Abstract
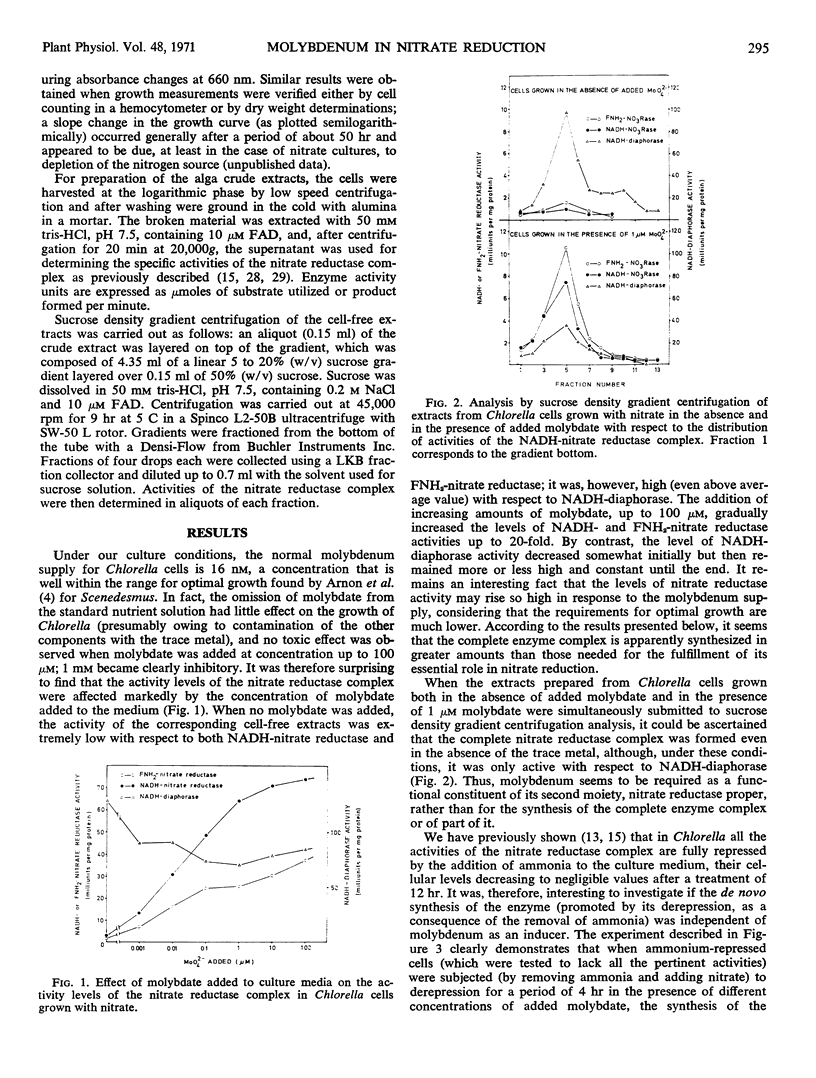
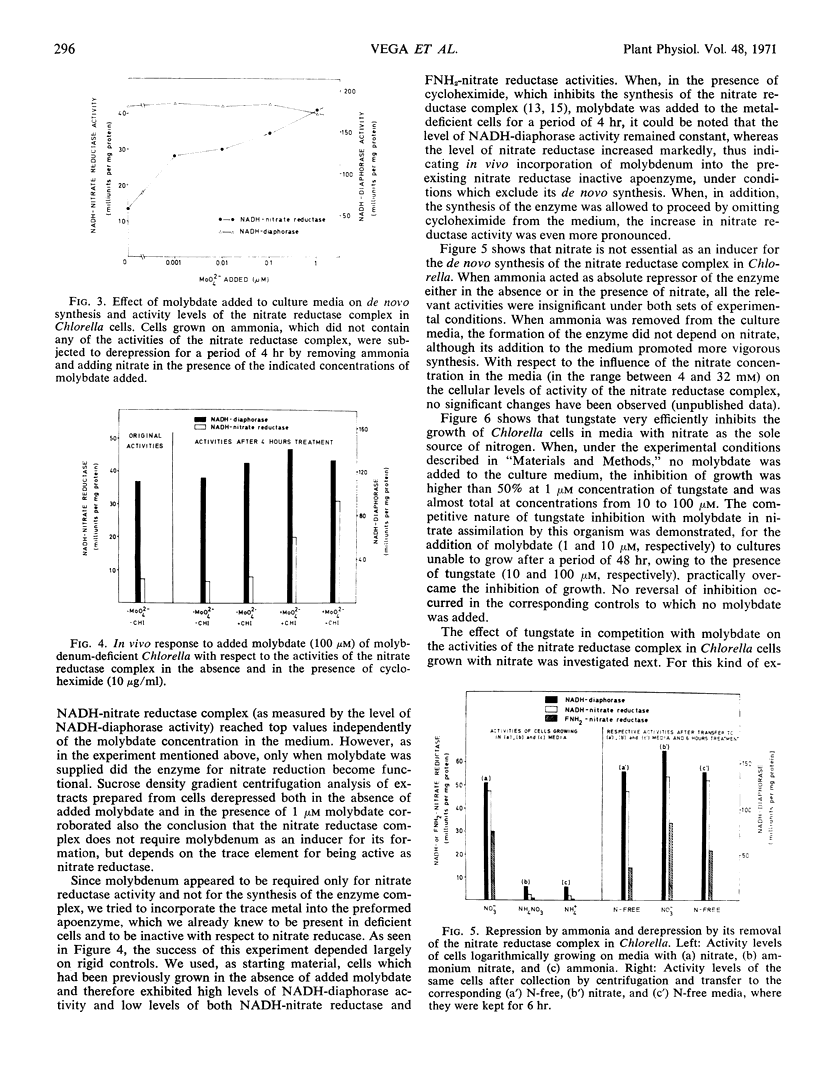
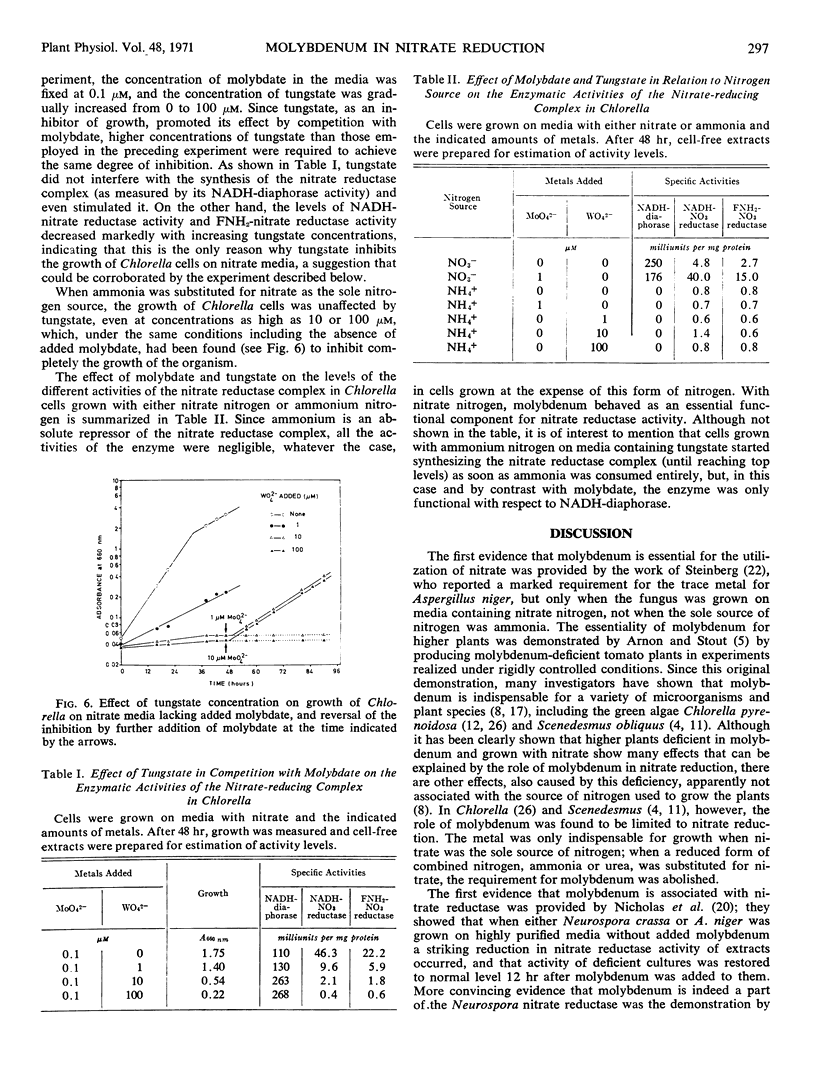
Molybdenum is absolutely required for the nitrate-reducing activity of the nicotinamide adenine dinucleotide nitrate reductase complex isolated from Chlorella fusca. The whole enzyme nicotinamide adenine dinucleotide nitrate reductase is formed by cells grown in the absence of added molybdate, but only its first activity (nicotinamide adenine dinucleotide diaphorase) is functional. The second activity of the complex, which subsequently participates also in the enzymatic transfer of electrons from nicotinamide adenine dinucleotide to nitrate (FNH2-nitrate reductase), depends on the presence of molybdenum. Neither molybdate nor nitrate is required for nitrate reductase synthesis de novo, but ammonia acts as a nutritional repressor of the complete enzyme complex. Under conditions which exclude de novo synthesis of nitrate reductase, the addition of molybdate to molybdenum-deficient cells clearly increases the activity level of this enzyme, thus suggesting in vivo incorporation of the trace metal into the pre-existing inactive apoenzyme.
Competition studies with tungstate corroborate these conclusions and indicate that the only role played by molybdenum in Chlorella is connected with the reduction of nitrate to nitrite. Tungsten seems to act by replacing molybdenum in the nitrate reductase complex, thus rendering inactive the FNH2-nitrate reductase portion of the nicotinamide adenine dinucleotide nitrate reductase complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTMAN K. I., SWISHER S. N. Incorporation of acetate-2-14C into human erythrocyte stroma as a function of storage. Nature. 1954 Sep 4;174(4427):459–460. doi: 10.1038/174459b0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Stout P. R. MOLYBDENUM AS AN ESSENTIAL ELEMENT FOR HIGHER PLANTS. Plant Physiol. 1939 Jul;14(3):599–602. doi: 10.1104/pp.14.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGGINS E. S., RICHERT D. A., WESTERFELD W. W. Tungstate antagonism of molybdate in Aspergillus niger. Proc Soc Exp Biol Med. 1956 Jul;92(3):509–511. doi: 10.3181/00379727-92-22527. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Wray J. L., Filner P. The effect of tungstate on nitrate assimilation in higher plant tissues. Plant Physiol. 1969 Aug;44(8):1197–1199. doi: 10.1104/pp.44.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada M., Paneque A., Aparicio P. J., Vega J. M., Cárdenas J., Herrera J. Inactivation and repression by ammonium of the nitrate reducing system in chlorella. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1009–1015. doi: 10.1016/0006-291x(70)90340-2. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Molybdenum and nitrate reductase. II. Molybdenum as a constituent of nitrate reductase. J Biol Chem. 1954 Mar;207(1):353–360. [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Molybdenum and nitrate reductase. II. Molybdenum as a constituent of nitrate reductase. J Biol Chem. 1954 Mar;207(1):353–360. [PubMed] [Google Scholar]
- Nicholas D. J., Nason A. Role of Molybdenum as a Constituent of Nitrate Reductase from Soybean Leaves. Plant Physiol. 1955 Mar;30(2):135–138. doi: 10.1104/pp.30.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI H., NASON A. Tungstate as competitive inhibitor of molybdate in nitrate assimilation and in N2 fixation by Azotobacter. Biochim Biophys Acta. 1957 Feb;23(2):433–435. doi: 10.1016/0006-3002(57)90351-7. [DOI] [PubMed] [Google Scholar]
- WALKER J. B. Inorganic micronutrient requirements of chlorella. I. Requirements for calcium (or strontium), copper, and molybdenum. Arch Biochem Biophys. 1953 Sep;46(1):1–11. doi: 10.1016/0003-9861(53)90163-5. [DOI] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Aparicio P. J., Paneque A., Losada M. Structural and functional role of FAD in the NADH-nitrate reducing system from Chlorella. FEBS Lett. 1970 Sep 6;9(3):157–160. doi: 10.1016/0014-5793(70)80342-8. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Paneque A., Aparicio P. J., Losada M. Mechanism of nitrate reduction in Chlorella. Biochem Biophys Res Commun. 1969 Sep 10;36(6):980–986. doi: 10.1016/0006-291x(69)90300-3. [DOI] [PubMed] [Google Scholar]



