Abstract
Adenine phosphoribosyltransferase deficiency is a rare autosomal recessive disorder manifesting as urolithiasis or crystalline nephropathy. It leads to the generation of large amounts of poorly soluble 2,8-dihydroxyadenine excreted in urine, yielding kidney injury and in some patients, kidney failure. Early recognition of the disease, institution of xanthine analog therapy to block the formation of 2,8-dihydroxyadenine, high fluid intake, and low purine diet prevent CKD. Because of symptom variability and lack of awareness, however, the diagnosis is sometimes extremely deferred. We describe a patient with adenine phosphoribosyltransferase deficiency who was diagnosed during evaluation of a poorly functioning second kidney allograft. This report highlights the risk of renal allograft loss in patients with undiagnosed adenine phosphoribosyltransferase deficiency and the need for improved early detection of this disease.
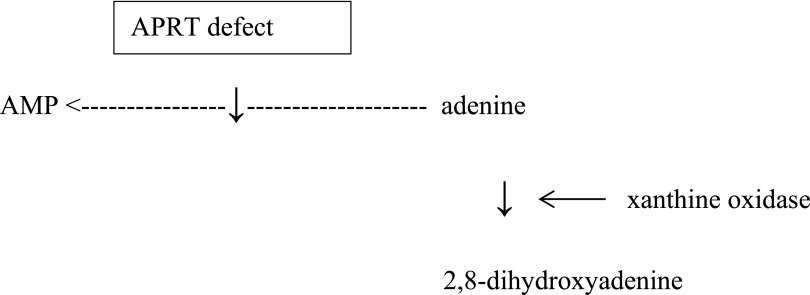
In humans, 2,8-dihydroxyadenine (DHA) does not normally occur as a metabolic product.1 However, in the autosomal recessive defect of adenine phosphoribosyltransferase (APRT) enzyme, adenine that is normally transformed into adenosine monophosphate, instead, is oxidized into DHA.1 This reaction is catalyzed by xanthine oxidase (Figure 1).1 Under normal circumstances, no DHA can be found in urine.1 DHA is protein-bound in plasma but extremely insoluble in urine, leading to the formation of crystals and subsequently, stones or precipitation in renal parenchyma, which causes crystalline nephropathy.2,3 Both these conditions can occur together or separately.3 The kidneys and urinary tract system seem to be the only organs affected by APRT deficiency.4
Figure 1.
Metabolism of adenine. The role of APRT deficiency in adenine metabolism in a simplified description. Modified from ref.1, with permission.
Prevalence of APRT deficiency is largely unknown, and some mutations have high frequency in certain countries.4 It has been suggested that prevalence of homozygotes is at least 1:50,000 to 1:100,000, meaning that the disease is poorly recognized.4,5 Lack of knowledge, inadequate evaluation of kidney stone patients, and confusion of DHA crystals or calculi with uric acid or calcium oxalate may contribute to lack of recognition.4
Case Report and Results
A 63-year-old white man born in the Middle East experienced recurrent kidney stones from childhood. After moving to Finland, his urine sediment showed remarkable spherical or multiformic crystals that were classified as urates in 1999. Kidney stones were identified as uric acid concrements, and no medication was commenced; plasma creatinine and urine sediment were normal in urological evaluation in 2003. He had no history of any other diseases. His grandfather had suffered from urinary stones, but otherwise, family history was negative. Because of recurrent stone problems, several interventional measures were needed during the subsequent years.
In 2007, he was referred to a nephrologist. Serum creatinine was 3.05 mg/dl (270 µmol/L; conversion factor=88.4), and urine sediment was negative. Serum calcium and parathyroid hormone concentrations, acid–base balance, and 24-hour excretions of calcium, magnesium, oxalate, and amino acids into urine were normal, but the excretion of uric acid was low. Computed tomography scan showed atrophy and several cysts in both kidneys and stones in the left kidney. Biopsy was not performed because of the small size of the kidneys, and maintenance dialysis was started 1 year later.
The first kidney transplantation from a deceased donor was performed in May of 2009. The lowest serum creatinine of 2.3 mg/dl was measured 2 months after the transplantation. The first allograft biopsy taken at 4 months showed normal glomerular and vascular pattern and only mild interstitial fibrosis and tubular atrophy, although serum creatinine was 3.3 mg/dl. Reanalysis of this biopsy after correct diagnosis revealed that a small area had crystals, but they had vanished, most likely during processing of the biopsy. The second biopsy was taken at 8 months and had mild tubular cell necrosis; also, tubuli were filled with amorphous material. Tubulointerstitium consisted of small lymphocytes, mononuclear cells, and some eosinophils. No crystals were found, even in reanalysis. However, the patient had periodic pain in native kidneys resembling previous stone problems, and he also reported almost weekly passing of stones into urine. Allograft dysfunction progressed further, and hemodialysis was restarted 9 months after transplantation.
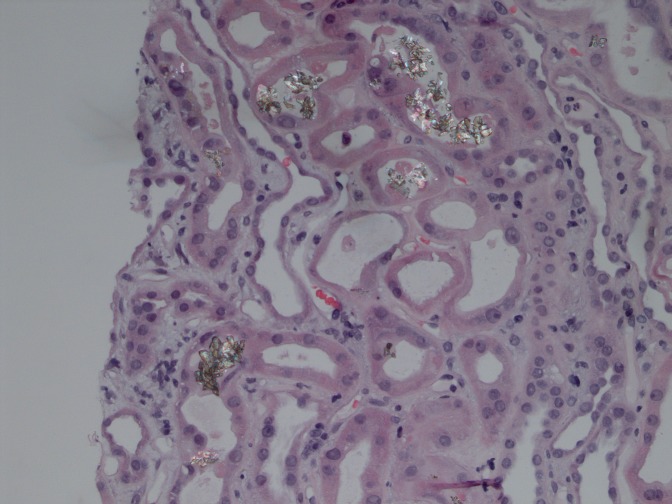
The second cadaveric transplantation was performed in September of 2012. Anuria persisted for 3 weeks, and although diuresis subsequently increased to a level of 1000 ml/d, he remained dialysis-dependent. Allograft biopsy was taken three times: 10, 19, and 38 days post-transplantation. Every biopsy revealed normal glomerular pattern and no signs of rejection but extensive acute tubular injury and intratubular obstruction by needle-shaped crystals of unknown type (Figure 2). These crystals were absent in the initial donor biopsy specimen. Analysis of urinary crystals now showed irregular round clusters reminiscent of purine molecules, and DHA was then raised as a possibility. APRT activity of red blood cells was markedly reduced to a level of 2.5 nmol/h per mg (reference=20–80 nmol/h per mg), consistent with APRT deficiency. Genetic analysis was performed after informed consent from the patient. A homozygous mutation in c.188G>A, p.G63D in the APRT gene was found, causing glycine (codon GGC) transformation into aspartate.
Figure 2.
Kidney biopsy shows extensive tubular epithelial cell injury and birefringent crystals within the tubular lumen.
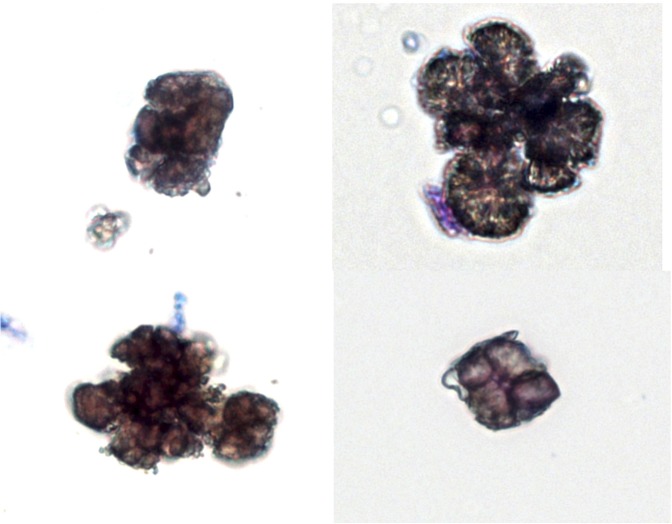
Allopurinol (300 mg/d) was started; however, kidney function has not improved, and 11 months after treatment, he is still dialysis-dependent. The current dose of allopurinol is 500 mg/d, because with the smaller dose, DHA crystals were still evident in the urine sample (Figure 3).
Figure 3.
The four images show the morphologic variability and irregularity of DHA crystals from the urine of our patient. Occasional crystals were still evident after the commencement of allopurinol treatment. Note the clustering and irregular shapes of the crystals, which resemble occasionally round urate crystals. Sternheimer supravital staining (Alcian blue-pyronin B) creates the brownish color (bright field image). Original magnification, ×400.
Discussion
Almost all patients have pathognomonic urinary DHA crystals, but crystals may be difficult to identify in case of diminished crystal clearance.4,6 Recurrent nephrolithiasis is a much more common presentation than crystalline nephropathy, and analysis of a stone consisting of DHA establishes the diagnosis.4 Urinary purine-type crystals are obvious years before development of renal failure, but they are easily confused with more common urate crystals (also purine). Infrared spectroscopy allows correct identification.3,4 By light microscopy, crystals may resemble calcium oxalate; however, they appear brown on the hematoxylin and eosin stain, whereas calcium oxalate crystals are optically clear.4,6 DHA crystals reflect light in polarized or phase-contrast microscopy (Figures 2 and 3). Measurement of APRT activity in red blood cells reveals almost absent enzyme activity in homozygotes and compound heterozygotes and markedly reduced activity to about one quarter of normal values in heterozygotes.4 Genetic testing is not clinically indicated in cases of abolished red blood cell APRT activity or if the diagnosis is otherwise clear from the stone analysis.4 Kidney biopsy may reveal crystalline nephropathy and variable degrees of tubulointerstitial injury. Crystals may lack the typical features of DHA.3,4 Clinical presentation can be AKI, CKD, or acute allograft dysfunction in patients with no previous stone history.4 Most cases have been reported from Iceland, Japan, and France.4
Our patient had mainly stone problems during the first transplant and crystalline nephropathy during the second transplant. Correct diagnosis was made only after evaluation of delayed graft function of the second transplant. Clinical suspicion rose when crystals were found in the biopsy, and it was substantiated by urinary DHA crystals. Red blood cell APRT activity was very low and genetic analysis revealed a homozygous mutation in the APRT gene. The diagnostic delays range from 6 months to 62 years, which our case also showed.7
The size of DHA stones is variable. Spontaneous stone passage is possible, but chemical dissolution is impossible under physiologic conditions.1 DHA formation can be inhibited by allopurinol, which acts by blocking xanthine dehydrogenase. Febuxostat has also been used successfully.1,4 Allopurinol dose is usually 5–10 mg/kg per day, with a target to decrease DHA excretion to a minimum and increase excretion of adenine.1,4 Low purine diet as well as sufficient fluid intake are also necessary.1,3,4 Optimal therapy prevents the development of kidney problems.3 Heterozygotes usually do not have crystalluria and remain asymptomatic, and, therefore, they do not need allopurinol therapy.3 However, analysis of urinary crystals is recommended, even for heterozygotes, in case of symptoms.3,8
Undiagnosed DHA nephropathy will lead to progressive allograft dysfunction within a few weeks.2,6,9,10 Less than 12 published cases of APRT deficiency are reported among patients with kidney allografts. A French publication showed that five of six patients were correctly diagnosed only after severe allograft dysfunction caused by intratubular and interstitial precipitation of DHA crystals. Despite treatment, two patients returned to dialysis, and the other four patients had severe but stable graft dysfunction. One patient had a correct diagnosis and subsequent allopurinol treatment only a few days after transplantation, which established good graft function.6 The possibility of renal recovery depends on the degree of acute tubular necrosis and chronic tubulointerstitial changes when treatment is initiated. With proper treatment, crystalluria decreases strongly, and it can also be used for treatment monitoring,6 which may prove to be especially important, because a higher than normal dose of allopurinol may be warranted.2,10 With vigorous treatment, even improvement of allograft dysfunction may be achieved.10,11 Asymptomatic individuals likely benefit from the same treatment.12 It is not known if individuals with APRT deficiency on dialysis benefit from allopurinol therapy, but all individuals undergoing renal transplantation require treatment with allopurinol.13
Our case emphasizes that patients with undiagnosed APRT deficiency undergoing transplantation are at high risk of losing their transplant. Early detection is critical, because kidney damage is easily prevented with proper medication.
Concise Methods
Urinary crystals were identified using standardized visual microscopy by both bright-field and phase contrast optics using Sternheimer staining in the Helsinki University Central Hospital Laboratory, Helsinki University Central Hospital Laboratory.14 Alltima C18 5µ 250×4.6-mm reversed phase analytical column and diode array detection in a Shimadzu 2010A HPLC system was used to measure red cell APRT activity at Erasmus University in Rotterdam, The Netherlands. After DNA extraction, sequencing was performed with dye terminators, and the fragments were separated by capillary electrophoresis (ABI Prism 3130) at the Center for Nephrology and Metabolic Disorders, Weisswasser, Germany.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hesse A, Tiselius H-G, Siener R, Hoppe B: 2,8-dihydroxyadenine stones. In: Urinary Stones Diagnosis, Treatment, and Prevention of Recurrence, 3rd Ed., Karger, 2009, pp 142–151 [Google Scholar]
- 2.Bertram A, Broecker V, Lehner F, Schwarz A: Kidney transplantation in a patient with severe adenine phosphoribosyl transferase deficiency: Obstacles and pitfalls. Transpl Int 23: e56–e58, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bollée G, Harambat J, Bensman A, Knebelmann B, Daudon M, Ceballos-Picot I: Adenine phosphoribosyltransferase deficiency. Clin J Am Soc Nephrol 7: 1521–1527, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Edvardsson VO, Goldfarb DS, Lieske JC, Beara-Lasic L, Anglani F, Milliner DS, Palsson R: Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol 28: 1923–1942, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahota A, Chen J, Boyadjiev SA, Gault MH, Tischfield JA: Missense mutation in the adenine phosphoribosyltransferase gene causing 2,8-dihydroxyadenine urolithiasis. Hum Mol Genet 3: 817–818, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Bollée G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, Deteix P, Daudon M, Knebelmann B, Ceballos-Picot I: Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol 21: 679–688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T: Clinical features and genotype of adenine phosphoribosyltransferase deficiency in iceland. Am J Kidney Dis 38: 473–480, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Sahota A, Chen J, Behzadian MA, Ravindra R, Takeuchi H, Stambrook PJ, Tischfield JA: 2,8-Dihydroxyadenine lithiasis in a Japanese patient heterozygous at the adenine phosphoribosyltransferase locus. Am J Hum Genet 48: 983–989, 1991 [PMC free article] [PubMed] [Google Scholar]
- 9.Benedetto B, Madden R, Kurbanov A, Braden G, Freeman J, Lipkowitz GS: Adenine phosphoribosyltransferase deficiency and renal allograft dysfunction. Am J Kidney Dis 37: E37, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Nasr SH, Sethi S, Cornell LD, Milliner DS, Boelkins M, Broviac J, Fidler ME: Crystalline nephropathy due to 2,8-dihydroxyadeninuria: An under-recognized cause of irreversible renal failure. Nephrol Dial Transplant 25: 1909–1915, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Stratta P, Fogazzi GB, Canavese C, Airoldi A, Fenoglio R, Bozzola C, Ceballos-Picot I, Bollée G, Daudon M: Decreased kidney function and crystal deposition in the tubules after kidney transplant. Am J Kidney Dis 56: 585–590, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Harambat J, Bollée G, Daudon M, Ceballos-Picot I, Bensman A; APRT Study Group: Adenine phosphoribosyltransferase deficiency in children. Pediatr Nephrol 27: 571–579, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Edvardsson V, Palsson R, Sahota A: Adenine Phosphoribosyltransferase Deficiency. GeneReviews at Genetests: Medical Genetics Information Resource 1997–2010, 2012. Available at: http://www.genetests.org Accessed January 8, 2013
- 14.Kouri T, Gant V, Fogazzi G, Hofmann W, Hallander H, Guder W; European Confederation of Laboratory Medicine: European urinalysis guidelines. Scand J Clin Lab Invest Suppl 231: 1–86, 2000 [DOI] [PubMed] [Google Scholar]