelamer is a large cationic polymer phosphate binder that binds endotoxin in both in vitro and in vivo studies.147,148 A cross-sectional study in hemodialysis patients showed that endotoxin level was lower in patients using sevelamer.148 Subsequently, a prospective, randomized, open-label study further confirmed that treatment with sevelamer reduced endotoxin and sCD14 levels in hemodialysis patients.149 Potential interaction between sevelamer and fat-soluble vitamins, including vitamin A, D, E, and K, has been proposed but remains to be determined.150
Synthetic TLR4 Antagonists
he biologic activity of LPS resides almost entirely in its lipid A component.151 The synthetic lipid A analogue eritoran (E5564) and the lipid A mimetic CRX-526152 inhibit LPS signaling.153,154 In healthy persons, E5564 blocked all of the effects of LPS, with significant reductions in white blood cell count, C-reactive protein levels, and cytokine levels (TNF-α and IL-6).155 More recently, C34, a 2-acetamidopyranoside, was developed. It inhibited TLR4 in enterocytes and macrophages in vitro and reduced systemic inflammation in mouse models of endotoxemia and necrotizing enterocolitis.156
Adsorption of Uremic Toxins
Oral Adsorbents
AST-120 is an oral adsorbent consisting of microspheres made from porous carbon material. Administration of AST-120 partially restored the epithelial tight-junction proteins and reduced plasma endotoxin and markers of oxidative stress and inflammation in CKD rats.157 In another study, AST-120 decreased serum levels of indoxyl sulfate and slowed the progression of CKD by reducing the profibrotic gene expression in the rat remnant kidney.158 In patients with CKD, administration of AST-120 significantly decreased the serum and urine levels of indoxyl sulfate and improved the slope of the 1/serum creatinine-time plot.159,160 AST-120 treatment of patients with CKD has also delayed the time to dialysis initiation.161
Miscellaneous
The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are lipid-lowering drugs with anti-inflammatory properties.162 Abe et al.163 demonstrated that statins partially attenuated the development of adipose tissue inflammation in obese mice, which might be associated with an inhibitory effect of statins on TLR4-triggered expression of IFN-β via MyD88-independent signaling pathway in macrophages. Atorvastatin is known to affect LPS indirectly by causing impaired TLR4 recruitment into the lipid raft, thereby affecting anti-inflammatory responses.164 In a small study, optimized BP control with antihypertensive agents decreases endotoxin levels.165 The mechanism of this beneficial effect is unknown.
Conclusions and Future Directions
Resident microbiota outnumber the human host cells by 10-fold, with metabolic activity in excess of that of the liver and a combined microbiome that is estimated to be 100 times greater than that of the human.166 In 2007, the Human Microbiome Project was established to characterize the human microbiome and analyze its role in health and disease.167 The project serves as a “roadmap” for discovering the roles these microorganisms play in human health and disease, with the goal of metagenomic characterization of microbial communities from 300 healthy individuals over time. Not long ago, the products of intestinal putrefaction were considered the primary uremic toxins. The recent explosion of knowledge on the metabolic potential of gut microbiome and its critical role in the pathogenesis of several chronic inflammatory diseases has led the nephrologist to refocus on the gut as a potential cause of CKD-related complication and a target organ for attenuating uremia-related complications. Therefore, it is time for more clinical and basic research studies to further our understanding of the role of the gut microbiome in progression of CKD and its associated complications. Finally, interventions aimed at establishing gut symbiosis and blocking microbiome-related pathogenic biochemical pathways should be explored in order to develop interventions to ameliorate uremic syndrome.
Disclosures
None.
Acknowledgments
D.S.R. is supported by National Institutes of Health grants 1R01DK073665-01A1, 1U01DK099924-01, and 1U01DK099914-01.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hooper LV, Gordon JI: Commensal host-bacterial relationships in the gut. Science 292: 1115–1118, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI: Host-bacterial mutualism in the human intestine. Science 307: 1915–1920, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Midtvedt T, Gordon JI: How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22: 283–307, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Ley RE, Turnbaugh PJ, Klein S, Gordon JI: Microbial ecology: Human gut microbes associated with obesity. Nature 444: 1022–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J: A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR: Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104: 13780–13785, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE: Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 26: 1727–1735, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y: Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74: 349–355, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Simenhoff ML, Dunn SR, Zollner GP, Fitzpatrick ME, Emery SM, Sandine WE, Ayres JW: Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab 22: 92–96, 1996 [PubMed] [Google Scholar]
- 11.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL: Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Lin CJ, Chen HH, Pan CF, Chuang CK, Wang TJ, Sun FJ, Wu CJ: p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J Clin Lab Anal 25: 191–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS: p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 26: 938–947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA: Diversity of the human intestinal microbial flora. Science 308: 1635–1638, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremaroli V, Bäckhed F: Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Hill MJ: Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev 6[Suppl 1]: S43–S45, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Hylemon PB, Harder J: Biotransformation of monoterpenes, bile acids, and other isoprenoids in anaerobic ecosystems. FEMS Microbiol Rev 22: 475–488, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS: Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol 68: 3841–3847, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E, Allergy and Endotoxin Study Team : Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med 347: 869–877, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI: The long-term stability of the human gut microbiota. Science 341: 1237439, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL: An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107–118, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lyte M, Bailey MT: Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J Surg Res 70: 195–201, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Samuel BS, Gordon JI: A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A 103: 10011–10016, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Flier LG, Clevers H: Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Farquhar MG, Palade GE: Junctional complexes in various epithelia. J Cell Biol 17: 375–412, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL: Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295: G1025–G1034, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB: The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1: 299–308, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO: Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018–C1030, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG: Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int 18: 586–590, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA: Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun 73: 5183–5188, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K: Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol 151: 528–535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C: Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Gupta P, Andrew H, Kirschner BS, Guandalini S: Is lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr 31: 453–457, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI: Molecular analysis of commensal host-microbial relationships in the intestine. Science 291: 881–884, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Steinert PM, Marekov LN: Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol Biol Cell 10: 4247–4261, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda K, Kaisho T, Akira S: Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Cario E, Gerken G, Podolsky DK: Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R: Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kramer HJ, Saranathan A, Luke A, Durazo-Arvizu RA, Guichan C, Hou S, Cooper R: Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol 17: 1453–1459, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Nerpin E, Risérus U, Ingelsson E, Sundström J, Jobs M, Larsson A, Basu S, Arnlöv J: Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care 31: 1550–1555, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI: Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102: 11070–11075, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ: Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 24: 4948–4959, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI: Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3: 213–223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI: The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 101: 15718–15723, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI: Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 104: 979–984, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV: Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455: 1109–1113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, Rizkalla S, Clément K: Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 59: 3049–3057, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, Yu P, Zhao C, Li L, Zhou A, Wang J, Moore JE, Millar BC, Xu J: Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 61: 69–78, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Aronsson B, Barany P, Nord CE, Nyström B, Stenvinkel P: Clostridium difficile-associated diarrhoea in uremic patients. Eur J Clin Microbiol 6: 352–356, 1987 [DOI] [PubMed] [Google Scholar]
- 51.Kang JY: The gastrointestinal tract in uremia. Dig Dis Sci 38: 257–268, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Vaziri ND, Dure-Smith B, Miller R, Mirahmadi MK: Pathology of gastrointestinal tract in chronic hemodialysis patients: An autopsy study of 78 cases. Am J Gastroenterol 80: 608–611, 1985 [PubMed] [Google Scholar]
- 53.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G: Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr 12: 17–31, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Hatch M, Freel RW, Vaziri ND: Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol 5: 1339–1343, 1994 [DOI] [PubMed] [Google Scholar]
- 55.Schena FP: Management of patients with chronic kidney disease. Intern Emerg Med 6[Suppl 1]: 77–83, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L: Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 5: e9836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jernberg C, Löfmark S, Edlund C, Jansson JK: Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156: 3216–3223, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Lefebvre HP, Ferré JP, Watson AD, Brown CA, Serthelon JP, Laroute V, Concordet D, Toutain PL: Small bowel motility and colonic transit are altered in dogs with moderate renal failure. Am J Physiol Regul Integr Comp Physiol 281: R230–R238, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, Shu KH, Tang MJ: Colonic transit time in long-term dialysis patients. Am J Kidney Dis 44: 322–327, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Goraya N, Wesson DE: Dietary management of chronic kidney disease: Protein restriction and beyond. Curr Opin Nephrol Hypertens 21: 635–640, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Goraya N, Wesson DE: Acid-base status and progression of chronic kidney disease. Curr Opin Nephrol Hypertens 21: 552–556, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Kooman JP, van der Sande FM, Leunissen KM: Role of sodium and volume in the pathogenesis of hypertension in dialysis patients. Reflections on pathophysiological mechanisms. Blood Purif 22: 55–59, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, Matsumori A: Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol 22: 811–813, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD: Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 353: 1838–1842, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Werner T, Wagner SJ, Martínez I, Walter J, Chang JS, Clavel T, Kisling S, Schuemann K, Haller D: Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut 60: 325–333, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Liguori L: Iron protein succinylate in the treatment of iron deficiency: Controlled, double-blind, multicenter clinical trial on over 1,000 patients. Int J Clin Pharmacol Ther Toxicol 31: 103–123, 1993 [PubMed] [Google Scholar]
- 67.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45: 1026–1033, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Pilkey RM, Morton AR, Boffa MB, Noordhof C, Day AG, Su Y, Miller LM, Koschinsky ML, Booth SL: Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis 49: 432–439, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Almqutst HJ, Stokstan ELR: The Gizzard factor of the chick. J Nutr 13: 339–350, 1937 [Google Scholar]
- 70.Fernandez F, Hill MJ: Proceedings: The production of vitamin K by human intestinal bacteria. J Med Microbiol 8: 1975 [PubMed] [Google Scholar]
- 71.Resta SC: Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J Physiol 587: 4169–4174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bentley R, Meganathan R: Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev 46: 241–280, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M: Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr Opin Biotechnol 24: 160–168, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Farhadi A, Banan A, Fields J, Keshavarzian A: Intestinal barrier: An interface between health and disease. J Gastroenterol Hepatol 18: 479–497, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Magnusson M, Magnusson KE, Sundqvist T, Denneberg T: Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: Effects of low- and high-protein diets. Nephron 56: 306–311, 1990 [DOI] [PubMed] [Google Scholar]
- 76.Magnusson M, Magnusson KE, Sundqvist T, Denneberg T: Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 32: 754–759, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, Nochi RJ, Jr: Bacterial translocation in experimental uremia. Urol Res 32: 266–270, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Gonçalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AE, Lima EG, Fuerbringer R, Sauthier SM, Riella MC: Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant 21: 2788–2794, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK: Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 3: 431–436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S: Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol 38: 99–103, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Ding LA, Li JS: Gut in diseases: physiological elements and their clinical significance. World J Gastroenterol 9: 2385–2389, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang F, Zhang P, Jiang H, Cheng S: Gut bacterial translocation contributes to microinflammation in experimental uremia. Dig Dis Sci 57: 2856–2862, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S: Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 17: 733–738, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ: Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176: 3070–3079, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J: Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: Prospective results from the Bruneck Study. J Am Coll Cardiol 34: 1975–1981, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Goto T, Edén S, Nordenstam G, Sundh V, Svanborg-Edén C, Mattsby-Baltzer I: Endotoxin levels in sera of elderly individuals. Clin Diagn Lab Immunol 1: 684–688, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caridis DT, Reinhold RB, Woodruff PW, Fine J: Endotoxaemia in man. Lancet 1: 1381–1385, 1972 [DOI] [PubMed] [Google Scholar]
- 88.Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, Glauser MP, Tobias PS, Ulevitch RJ: CD14 is a pattern recognition receptor. Immunity 1: 509–516, 1994 [DOI] [PubMed] [Google Scholar]
- 89.Tobias PS, Soldau K, Ulevitch RJ: Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem 264: 10867–10871, 1989 [PubMed] [Google Scholar]
- 90.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK: Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feroze U, Kalantar-Zadeh K, Sterling KA, Molnar MZ, Noori N, Benner D, Shah V, Dwivedi R, Becker K, Kovesdy CP, Raj DS: Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J Ren Nutr 22: 317–326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raj DS, Carrero JJ, Shah VO, Qureshi AR, Bárány P, Heimbürger O, Lindholm B, Ferguson J, Moseley PL, Stenvinkel P: Soluble CD14 levels, interleukin 6, and mortality among prevalent hemodialysis patients. Am J Kidney Dis 54: 1072–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valtonen VV: Infection as a risk factor for infarction and atherosclerosis. Ann Med 23: 539–543, 1991 [DOI] [PubMed] [Google Scholar]
- 94.Stoll LL, Denning GM, Weintraub NL: Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 24: 2227–2236, 2004 [DOI] [PubMed] [Google Scholar]
- 95.Reidy MA, Bowyer DE: Distortion of endothelial repair. The effect of hypercholesterolaemia on regeneration of aortic endothelium following injury by endotoxin. A scanning electron microscope study. Atherosclerosis 29: 459–466, 1978 [DOI] [PubMed] [Google Scholar]
- 96.Eggesbø JB, Hjermann I, Ovstebø R, Joø GB, Kierulf P: LPS induced procoagulant activity and plasminogen activator activity in mononuclear cells from persons with high or low levels of HDL lipoprotein. Thromb Res 77: 441–452, 1995 [DOI] [PubMed] [Google Scholar]
- 97.Rice JB, Stoll LL, Li WG, Denning GM, Weydert J, Charipar E, Richenbacher WE, Miller FJ, Jr, Weintraub NL: Low-level endotoxin induces potent inflammatory activation of human blood vessels: Inhibition by statins. Arterioscler Thromb Vasc Biol 23: 1576–1582, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Stoll LL, Denning GM, Li WG, Rice JB, Harrelson AL, Romig SA, Gunnlaugsson ST, Miller FJ, Jr, Weintraub NL: Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: Expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. J Immunol 173: 1336–1343, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zalai CV, Kolodziejczyk MD, Pilarski L, Christov A, Nation PN, Lundstrom-Hobman M, Tymchak W, Dzavik V, Humen DP, Kostuk WJ, Jablonsky G, Pflugfelder PW, Brown JE, Lucas A: Increased circulating monocyte activation in patients with unstable coronary syndromes. J Am Coll Cardiol 38: 1340–1347, 2001 [DOI] [PubMed] [Google Scholar]
- 100.Amar J, Ruidavets JB, Bal Dit Sollier C, Bongard V, Boccalon H, Chamontin B, Drouet L, Ferrières J: Soluble CD14 and aortic stiffness in a population-based study. J Hypertens 21: 1869–1877, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Raj DS, Shah VO, Rambod M, Kovesdy CP, Kalantar-Zadeh K: Association of soluble endotoxin receptor CD14 and mortality among patients undergoing hemodialysis. Am J Kidney Dis 54: 1062–1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bone E, Tamm A, Hill M: The production of urinary phenols by gut bacteria and their possible role in the causation of large bowel cancer. Am J Clin Nutr 29: 1448–1454, 1976 [DOI] [PubMed] [Google Scholar]
- 103.Macfarlane GT, Macfarlane S: Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 95: 50–60, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez AW, Recht NS, Hostetter TH, Meyer TW: Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol 16: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Cummings JH: Fermentation in the human large intestine: Evidence and implications for health. Lancet 1: 1206–1209, 1983 [DOI] [PubMed] [Google Scholar]
- 107.de Loor H, Meijers BK, Meyer TW, Bammens B, Verbeke K, Dehaen W, Evenepoel P: Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J Chromatogr A 1216: 4684–4688, 2009 [DOI] [PubMed] [Google Scholar]
- 108.Satoh M, Hayashi H, Watanabe M, Ueda K, Yamato H, Yoshioka T, Motojima M: Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron, Exp Nephrol 95: e111–e118, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Miyazaki T, Ise M, Seo H, Niwa T: Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int Suppl 62: S15–S22, 1997 [PubMed] [Google Scholar]
- 110.Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, Nolph KD, Pollock CA, Prowant B, Farrell PC: The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 37: 598–604, 1991 [PubMed] [Google Scholar]
- 111.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T: Uraemic toxins induce proximal tubular injury via organic anion transporter 1-mediated uptake. Br J Pharmacol 135: 555–563, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T: Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int 63: 1671–1680, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P: The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5: 1302–1308, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Amabile N, Guérin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM: Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 16: 3381–3388, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P: The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442–451, 2004 [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto H, Tsuruoka S, Ioka T, Ando H, Ito C, Akimoto T, Fujimura A, Asano Y, Kusano E: Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int 69: 1780–1785, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 119.Dou L, Cerini C, Brunet P, Guilianelli C, Moal V, Grau G, De Smet R, Vanholder R, Sampol J, Berland Y: P-cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int 62: 1999–2009, 2002 [DOI] [PubMed] [Google Scholar]
- 120.Cerini C, Dou L, Anfosso F, Sabatier F, Moal V, Glorieux G, De Smet R, Vanholder R, Dignat-George F, Sampol J, Berland Y, Brunet P: P-cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb Haemost 92: 140–150, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Gibson GR, Roberfroid MB: Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr 125: 1401–1412, 1995 [DOI] [PubMed] [Google Scholar]
- 122.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB: Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr Res Rev 17: 259–275, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR: Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 29: 508–518, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM: Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50: 2374–2383, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Gibson GR, Beatty ER, Wang X, Cummings JH: Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108: 975–982, 1995 [DOI] [PubMed] [Google Scholar]
- 126.Pylkas AM, Juneja LR, Slavin JL: Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J Med Food 8: 113–116, 2005 [DOI] [PubMed] [Google Scholar]
- 127.Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S: High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 25: 219–224, 2010 [DOI] [PubMed] [Google Scholar]
- 129.Reimer RA, McBurney MI: Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology 137: 3948–3956, 1996 [DOI] [PubMed] [Google Scholar]
- 130.Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC: Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology 139: 3780–3786, 1998 [DOI] [PubMed] [Google Scholar]
- 131.Drucker DJ: The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Grudell AB, Camilleri M: The role of peptide YY in integrative gut physiology and potential role in obesity. Curr Opin Endocrinol Diabetes Obes 14: 52–57, 2007 [DOI] [PubMed] [Google Scholar]
- 133.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V: Attenuated GLP-1 secretion in obesity: Cause or consequence? Gut 38: 916–919, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR: Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Delzenne NM, Cani PD, Daubioul C, Neyrinck AM: Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr 93[Suppl 1]: S157–S161, 2005 [DOI] [PubMed] [Google Scholar]
- 136.Food and Agriculture Organization of the United Nations (FAO). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. 2001. Available at: http://www.who.int/foodsafety/publications/fs_management/probiotics/en/ Accessed October 30, 2013
- 137.Rastall RA, Gibson GR, Gill HS, Guarner F, Klaenhammer TR, Pot B, Reid G, Rowland IR, Sanders ME: Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: An overview of enabling science and potential applications. FEMS Microbiol Ecol 52: 145–152, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y: S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci U S A 105: 19474–19479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, Hooiveld GJ, Brummer RJ, Kleerebezem M, van BP : Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A 106: 2371–2376, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ranganathan N, Patel B, Ranganathan P, Marczely J, Dheer R, Chordia T, Dunn SR, Friedman EA: Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. ScientificWorldJournal 5: 652–660, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen L, Liu W, Li Y, Luo S, Liu Q, Zhong Y, Jian Z, Bao M: Lactobacillus acidophilus ATCC 4356 attenuates the atherosclerotic progression through modulation of oxidative stress and inflammatory process. Int Immunopharmacol 17: 108–115, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Prakash S, Chang TM: Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats. Nat Med 2: 883–887, 1996 [DOI] [PubMed] [Google Scholar]
- 143.Evenepoel P, Bammens B, Verbeke K, Vanrenterghem Y: Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: a pilot study. Kidney Int 70: 192–198, 2006 [DOI] [PubMed] [Google Scholar]
- 144.Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, Guigo R, Knight R, Guarner F: Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res 20: 1411–1419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Persky SE, Brandt LJ: Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 95: 3283–3285, 2000 [DOI] [PubMed] [Google Scholar]
- 146.Hawrelak JA, Cattley T, Myers SP: Essential oils in the treatment of intestinal dysbiosis: A preliminary in vitro study. Altern Med Rev 14: 380–384, 2009 [PubMed] [Google Scholar]
- 147.Perianayagam MC, Jaber BL: Endotoxin-binding affinity of sevelamer hydrochloride. Am J Nephrol 28: 802–807, 2008 [DOI] [PubMed] [Google Scholar]
- 148.Sun PP, Perianayagam MC, Jaber BL: Sevelamer hydrochloride use and circulating endotoxin in hemodialysis patients: A pilot cross-sectional study. J Ren Nutr 19: 432–438, 2009 [DOI] [PubMed] [Google Scholar]
- 149.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Donate-Correa J, Cazaña-Pérez V, García-Pérez J: Effect of phosphate binders on serum inflammatory profile, soluble CD14, and endotoxin levels in hemodialysis patients. Clin J Am Soc Nephrol 6: 2272–2279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Susantitaphong P, Jaber BL: Potential interaction between sevelamer and fat-soluble vitamins: A hypothesis. Am J Kidney Dis 59: 165–167, 2012 [DOI] [PubMed] [Google Scholar]
- 151.Bortoluci KR, Medzhitov R: Control of infection by pyroptosis and autophagy: Role of TLR and NLR. Cell Mol Life Sci 67: 1643–1651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johnson DA: Synthetic TLR4-active glycolipids as vaccine adjuvants and stand-alone immunotherapeutics. Curr Top Med Chem 8: 64–79, 2008 [DOI] [PubMed] [Google Scholar]
- 153.Barochia A, Solomon S, Cui X, Natanson C, Eichacker PQ: Eritoran tetrasodium (E5564) treatment for sepsis: Review of preclinical and clinical studies. Expert Opin Drug Metab Toxicol 7: 479–494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fort MM, Mozaffarian A, Stöver AG, Correia JS, Johnson DA, Crane RT, Ulevitch RJ, Persing DH, Bielefeldt-Ohmann H, Probst P, Jeffery E, Fling SP, Hershberg RM: A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol 174: 6416–6423, 2005 [DOI] [PubMed] [Google Scholar]
- 155.Lynn M, Rossignol DP, Wheeler JL, Kao RJ, Perdomo CA, Noveck R, Vargas R, D’Angelo T, Gotzkowsky S, McMahon FG: Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis 187: 631–639, 2003 [DOI] [PubMed] [Google Scholar]
- 156.Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, Afrazi A, Prindle T, Jr, Ma C, Branca M, Ozolek J, Brodsky JL, Wipf P, Hackam DJ: Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS ONE 8: e65779, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vaziri ND, Yuan J, Khazaeli M, Masuda Y, Ichii H, Liu S: Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am J Nephrol 37: 518–525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miyazaki T, Aoyama I, Ise M, Seo H, Niwa T: An oral sorbent reduces overload of indoxyl sulphate and gene expression of TGF-beta1 in uraemic rat kidneys. Nephrol Dial Transplant 15: 1773–1781, 2000 [DOI] [PubMed] [Google Scholar]
- 159.Niwa T, Emoto Y, Maeda K, Uehara Y, Yamada N, Shibata M: Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant 6: 105–109, 1991 [DOI] [PubMed] [Google Scholar]
- 160.Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S: The protein metabolite hypothesis, a model for the progression of renal failure: An oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int Suppl 62: S23–S28, 1997 [PubMed] [Google Scholar]
- 161.Hatakeyama S, Yamamoto H, Okamoto A, Imanishi K, Tokui N, Okamoto T, Suzuki Y, Sugiyama N, Imai A, Kudo S, Yoneyama T, Hashimoto Y, Koie T, Kaminura N, Saitoh H, Funyu T, Ohyama C: Effect of an Oral Adsorbent, AST-120, on Dialysis Initiation and Survival in Patients with Chronic Kidney Disease. Int J Nephrol 2012: 376128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takemoto M, Liao JK: Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 21: 1712–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Abe M, Matsuda M, Kobayashi H, Miyata Y, Nakayama Y, Komuro R, Fukuhara A, Shimomura I: Effects of statins on adipose tissue inflammation: Their inhibitory effect on MyD88-independent IRF3/IFN-beta pathway in macrophages. Arterioscler Thromb Vasc Biol 28: 871–877, 2008 [DOI] [PubMed] [Google Scholar]
- 164.Chansrichavala P, Chantharaksri U, Sritara P, Ngaosuwankul N, Chaiyaroj SC: Atorvastatin affects TLR4 clustering via lipid raft modulation. Int Immunopharmacol 10: 892–899, 2010 [DOI] [PubMed] [Google Scholar]
- 165.John SG, Owen PJ, Harrison LE, Szeto CC, Lai KB, Li PK, McIntyre CW: The impact of antihypertensive drug therapy on endotoxemia in elderly patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2389–2394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shanahan F: The host-microbe interface within the gut. Best Pract Res Clin Gastroenterol 16: 915–931, 2002 [DOI] [PubMed] [Google Scholar]
- 167.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI: The human microbiome project. Nature 449: 804–810, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lotz M, Gütle D, Walther S, Ménard S, Bogdan C, Hornef MW: Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 203: 973–984, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P: Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology 124: 1265–1276, 2003 [DOI] [PubMed] [Google Scholar]
- 170.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS: Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J 26: 4457–4466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Macpherson AJ, Uhr T: Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303: 1662–1665, 2004 [DOI] [PubMed] [Google Scholar]
- 172.Bode JG, Ehlting C, Häussinger D: The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal 24: 1185–1194, 2012 [DOI] [PubMed] [Google Scholar]
- 173.Ranganathan N, Patel B, Ranganathan P, Marczely J, Dheer R, Chordia T, Dunn SR, Friedman EA: Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. ScientificWorldJournal 5: 652–660, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R: Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr Med Res Opin 25: 1919–1930, 2009 [DOI] [PubMed] [Google Scholar]
- 175.Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, Tam P, Rao AV, Anteyi E, Musso CG: Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther 27: 634–647, 2010 [DOI] [PubMed] [Google Scholar]
- 176.de Preter V, Vanhoutte T, Huys G, Swings J, Rutgeerts P, Verbeke K: Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment Pharmacol Ther 27: 504–513, 2008 [DOI] [PubMed] [Google Scholar]
- 177.Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, Yoshida M: Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol Dial Transplant 26: 1094–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 178.Swanson KS, Grieshop CM, Flickinger EA, Bauer LL, Wolf BW, Chow J, Garleb KA, Williams JA, Fahey GC, Jr: Fructooligosaccharides and Lactobacillus acidophilus modify bowel function and protein catabolites excreted by healthy humans. J Nutr 132: 3042–3050, 2002 [DOI] [PubMed] [Google Scholar]
- 179.Uchida M, Ishii I, Inoue C, Akisato Y, Watanabe K, Hosoyama S, Toida T, Ariyoshi N, Kitada M: Kefiran reduces atherosclerosis in rabbits fed a high cholesterol diet. J Atheroscler Thromb 17: 980–988, 2010 [DOI] [PubMed] [Google Scholar]
- 180.Oxman T, Shapira M, Klein R, Avazov N, Rabinowitz B: Oral administration of Lactobacillus induces cardioprotection. J Altern Complement Med 7: 345–354, 2001 [DOI] [PubMed] [Google Scholar]
- 181.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H: Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr 76: 1249–1255, 2002 [DOI] [PubMed] [Google Scholar]
- 182.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM: Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 6: e20944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, Delzenne NM: Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62: 1112–1121, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Møller K, Svendsen KD, Jakobsen M, Pedersen BK: Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 104: 1831–1838, 2010 [DOI] [PubMed] [Google Scholar]
- 185.Schiffrin EJ, Thomas DR, Kumar VB, Brown C, Hager C, Van’t Hof MA, Morley JE, Guigoz Y: Systemic inflammatory markers in older persons: The effect of oral nutritional supplementation with prebiotics. J Nutr Health Aging 11: 475–479, 2007 [PubMed] [Google Scholar]
- 186.Anderson AD, McNaught CE, Jain PK, MacFie J: Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 53: 241–245, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E: Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: Early results of a randomized controlled trial. World J Surg 30: 1848–1855, 2006 [DOI] [PubMed] [Google Scholar]