Abstract
Steroid hormones play an important role in reproduction and the receptors through which they signal change in a developmental time, follicle stage, and cell-specific manner. Disruption in steroid receptor expression affects follicle formation and differentiation. In this study, using prenatal testosterone (T) and dihydrotestosterone (DHT)-treated female sheep as model systems we tested the hypothesis that prenatal androgen excess disrupts the developmental ontogeny of ovarian steroid receptor protein expression. Pregnant Suffolk ewes were injected twice weekly with T propionate or DHT propionate (a non-aromatizable androgen) in cottonseed oil from day 30 to 90 of gestation. Changes in ovarian estrogen receptors (ERα, ERβ), androgen receptor (AR) and progesterone receptor (PR) proteins were determined at fetal (day 90 and 140), postpubertal (10 months) and adult (21 months; only prenatal T-treated sheep studied) ages by immunohistochemistry. Prenatal T and DHT treatment induced selective increase in AR but not ER or PR expression in the stroma and granulosa cells of fetal day 90 and 140 ovaries. An increase in ERα and decrease in ERβ immunostaining coupled with increased AR expression were evident in granulosa cells of antral follicles of 10 and 21-month old prenatal T but not DHT-treated females (analyzed only at 10 months). These findings provide evidence that an early increase in ovarian AR is the first step in the altered ovarian developmental trajectory of prenatal T-treated females and manifestation of postnatal ovarian dysfunction are likely facilitated via altered equilibrium of antral follicular granulosa cell ER / AR protein expression.
Keywords: Androgen receptor, estrogen receptor, progesterone receptor, folliculogenesis
Introduction
Steroids play a key role in the growth, differentiation and function of female reproductive tissues (Drummond et al., 2002, Yeh et al. 2002, Simpson et al. 2005, Padmanabhan et al. 2006). As such, inappropriate activation of the reproductive system by exposure to excess steroid hormones is a major concern, especially in the female. At risk is the female fetus whose mother has been exposed to exogenous steroids for a variety of reasons: failed contraception and continued exposure to contraceptive steroids, use of anabolic steroids or inadvertent exposure to environmental compounds with estrogenic or androgenic activity (Bahrke at al. 1998, Crews & McLachlan 2006, Crain et al. 2008, Jellson et al. 2008, Woodruff & Walker 2008). Evidence exists in support of excess native or environmental steroid exposure. For instance, serum T levels in 40% of female fetuses at mid gestation were found to be in the male range (Beck-Peccoz et al. 1991). Amniotic fluid T levels were found to be also elevated in diabetic pregnancies (Barbieri et al. 1986). Furthermore, female stillbirth offspring of diabetic mothers have hirsutism, ovarian theca-lutein cysts and thecal cell hyperplasia (Driscoll et al. 1960) indicative of excess androgen exposure. Evidence exists in support of biologically significant levels of unconjugated bisphenol-A, an environmental estogen mimic, in maternal and fetal serum (Padmanabhan et al. 2008, Schonfelder et al. 2002). Experimentally, exposure of sheep or monkey fetuses to excess T during gestation culminates in a metabolic and reproductive phenotype (Yeh et al. 2002, Abbott et al. 2006, Padmanabhan et al. 2006, Dumesic et al. 2007) similar to that of women with polycystic ovary syndrome (PCOS) (Dunaif 1997, Rosenfield 1997, Franks et al. 2006), the most common cause of anovulatory infertility in women. Extensive investigations have been undertaken in sheep comparing prenatal T (signals through androgen receptor [AR] or estrogen receptor [ER], due to aromatization to estrogen), and DHT (non aromatizable androgen; signals mainly through AR) to delineate the roles of androgen and estrogen in disrupting neuroendocrine feedback systems (Wood & Foster 1998, Robinson et al. 2002) and more recently ovarian follicular recruitment (Steckler et al. 2005, Smith et al. 2009) and persistence (Manikkam et al. 2006, Steckler et al. 2007). These studies have shown that androgens program the disruptive effects of prenatal T excess on estradiol (E2) negative but not positive feedback at the neuroendocrine level and follicular recruitment but not persistence at the ovarian level.
Considering that the genomic effects of steroids are mediated via intracellular receptors (Chan & O’Malley 1976, Tenbaum & Beniahmad 1997, Beato & Klug 2002), and steroids have the potential to up or down regulate their own receptors (Chadha et al. 1994, Tetsuka & Hillier 1996, Drummond et al. 1999), the disruptive effects of prenatal T excess at the ovarian level may be mediated via altered developmental expression of ovarian steroid receptors. Changes in receptor expression would result in altered steroid signaling culminating in changes in follicle formation and differentiation (Rosenfeld et al. 2001, Drummond et al. 2002, Walters et al. 2008). During fetal ontogeny in sheep, expression of AR, ER alpha (ERα), ER beta (ERβ) and progesterone receptor (PR) undergo progressive follicular type, cell and time specific changes (Juengel et al. 2006). In this study, using prenatal T- and DHT-treated female sheep as model systems, we tested the hypothesis that prenatal androgen excess disrupts the normal developmental progression of ovarian steroid receptor expression.
Results
Antibody specificity
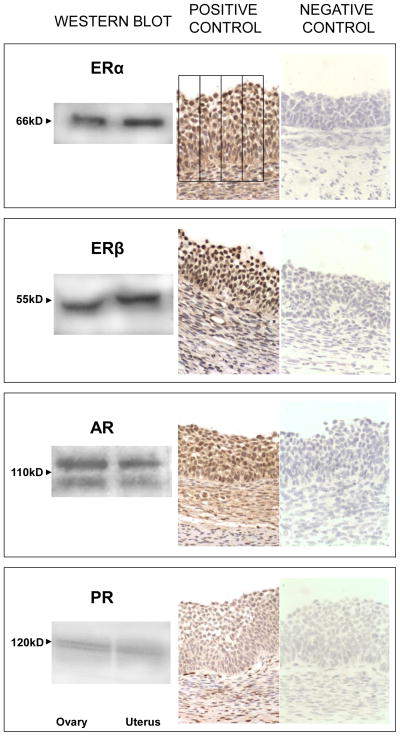
Results from Western Blot analyses of ovarian and uterine (positive control) homogenate (Left) and immunohistochemical recognition of the 4 steroid receptors in ovarian sections (Middle) are summarized in Fig. 1. The negative controls (Right) demonstrate the specificity of the antibody. Western blot analysis only found positive bands of appropriate sizes for each of the receptors studied (ERα, ERβ, AR and PR). The ERα and ERβ antibodies detected a single band at 66 kDa and 55 kDa, respectively. PR (isoform B) was detected as a single band at 116 kDa while two bands were observed for AR around 110 kDa. In the absence of the primary antibodies no specific bands were detected (not shown). Specific nuclear staining was detected for all four nuclear receptors studied in the ovaries (Fig. 1). Faint cytoplasmic stain was also evident and this was subtracted as background in the image analysis.
Fig. 1.
Verification of antibody specificity by Western Blot analyses of ovarian and uterine homogenate (Left) and immunohistochemical recognition of the 4 steroid receptors in ovarian sections (Middle). Negative controls demonstrating the specificity of the antibody are also shown (Right). GR, granulosa cells; TI, theca interna; TE, theca externa; Bar = 25μm.
Ovarian ERα, ERβ, AR and PR localization
In control animals pattern of AR, ERα, ERβ and PR immunostaining in the various ovarian compartments and follicular classes followed what has been reported earlier (Juengel et al. 2006). Representative patterns of AR immunostaining in primordial, primary, and preantral follicles from fetal Day 140 ovaries and AR, ERα, and ERβ in antral follicles of 10-month old control and prenatal T-treated females are shown in Fig. 2. In general, changes in steroid receptor expression induced by prenatal steroid treatment was evident only at the level of AR at all 3 fetal time points studied. In 10-month and 22-month-old animals prenatal steroid-induced changes were evident at the level of AR, ERα, and ERβ. No changes in PR was evident at any of the developmental time points studied. Prenatal steroid induced changes in steroid receptor expression in the various ovarian compartments and follicular classes are discussed below within each developmental time-point.
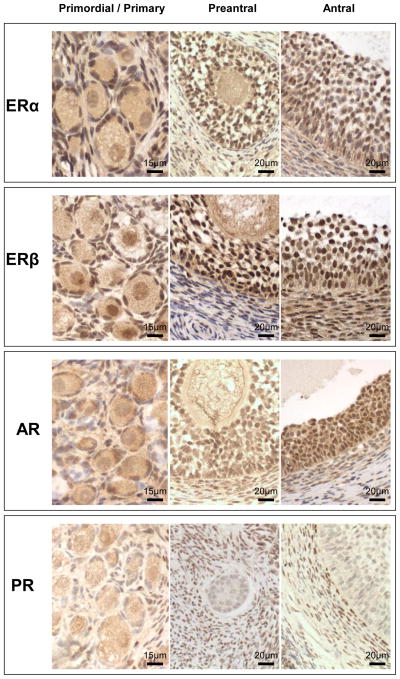
Fig. 2.
Representative images of AR in primordial / primary and preantral (fetal day 140) and ERα, ERβ and AR in antral follicles (10-month-old). GR, granulosa cells; T, theca; TI, theca interna; TE, theca externa; Str, stroma; O, oocyte. Bar = 30μm.
Fetal
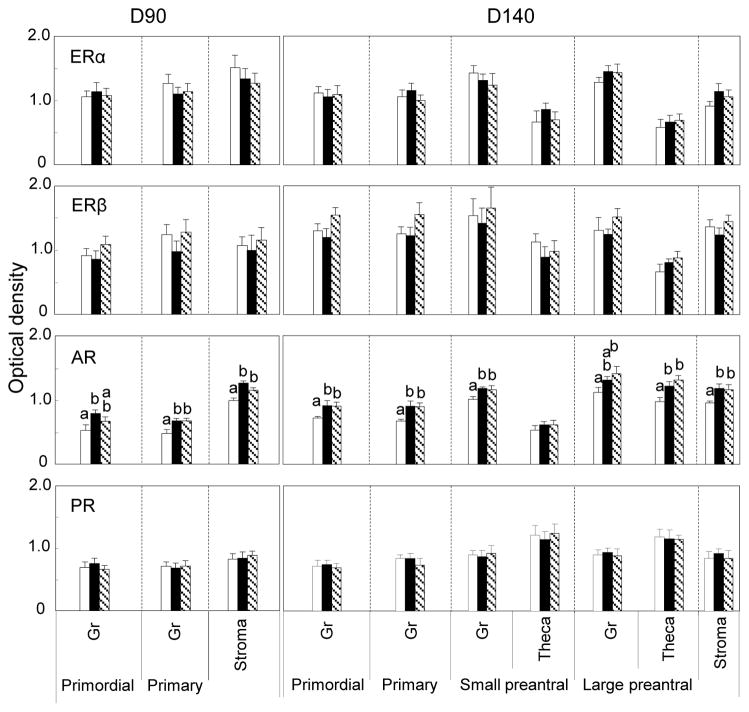
Relative expression of ERα, ERβ, AR and PR-B protein in the ovaries of fetal days 90 and 140 are summarized in Fig. 3. ERα and ERβ proteins were expressed predominantly in granulosa of primordial, primary and preantral (day 140 only) follicles and stroma at both fetal ages with very little expression in the oocytes. Expression level of these two receptor proteins in prenatal T and DHT treated females in all ovarian compartments did not differ (P>0.05) from that of controls at these two ages. AR protein was expressed in the granulosa, theca and stromal compartments (Fig. 2). In fetal D90 ovaries, expression was highest in the stroma (p<0.05) (Fig. 3). Comparing across treatments, prenatal T and DHT excess increased (p<0.05) AR expression in granulosal, thecal and stromal compartments (Fig. 3). PR-B was expressed in granulosa, theca and stroma with expression being highest in theca (p<0.05). Prenatal T and DHT did not alter expression pattern of PR-B. Only a few antral follicles were present at fetal day 140 and hence were not quantified.
Fig. 3.
Relative expression (measured as optical density) of ERα, ERβ, AR and PR-B in ovaries of control (open bars), prenatal T-treated (black bars) and prenatal DHT-treated (hatched bars) day 90 and day 140 fetuses. For each cellular compartment within each follicle type, values with different letters are significantly different (p< 0.05). Expression of these receptors were low in oocytes and did not differ between treatment or follicular classes (not shown). Values represent mean ± SEM. Day 90 (6 C fetuses from 6 dams, 6 T-treated fetuses from 6 dams and 6 DHT-treated fetuses from 5 dams). Day 140 (6 control fetuses from 5 dams, 7 T-treated from 7 dams and 5 DHT-treated from 5 dams). Published summary of ovarian stereology results (Smith et al 2009) from the contralateral ovary documenting enhanced follicular recruitment in prenatal T- and DHT-treated day 140 but not day 90 fetuses, are provided in Table below for comparison (Asterisks indicate significant differences from controls).
| Control | Prenatal T | Prenatal DHT | |
| Fetal Day 90 | |||
| Primordial Follicles | 49,433 ± 8,683 | 54,447 ± 6,609 | 62,602 ± 9,506 |
| Growing follicles | 1,732±640 | 2297±1613 | 738±543 |
| Fetal Day 140 | |||
| Primordial Follicles | 88,629 ± 11,832 | 86,219 ± 11,375 | 64,239 ± 12,622 |
| Growing follicles | 1638±337 | 4071±802* | 3547±862* |
Postpubertal
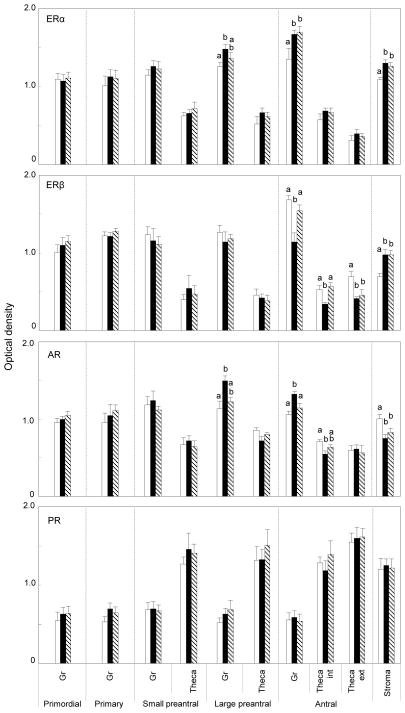
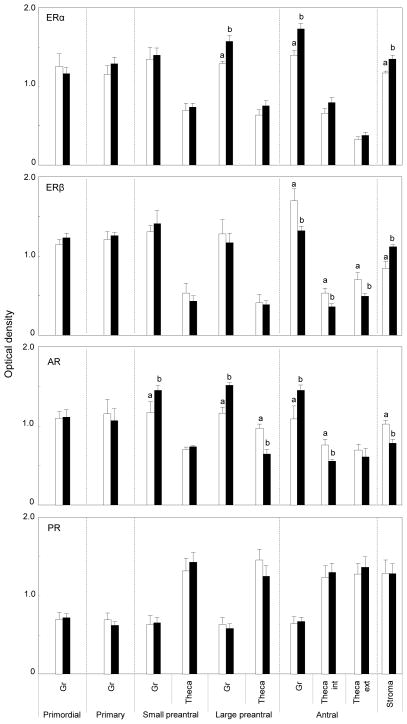
Mean changes in expression of the 4 receptor proteins in the various ovarian compartments of postpubertal females (10 months) are summarized in Fig. 4. As was the case with fetal ovaries, ERα and ERβ immunostaining was predominant in granulosa of primordial, primary and preantral follicles, with amounts in theca being lower than in granulosa (p<0.05). Similar direction of changes was observed in the antral follicles with expression being high in granulosa, very low in oocytes and intermediate in theca interna and externa (Fig. 2). Comparing across treatments, effects of prenatal T and DHT excess were unidirectional with both increasing ERα expression in granulosa of large preantral and antral follicles as well as in the stromal compartment (p<0.05) (representative patterns in control and prenatal T-treated females in Fig. 2, right). ERβ expression followed a completely different trajectory and was manifested in a tissue and steroid specific manner. Prenatal steroid excess induced changes in ERβ expression only in antral follicles and the stromal compartment (p<0.05) (representative patterns in control and prenatal T-treated females in Fig. 2, right). Prenatal T but not DHT excess reduced ERβ expression in both granulosa and theca interna (p<0.05). In contrast, prenatal T and DHT excess increased ERβ expression in the stroma (p<0.05). In theca externa, both treatments reduced level of ERβ immunostaining (p<0.05).
Fig. 4.
Relative expression of ERα, ERβ, AR and PR-B in the ovaries of 10-month-old control (open bars), prenatal T-treated (black bars) and prenatal DHT-treated (hatched bars) females. For each cellular compartment within each follicle type, values with different letters are significantly different (p< 0.05). Values represent mean ± SEM of 5 control animals from 5 dams, 6 T-treated animals from 6 dams, and 5 DHT-treated animals from 5 dams. Published summary of ovarian stereology results (Smith et al 2009) from the same ovary used in this study, which document enhanced follicular depletion and recruitment in prenatal T- but not DHT-treated 10-month old females, are provided in Table below for comparison (Asterisks indicate significant differences from controls).
| Control | Prenatal T | Prenatal DHT | |
| Type 1 Follicles | 58,731 ± 9,602 | 24,250 ± 3,364* | 38,298 ± 10,192 |
| Growig follicles | 158±35 | 240±15* | 139±28 |
AR immunostaining followed a similar trend as in the fetuses with higher amounts found in granulosa and stroma (p<0.05). In contrast to the fetal ovaries, prenatal T and DHT excess had no effect on AR protein expression in granulosa of primordial, primary and small prenatal follicles. However, prenatal T excess increased granulosal expression of AR protein in large preantral and antral follicles while reducing expression in stromal cells (p<0.05). Effects of prenatal DHT excess parallel that of prenatal T at the stromal level with both reducing AR immunostaining (p<0.05). Changes in PR-B for the most part were similar to that of fetal ovaries. Predominant PR-B protein expression at this age was evident in thecal and stromal cells, with almost twice the amount as seen in granulosa cells (p<0.05). Prenatal T and DHT treatment had no effect on PR-B immunostaining in any ovarian compartment at this age (p>0.05).
Adult
Changes in expression pattern of the 4 receptor proteins in the ovarian compartments of adult females are shown in Fig. 5. Note that the comparison is only between control and prenatal T as insufficient prenatal DHT-females were born for including an adult group. Expression of ERα protein in follicular classes at this age paralleled that of fetal and 10-month-old animals. Prenatal T excess increased ERα immunostaining in granulosa and stroma (p<0.05) as was the case with 10-month-old animals. Expression pattern of ERβ protein also paralleled that of 10-month-old animals with prenatal T excess reducing expression in granulosa and theca of antral follicles but increasing expression in stroma (p<0.05). Both AR and PR-B immunostaining also followed similar changes as in the 10-month-old females. While no effect of prenatal T excess was seen in PR-B, AR immunostaining was increased in granulosa cells of preantral and antral follicles but reduced in theca and stroma (p<0.05).
Fig. 5.
Relative expression of ERα, ERβ, AR and PR-B in the ovaries of 21-month-old control (open bars) and prenatal T-treated (black bars) females. For each cellular compartment within each follicle type, values with different letters are significantly different (p< 0.05). Values represent mean ± SEM of 5 control animals from 5 dams, 8 prenatal T-treated animals from 8 dams. Note stereology has not been undertaken with 21-month old animals.
Discussion
Findings from this study provide evidence that prenatal T and DHT excess alters the ontogeny of ERα, ERβ and AR but not PR-B protein expression in the ovary, in a developmental time, steroid, and follicular stage and cell-specific manner. Furthermore, the finding that the changes are manifested during fetal life exclusively at the level of AR but not ER/PR indicates that resulting adult ovarian perturbations that the prenatal T-treated females manifest, namely disrupted ovarian morphology, follicular persistence and depletion (Manikkam et al. 2006, Steckler et al. 2007, Smith et al. 2009), are reprogrammed via altered AR signaling. The significance of the changes in steroid receptor proteins observed during the different developmental time points is discussed below.
Fetal
The selective increase in AR but not ER or PR-B immunostaining in the stroma and granulosa of primordial and primary follicles of fetal day-90 ovary at the end of T treatment suggest that this is a key step in programming adult dysfunction. Administration of T propionate to prepuberal rats on day 5 of postnatal life also increased ovarian nuclear AR expression (Bukovsky et al. 2002). Developmentally, complete follicular differentiation (primordial to antral) is completed postnatally in rodents as opposed to sheep, in which it is completed in utero (Padmanabhan et al. 2007). The increased AR expression in fetal day-90 ovaries of prenatal T-treated sheep appears to be mediated by androgenic actions of T because the non-aromatizable androgen, DHT, also increased AR immunostaining in stromal as well as granulosa of primordial / primary follicles of day 90 fetuses.
Increase in AR immunostaining in stromal and granulosa compartments of fetal day-140 ovaries, coupled with enhanced follicular recruitment seen at this developmental time point in these very same animals (Smith et al. 2009) is consistent with androgens playing a role in early follicular differentiation (Hillier & Tetsuka, 1997, Vendola et al. 1998, Walters et al. 2008). Androgens have been shown to recruit primordial follicles into the growing pool of developing follicles (Hillier & Tetsuka, 1997, Vendola et al. 1998, McGee & Hsueh 2000). Recent studies using a well-characterized culture system found that T stimulates primary to secondary follicle transition via an androgen receptor-dependent mechanism (Yang & Fortune 2006). Earlier findings that the androgen receptor knockout mice show accelerated follicle depletion at older ages (Shiina et al. 2006) and the testicular-feminized mice, which express a truncated non-functional AR, have a shortened reproductive life span (Young et al. 1989, Gaspar et al. 1991) indicate that AR might play a physiological role in determining the reproductive life-span of the ovary. These findings are at odds with enhanced follicular depletion the 10-month old prenatal T-treated sheep manifest (Smith et al. 2009) in the face of increased AR during fetal life (this study). One possibility is that there is a threshold requirement of AR for occurrence of normal ovarian differentiation, amounts of AR above or below which would be detrimental to follicular survival.
Postpubertal and Adult
Pattern of ERα, ERβ and AR immunostaining in ovarian compartments of adult controls were consistent for the most part with what has been described for sheep (Juengel et al. 2006). The altered equilibrium of ERα to ERβ protein coupled with increased AR expression evident mainly in antral follicles of 10-month and 21-month-old prenatal T-treated females might be a contributing factor in the development of follicular persistence that the prenatal T-treated females have been found to manifest in our earlier studies (Manikkam et al. 2006, Smith et al. 2007, Steckler et al. 2007). Antral follicles represent a stage in follicular development, at which maximal proliferation is occurring. Rodent studies have found E2 synergizes with FSH in stimulating granulosal cell proliferation and steroidogenesis (Palter et al. 2001, Drummond 2006). The similarity in the direction of changes in ERα to ERβ immunostaining in ovaries of 10-month and 21-month-old animals is supportive of inherent reprogramming of the ovary.
Interestingly, ERα over-expressed mice are subfertile and show down regulation of the ERβ gene (Tomic et al. 2007). This observation raises the possibility that the decrease in ERβ seen in granulosa of antral follicles of prenatal T-treated females may be secondary to ERα up-regulation. The increased numbers of antral follicles observed in ERα over-expressed mice (Tomic et al. 2007) parallel the increased number of antral follicles seen in the 10-month old prenatal T-treated female sheep (same animals used in this study; Smith et al. 2009). These findings are consistent with a role for estrogen in promoting follicular growth via ERα receptor. Studies with ERα knockout mice that were anovulatory also indicated that ERα is not required for follicular recruitment or early differentiation but necessary for subsequent follicular growth (Dupont et al. 2000).
Another possibility to consider is that it is not the increased ERα but the relative expression of ERα to ERβ that is more important, because the 10-month-old prenatal DHT-treated females, which failed to show the decrease in ERβ (this study), did not show increased presence of antral follicles (Smith et al 2009). Because ERα and ERβ can form homodimers as well as heterodimers (Pettersson et al. 1997) and both ERα and ERβ are expressed in the granulosa, theoretically they should be capable of forming ERα: ERα and ERβ: ERβ homodimers as well as ERα: ERβ heterodimers and alter signal transduction. ERα and ERβ homodimers and heterodimers can have different affinities/specificities for estrogen or other potential estrogen-like ligands (Sun et al. 1999) and cause differential gene activation (McInerney et al. 1998, Pettersson et al. 2000). Earlier studies have found that ERβ, when present within a heterodimer, repressed ERα activity and sensitivity to E2 (Hall & McDonnell 1999). More recent studies suggest that the main determinants of the transcriptional activity of ERα and ERβ are not their binding ability but rather the individual concentration of the two receptors in target cells and the structure of the estrogen ligand (Gougelet et al. 2007, Bhavnani et al. 2008). As such, a given ligand could exert opposite activities depending on the type of ER expressed. Therefore, regulation of ovarian differentiation and subsequent function in adulthood by estrogen appears to be complex and involve intricate interactions/cell signaling pathways coordinated by different receptor-receptor interactions (Pettersson et al. 1997, McInerney et al. 1998, Hall & McDonnell 1999, Sun et al. 1999, Pettersson et al. 2000, Gougelet et al. 2007, Bhavnani et al. 2008). If so, induction of changes in the ERα: ERβ ratio such as that seen in prenatal T-treated females would perturb the effects of estrogen in regulating ovarian function.
The disruptive effects of altered ER signaling may be compounded further by altered AR signaling in antral follicles of 10-month and 21-month-old females. Findings from this study coupled with earlier findings of multifollicular ovaries, reduced inhibin / activin βB mRNA expression (West et al. 2001), enhanced follicular recruitment (Steckler et al. 2005, Smith et al. 2009 [same animals as used in this study]), and follicular persistence (Manikkam et al. 2006, Steckler et al. 2007), in prenatal T-treated sheep all point to disrupted intra-follicular androgen signaling. It is known that androgens promote atresia and/or arrest of follicles (Hillier & Tetsuka 1997, Vendola et al. 1998, McGee & Hsueh 2000). Furthermore, T antagonizes the anti-apoptotic effects of E2 in rat granulosal cells in early antral and preantral follicles (Billig et al. 1993). Our earlier findings of follicular persistence in prenatal T- but not DHT-treated females (Manikkam et al. 2006, Steckler et al. 2007) is therefore consistent with increased AR protein in granulosa cells of antral follicles of prenatal T- but not DHT-treated females. The fact that the direction of changes in AR expression was tissue / follicle stage-specific and a function of the nature of prenatal steroid exposure, emphasizes the specificity of this regulation and the importance of these findings to ovarian function.
The finding that AR expression was increased only in granulosa cells of antral follicles from adult animals is also consistent with reduced ERβ expression seen in prenatal T-treated females. Earlier studies have found that AR expression in the granulosa of late antral follicles is repressed by the activation of ERβ (Cheng et al. 2002). Repression of AR changes the follicular environment from androgen to estrogen dominance, a critical step for survival of the follicle (Billig et al. 1993, Hillier & Tetsuka 1997, Britt & Findlay 2002, Drummond 2006, Yang & Fortune 2006). If AR expression remains high, as is the case in prenatal T-treated females, antral follicles cannot achieve estrogen dominance and their fate is atresia or arrest. However, parallel studies performed using the same ovaries as used in this study (Smith et al. 2009) found no differences in the percentage of atretic antral follicles between control and prenatal T-treated females at 10-months of age. On the contrary our earlier finding of follicular persistence in the prenatal T-treated females (Manikkam et al. 2006, Steckler et al. 2007) provide evidence in support of arrested follicular development. It remains to be determined if this arrest is mediated via a shift in the balance of apoptotic to antiapoptotic factors in these follicles. It is of interest that granulosa cells of antral follicles derived after gonadotropin stimulation from women with PCOS, whose reproductive attributes the prenatal T-females duplicate, also exhibit increased AR expression compared to controls (Catteau-Jonard et al. 2008) and flutamide, an antiandrogen, restores ovulatory function in anovulatory women with polycystic ovarian syndrome (PCOS) (De Leo et al. 1998).
While changes in ER and AR likely play a role in manifestation of ovarian disruptions in the adult prenatal T-treated females, lack of changes in PR-B indicate that this isoform of PR is not involved. However, the finding that double PR (PR-A and PR-B) knockout mice fail to ovulate (Lydon et al. 1995) provide evidence that other PR isoforms may be involved. Consistent with this premise, cystic ovaries of rats manifested higher expression of PR-A, lower expression of the PR-C and no change in PR-B isoform (Salvetti et al. 2008). Changes in expression of other PR forms were not evaluated in this study.
The fetal ovarian changes in AR and changes in AR/ER at 10-months of age are consistent with the effects of prenatal T and DHT treatment on follicular recruitment, depletion and ovarian morphology reported in these very same animals (Smith et al. 2009). The increased fetal ovarian AR expression (this study) coupled with the increased follicular recruitment (Smith et al. 2009). in fetal Day 140 prenatal T- and DHT-treated fetuses support a role for androgen in early follicular activation. Similarly, increased follicular depletion and ovarian disruptions seen in the 10-month-old prenatal T-treated but not DHT-treated females (same animals used in this study; Smith et al. 2009) coupled with selective changes in expression of ERβ in antral follicles of 10-month-old prenatal T- but not DHT-treated females (this study) are also consistent with a role for estrogen. In distinguishing between androgenic vs. estrogenic contribution, similarity of responses between prenatal T (aromatizable androgen) and prenatal DHT-treated (non- aromatizable androgen) females was used as an index to imply androgenic action. While evidence exists that DHT can be metabolized into 5α-androstane-3β,17β-diol (3β-diol) and has the ability to act via the ERβ receptor (Handa et al. 2008), there is very little informaton available regarding the extent and impact of this conversion at the ovarian level. Nonetheless, selective changes in AR and ER expression seen in this study help distinguish which receptor signling are involved and the developmental time point when this occurs.
Finally, it is unclear how the fetal changes in AR induced by prenatal T excess reprogram the ovary to culminate in adult ovarian dysfunction. A likely possibility involves epigenetic modifications of key ovarian regulatory genes. Earlier studies have found that sex steroids, have the ability to influence the methylation state of DNA sequences and both ER and AR acetylation can be regulated by physiologic stimuli (Leader et al. 2006a, 2006b, Vottero et al. 2006, Xue et al. 2007). The findings from this study are consistent with the recent proposal of a two step process (Tang et al. 2008) whereby early insult from in utero T treatment resets the course of ovarian development and the manifestation of adult ovarian phenotype requires subsequent exposure to ovarian steroids such as that occurring during puberty (Tang et al. 2008).
In summary, this comprehensive reproductive lifespan study relates the impact of prenatal exposure to excess sex steroids on the developmental trajectory of ovarian steroid receptors. The findings provide evidence that the first step in the altered trajectory of ovarian differentiation of prenatal T-treated females involves an early increase in ovarian AR and that manifestation of postnatal ovarian dysfunction is likely mediated via an increase in ratio of ERα / ERβ receptor coupled with an increase in granulosal expression of AR resulting in increased AR signaling and consequent follicular persistence. The findings may be of translational significance to women with polycystic ovarian disease, the reproductive and metabolic phenotype of whom the prenatal T-treated females manifest.
Material and Methods
Breeding and prenatal treatment
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of the University of Michigan and were consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Two to three year-old Suffolk ewes were purchased locally and bred in a farm receiving oversight from University of Michigan Department of Laboratory Animal Medicine. Day of mating was determined by visual confirmation of a paint mark left by an intact ram on the hindquarter of bred ewes. Beginning on day 30 of gestation and continuing until day 90 of gestation pregnant ewes were injected im twice weekly with 100 mg of T propionate (1.2 mg/kg; Sigma-Aldrich Corp., St. Louis, MO) or 100 mg DHT propionate (Steraloids, Inc., Newport, RI) suspended in cottonseed oil (Sigma-Aldrich Corp.). The dose and mode of treatment were chosen based on the large body of data available documenting postnatal reproductive disruptions (Wood & Foster 1998, Robinson et al. 2002, Manikkam et al. 2004, Steckler et al. 2007). Prenatal T treatment produces circulating concentrations of T in pregnant sheep and female fetuses in the range seen in intact adult males and male fetuses, respectively (Wood et al. 1991). Details of prenatal treatments, husbandry, and nutrition of maternal sheep as well as newborn and growing lambs until 4 months of age have been published (Manikkam et al. 2004).
Ovaries procured from control, prenatal T and DHT-treated females on fetal day 90 (6 control fetuses from 6 dams, 6 T-treated fetuses from 6 dams and 6 DHT-treated fetuses from 5 dams), fetal day 140 (6 control fetuses from 5 dams, 7 T-treated from 7 dams and 5 DHT-treated from 5 dams), 10 months of age (5 control animals from 5 dams, 6 T-treated animals from 6 dams, and 5 DHT-treated animals from 5 dams) and 21 months of age (5 control animals from 5 dams, 8 T-treated animals from 8 dams) were utilized in this study. There were insufficient DHT-treated females born to include a 21 month-old prenatal DHT-treated group.
For collection of ovaries from fetuses, dams were euthanized by administration of a barbiturate overdose (Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI) and fetuses removed. Biweekly progesterone monitoring found all controls were cycling at both 10 and 21 month age. Barring one prental T-treated female, all prental T- and DHT- tretaed females were cycling at 10 months of age although the T animals manifested cycle irregularities. At 21 months of age, 3 prental T-treated animals were anovulatory and the other 5 showed irregular cycles. To avoid influence of cycle stage in cycling animals, two 20 mg injections of prostaglandin F2α (PGF2α, 5 mg/mL Lutalyse; Pfizer Animal Health, New York, NY, USA) were administered 11 days apart to induce luteolysis and synchronize estrus in cycling females. Ewes were euthanized 28 h after second PGF2 α injection and ovaries collected. There were no differences in dam weights among treatment groups at the time of fetal collection or between control and experimental females at the time of study (data not shown). For immunohistochemical determination of steroid receptors, one ovary from each fetus was fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), pH 7.4, embedded in paraffin and the entire ovary serially sectioned at 5 μm thickness. The second ovary from fetal ages was embedded in plastic stereology (Smith et al. 2009). For adult ovaries, one ovary was fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), pH 7.4, embedded in paraffin and the entire ovary serially sectioned and sections used for morphometry in a parallel study (Smith et al. 2009) and steroid receptor quantification (this study). The other ovary was stored frozen for future in situ hybridization studies.
Western blotting
Details of antibodies used are summarized in Table 1. To test specificity of the antibodies, sheep ovarian tissues were homogenized in a radio-immunoprecipitation assay lysis buffer consisting of 1% v/v IGEPAL CA630 (octylphenyl-polyethylene glycol), 0.5% (w/v) sodium desoxycolate, 0.1% w/v SDS, 1mM EDTA, 50 mM sodium fluoride (all from Sigma-Aldrich Corp.), 0.1 M PBS and a protease inhibitor cocktail (Complete Mini Protease Inhibitor Cocktail Tablets, Roche, Mannheim, Germany). Ovarian homogenates were centrifuged at 14,000 rpm for 20 min and supernatant stored frozen at −80ºC. Forty mg of proteins were separated by SDS-PAGE (10% resolving gel). Proteins were transferred to nitrocellulose membranes (Amersham, Buckinghamshire, UK), blocked for 1 h in 2% w/v non-fat milk in Tris-buffered saline (TBS) containing 0.05% v/v Tween20 (Sigma-Aldrich Corp.), and then incubated overnight at 4ºC with specific primary antibodies (Table 1). Following washing, membranes were treated for 1 h with corresponding secondary peroxidase-conjugated antibody (Table 1). Immunopositive bands were visualized with a chemiluminescent detection kit (ECL, Amersham, Buckinghamshire, UK).
Table 1.
Antibodies used for immunohistochemistry (IH) and Western blot (WB).
| Antigen | Clone and Source | Dilution | IH Antigen retrieval | Secondary antibody and dilution |
|---|---|---|---|---|
| ERα | 33 (MA1-310, Affinity Bioreagents, Golden, CO, USA) | IH: 1:50 WB: 1:200 |
pressure-cooker | IH: Biotinylated Goat-anti-mouse IgG (Zymed, San Francisco, CA, USA), 1:100 WB: Goat-anti-mouse IgG peroxidase (Amersham, Buckinghamshire, UK), 1:500 |
| ERβ | Polyclonal (PA1-311, Affinity Bioreagents, Golden, CO, USA) | IH: 1:50 WB: 1:200 |
microwave | IH: Biotinylated Goat-anti-rabbit IgG (Zymed, San Francisco, CA, USA), 1:100 WB: Goat-anti-rabbit IgG peroxidase (Amersham, Buckinghamshire, UK), 1:500 |
| AR | Polyclonal (PA1-110, Affinity Bioreagents, Golden, CO, USA) | IH: 1:200 WB: 1:300 |
microwave | IH: Biotinylated Goat-anti-rabbit IgG (Zymed, San Francisco, CA, USA), 1:100 WB: Goat-anti-rabbit IgG peroxidase (Amersham, Buckinghamshire, UK), 1:500 |
| PR-Ba | Alpha PR6 (MA1-411, Affinity Bioreagents, Golden, CO, USA) | IH: 1:50 WB: 1:20 |
pressure-cooker | IH: Biotinylated Goat-anti-mouse IgG (Zymed, San Francisco, CA, USA), 1:100 WB: Goat-anti-mouse IgG peroxidase (Amersham, Buckinghamshire, UK), 1:500 |
PR isoform B
Immunohistochemistry
A streptavidin-biotin immunoperoxidase method as described previously (Salvetti et al. 2007) was used for immunohistochemical detection. After deparaffinizing and antigen retrieval in 10 mM sodium citrate solution (pH 6,0) by boiling in pressure cooker (ERα, PR) or microwaving (AR, ERβ) (Table 1), endogenous peroxidase activity in ovarian sections was quenched with 1% (v/v) H2O2 in methanol. To eliminate nonspecific binding, sections were incubated with 10% (v/v) normal goat serum for 20 min at room temperature before incubating with the primary antibodies for 18 h at 4ºC and then with respective biotinylated secondary antibody for 30 min at room temperature. The visualization of antigens was achieved with streptavidin-peroxidase (BioGenex, San Ramon, CA, USA) using 3.3-diaminobenzidine (DAB; Dako, Carpinteria, CA, USA) as chromogen. Ovarian sections were counterstained with Mayer’s haematoxylin, dehydrated and mounted. Quenching of residual endogenous peroxidase activity was confirmed by incubating some sections with DAB alone. The specificity of the secondary antibodies was tested with negative control sections processed as above except for replacement of primary antibodies with non-immune serum. As multiple series of histological processing were involved, serial sections of a non-experimental set of sheep ovaries were included with each series to allow normalization across series. Each immunohistochemical series included randomly selected slides with ovarian sections from different ages and treatments. Follicle classes were distinguished using the following criteria: primordial - single layer of flattened granulosa cells, primary - partial or one complete layer of cuboidal granulosa cells, small preantral - >1 to five layers granulosa cells, large preantral - > 5 layers of cuboidal granulosa cells, and antral - those with an antral cavity.
Image Analysis
Two sections, the first 1/3 into the ovary and the second 2/3 into the ovary, were used for immunohistochemical quantification. Considering the follicular sizes (McNatty et al. 1999) and the distance between the 2 sections (~200 μm in fetal and >10 mm in adult), there is little likelihood of follicular overlap. All growing follicles in both sections were analyzed (ranged between 8 and 15 in follicular classes). For primordial follicles, the slides were scanned left to right from the top and the first 20 primordial follicles that were distinct and showed no overlap with neighboring follicles were utilized. Only healthy follicles without atretic signs (apoptotic / pycnotic nucleus and loss of cell adhesion in granulosa) were evaluated. Image analysis was performed using Image Pro-Plus 3.0.1 system (Media Cybernetics, Silver Spring, MA, USA). Images were digitized at × 40 magnification using an Olympus C5060 digital camera mounted on a conventional light microscope (Olympus BH-2, Olympus Co., Japan). Details of image analysis have been described earlier (Shan et al. 1997, Wang et al. 1999, 2000, Zhu et al. 2000, Salvetti et al. 2007). The nuclei were visualized and identified using AutoPro macro language, an automated sequence operation created to measure the optical density (OD). The images of immunostained slides were converted to an 8-bit gray scale, with the background staining of the histological slides set at zero and the most intense staining set at two. This calibration was carried out using specific tools of the software that determine through an histogram analysis of the images, the intensity values corresponding to background (represented mainly by cytoplasmic and antral staining) and most intense staining (in nucleus), independently for each receptor and considering the respective positive and negative controls in each assay. These values were verified and normalized with the controls carried across various runs and the same region (verified by image comparison) was used for calibration. The optical density (OD) was measured as a mean grey intensity of each pixel divided by the total number of pixels measured. The OD was calculated separately for each follicular compartment (oocyte, granulosa, theca interna, and theca externa) and stroma.
For primordial, primary and small preantral follicles, all granulosa cells within each follicle were analyzed. For large preantral and antral follicles, 100 cells / compartment / follicle were quantified. To avoid subjectivity and differences in location of cells relative to antrum and theca, vertical rows of all cells spanning between the theca and antrum were quantified until 100 cells were counted (Fig. 1). For each ovary, 200 stromal cells were analyzed, in the centre of the ovary. Sections were analyzed with the observer blinded to treatment.
The major strength of the imaging approach used in this study is visualization of in situ localization of proteins within cells of interest. In the past decade, computerized image analysis systems have been developed to obtain objective and accurate quantification of nuclear markers (Lejeune et al. 2008). This approach has been successfully applied by other investigators to quantify steroid receptors in different tissues and validated for diagnostic, prognostic and therapeutic purposes (Shan et al. 1997, Wang et al. 1999, 2000, Zhu et al. 2000, Salvetti et al. 2007, Lejeune et al. 2008). Since the immunostaining and image analyses are optimized for each protein, quantitative comparisons across proteins are not possible.
Statistical analyses
The OD of all cells within each follicular compartment (granulosa, theca, and oocyte) within a follicle class was first averaged and then a group mean across follicles was derived for each follicle type within an animal. When more than one fetus was studied per dam, the data were averaged before analyses. For 21 month old females, because comparison of data from cycling and anovulatory prenatal T-treaeted animals revealed no differences in steroid receptor expression, data from cycling and anovulatory animals were treated as one group for analysis. A statistical software package (SPSS 11.0 for Windows, SPSS Inc., Chicago, IL, USA) was used for performing the statistical tests. When more than two groups were involved (control, T-treated, DHT-treated; all ages except 21 months), data were compared by analyses of variance, followed by Duncan’s multiple range tests. For comparing results between two groups (control vs. T-treated, 21 months) student’s t test was utilized. A p<0.05 value was considered significant. Results are expressed as mean ± SEM.
Acknowledgments
Funding
This study was supported by USPHS grants P01-HD44232 to VP.
We are grateful to Mr. Douglas Doop for his expert animal care, facility management and help with generation of the experimental lambs; Drs. Mohan Manikkam, Almudena Veiga-Lopez, Teresa Steckler, Mr. James Lee, Ms. Carol Herkimer, Ms. Olga Astapova for assistance with prenatal steroid treatment, and/or collection, processing and sectioning of ovaries; Dr. Keith Inskeep for editorial help. We also thank the staff members of the Laboratory of Cellular Biology (FCV-UNL) for their technical support during processing of the slides.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, editors. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. 2. Totowa, New Jersey JR: Humana Press Inc; 2006. pp. 259–272. [Google Scholar]
- Bahrke MS, Yesalis CE, Brower KJ. Anabolic-androgenic steroid abuse and performance-enhancing drugs among adolescents. Child and Adolescent Psychiatric Clinics of North America. 1998;7:821–838. [PubMed] [Google Scholar]
- Barbieri RL, Saltzman DH, Torday JS, Randall RW, Frigoletto FD, Ryan KJ. Elevated concentrations of the beta-subunit of human chorionic gonadotropin and testosterone in amniotic fluid of gestations of diabetic mothers. Am J Obstet Gynecol. 1986;154:1039–1043. doi: 10.1016/0002-9378(86)90746-5. [DOI] [PubMed] [Google Scholar]
- Beato M, Klug J. Steroid hormone receptors: an update. Human Reproduction Update. 2002;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73:525–32. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR, Tam SP, Lu XF. Structure activity relationships and differential interactions and functional activity of various equine estrogens mediated via estrogen receptors ERα and ERβ. Endocrinology. 2008;149:4857–4870. doi: 10.1210/en.2008-0304. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Hsueh AJW. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993;133:2204–2212. doi: 10.1210/endo.133.5.8404672. [DOI] [PubMed] [Google Scholar]
- Britt KL, Findlay JK. Estrogen actions in the ovary revisited. Journal of Endocrinology. 2002;175:269–276. doi: 10.1677/joe.0.1750269. [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Ayala ME, Dominguez R, Keenan JA, Wimalasena J, Elder RF, Caudle MR. Changes of ovarian interstitial cell hormone receptors and behavior of resident mesenchymal cells in developing and adult rats with steroid-induced sterility. Steroids. 2002;67:277–289. doi: 10.1016/s0039-128x(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian Hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 2008;93:4456–4461. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- Chadha S, Pache TD, Huikeshoven JM, Brinkmann AO, van der Kwast TH. Androgen receptor expression in human ovarian and uterine tissue of long-term androgen-treated transsexual women. Human Pathology. 1994;25:1198–1204. doi: 10.1016/0046-8177(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Chan L, O’Malley BW. Mechanism of action of the sex steroid hormones (first of three parts) The New England Journal of Medicine. 1976;294:1322–1328. doi: 10.1056/NEJM197606102942405. [DOI] [PubMed] [Google Scholar]
- Cheng G, Weihua Z, Makinen S, Makela S, Saji S, Warner M, Gustafsson JA, Hovatta O. A role for the androgen receptor in follicular atresia of estrogen receptor beta knockout mouse ovary. Biology of Reproduction. 2002;66:77–84. doi: 10.1095/biolreprod66.1.77. [DOI] [PubMed] [Google Scholar]
- Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147(6 Suppl):S4–S10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- De Leo V, Lanzetta D, D’Antona D, Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 1998;83:99–102. doi: 10.1210/jcem.83.1.4500. [DOI] [PubMed] [Google Scholar]
- Driscoll SG, Benirschke K, Curtis GW. Neonatal deaths among infants of diabetic mothers. Postmortem findings in ninety-five infants. Am J Dis Child. 1960;100:818–835. doi: 10.1001/archpedi.1960.04020040820004. [DOI] [PubMed] [Google Scholar]
- Drummond AE. The role of steroids in follicular growth. Reproductive biology and endocrinology. 2006;4:16. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AE, Baillie AJ, Findlay JK. Ovarian estrogen receptor alpha and beta mRNA expression: impact of development and estrogen. Molecular and Cellular Endocrinology. 1999;149:153–161. doi: 10.1016/s0303-7207(98)00247-0. [DOI] [PubMed] [Google Scholar]
- Drummond AE, Britt KL, Dyson M, Jones ME, Kerr JB, O’Donnell L, Simpson ER, Findlay JK. Ovarian steroid receptors and their role in ovarian function. Molecular and Cellular Endocrinology. 2002;191:27–33. doi: 10.1016/s0303-7207(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Reviews in Endocrine and Metabolic Disorders. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocrine Reviews. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors a (ERα) and b (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Franks S, McCarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. International Journal of Andrology. 2006;29:278–285. doi: 10.1111/j.1365-2605.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- Gaspar ML, Meo T, Bourgarel P, Guenet JL, Tosi M. A single base deletion in the Tfm androgen receptor gene creates a short-lived messenger RNA that directs internal translation initiation. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8606–8610. doi: 10.1073/pnas.88.19.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougelet A, Mueller SD, Korach KS, Renoir JM. Oestrogen receptors pathways to oestrogen responsive elements: Transcriptional function-1 acts as the keystone of oestrogen receptor (ER) beta-mediated transcriptional repression of ERalpha. The Journal of Steroid Biochemistry and Molecular Biology. 2007;104:110–122. doi: 10.1016/j.jsbmb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SG, Tetsuka M. Role of androgens in follicle maturation and atresia. Baillière’s Clinical Obstetrics and Gynaecology. 1997;11:249–260. doi: 10.1016/s0950-3552(97)80036-3. [DOI] [PubMed] [Google Scholar]
- Jellesen R, Strandberg-Larsen K, Jørgensen T, Olsen J, Thulstrup AM, Andersen AM. Maternal use of oral contraceptives and risk of fetal death. Paediatric and Perinatal Epidemiology. 2008;22:334–340. doi: 10.1111/j.1365-3016.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Heath DA, Quirke LD, McNatty KP. Oestrogen receptor alpha and beta, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction. 2006;131:81–92. doi: 10.1530/rep.1.00704. [DOI] [PubMed] [Google Scholar]
- Leader JE, Wang C, Fu, Pestell RG. Epigenetic regulation of nuclear steroid receptors. Biochemical Pharmacology. 2006a;72:1589–1596. doi: 10.1016/j.bcp.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Leader JE, Wang C, Popov VM, Fu M, Pestell RG. Epigenetics and the estrogen receptor. Annals of the New York Academy of Sciences. 2006b;1089:73–87. doi: 10.1196/annals.1386.047. [DOI] [PubMed] [Google Scholar]
- Lejeune M, Jaén J, Pons L, López C, Salvadó MT, Bosch R, García M, Escrivà P, Baucells J, Cugat X, et al. Quantification of diverse subcellular immunohistochemical markers with clinicobiological relevancies: validation of a new computer-assisted image analysis procedure. Journal of Anatomy. 2008;212:868–878. doi: 10.1111/j.1469-7580.2008.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung ST, Reynolds TS, Wathes DC. Regulation of oxytocin receptor in the placentome capsule throughout the pregnancy in the ewe: the possible role of estrogen receptor, progesterone receptor and aromatase. Journal of Endocrinology. 1998;158:173–181. doi: 10.1677/joe.0.1580173. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, OMalley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Development. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocrine Reviews. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- McInerney EM, Weis KE, Sun J, Mosselman S, Katzenellenbogen BS. Transcription activation by the human estrogen receptor subtype β (ERβ) studied with ERβ and ERα receptor chimeras. Endocrinology. 1998;139:4513–4522. doi: 10.1210/endo.139.11.6298. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Heath DA, Lundy T, Fidler AE, Quirke L, O’Connell A, Smith P, Groome N, Tisdall DJ. Control of early ovarian follicular development. Journal of Reproduction and Fertility (Suppl) 1999;54:3–16. [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Molecular and Cellular Endocrinology. 2006;246:165–174. doi: 10.1016/j.mce.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga_Lopez, Abbott DH, Dumesic DA. Developmental programming of ovarian disruption. In: Gonzalez-Bulnes A, editor. Novel concepts in ovarian endocrinology. Research Signpost; India: 2007. pp. 329–352. [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A exposure: an environmental risk? J of Perinatology. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palter SF, Tavares AB, Hourvitz A, Veldhuis JD, Adashi EY. Are estrogens of import to primate/human ovarian folliculogenesis? Endocrine Reviews. 2001;22:389–424. doi: 10.1210/edrv.22.3.0433. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Molecular Endocrinology. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Foster DL, Padmanabhan V. Prenatal exposure of the ovine fetus to androgens sexually differentiates the steroid feedback mechanisms that control gonadotropin releasing hormone secretion and disrupts ovarian cycles. Archives of Sexual Behavior. 2002;31:35–41. doi: 10.1023/a:1014075016956. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Roberts RM, Lubahn DB. Estrogen receptor- and aromatase-deficient mice provide insight into the roles of estrogen within the ovary and uterus. Molecular Reproduction Development. 2001;59:336–346. doi: 10.1002/mrd.1039. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Is polycystic ovary syndrome a neuroendocrine or an ovarian disorder? Clinical Endocrinology (Oxf) 1997;47:423–424. doi: 10.1046/j.1365-2265.1997.2911093.x. [DOI] [PubMed] [Google Scholar]
- Salvetti NR, Acosta JC, Gimeno EJ, Müller LA, Mazzini RA, Taboada AF, Ortega HH. Estrogen receptors alpha and beta and progesterone receptors in normal bovine ovarian follicles and cystic ovarian disease. Veterinary Pathology. 2007;44:373–378. doi: 10.1354/vp.44-3-373. [DOI] [PubMed] [Google Scholar]
- Salvetti NR, Baravalle C, Mira GA, Gimeno EJ, Dallard BE, Rey F, Ortega HH. Heat shock protein 70 and sex steroid receptors in the follicular structures of induced ovarian cysts. Reproduction in Domestic Animals. 2008 doi: 10.1111/j.1439-0531.2008.01086.x. In Press. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan LX, Bardin CW, Hardy MP. Immunohistochemical analysis of androgen effects on androgen receptor expression in developing Leydig and Sertoli cells. Endocrinology. 1997;138:1259–1266. doi: 10.1210/endo.138.3.4973. [DOI] [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, et al. Premature ovarian failure in androgen receptor deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen: the good, the bad, and the unexpected. Endocrine Reviews. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- Smith P, Steckler T, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology. Biol Reprod 2008. 2009 Dec 17; doi: 10.1095/biolreprod.108.072801. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Novel ligands that function as selective estrogens or antiestrogen for estrogen receptor- α or estrogen receptor-β. Endocrinology. 1999;140:800–804. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;31:2008. doi: 10.1210/en.2008-0682. In press Published online ahead of print July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenbaum S, Baniahmad A. Nuclear receptors: structure, function and involvement in disease. The International Journal of Biochemistry and Cell Biology. 1997;29:1325–1341. doi: 10.1016/s1357-2725(97)00087-3. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Hillier SG. Androgen receptor gene expression in rat granulosa cells: the role of follicle-stimulating hormone and steroid hormones. Endocrinology. 1996;137:4392–4397. doi: 10.1210/endo.137.10.8828500. [DOI] [PubMed] [Google Scholar]
- Tomic D, Frech MS, Babus JK, Symonds D, Furth PA, Koos RD, Flaws JA. Effects of ER alpha overexpression on female reproduction in mice. Reproductive Toxicology. 2007;23:317–325. doi: 10.1016/j.reprotox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. Journal of Clinical Investigation. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vottero A, Capelletti M, Giuliodori S, Viani I, Ziveri M, Neri TM, Bernasconi S, Ghizzoni L. Decreased androgen receptor gene methylation in premature pubarche: a novel pathogenetic mechanism? The Journal of Clinical Endocrinology and Metabolism. 2006;91:968–972. doi: 10.1210/jc.2005-2354. [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biology of Reproduction. 2008;78:380–389. doi: 10.1095/biolreprod.107.064089. [DOI] [PubMed] [Google Scholar]
- Wang H, Masironi B, Eriksson H, Sahlin L. A comparative study of estrogen receptors α and β in the rat uterus. Biology of Reproduction. 1999;61:955–964. doi: 10.1095/biolreprod61.4.955. [DOI] [PubMed] [Google Scholar]
- Wang H, Eriksson H, Sahlin L. Estrogen receptors α and β in the female reproductive tract of the rat during the estrous cycle. Biology of Reproduction. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra follicular activin availability is altered in prenatally-androgenized lambs. Molecular and Cellular Endocrinology. 2001;185:51–59. doi: 10.1016/s0303-7207(01)00632-3. [DOI] [PubMed] [Google Scholar]
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function. Reviews of Reproduction. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
- Wood RI, Ebling FJ, I’Anson H, Bucholtz DC, Yellon SM, Foster DL. Prenatal androgens time neuroendocrine sexual maturation. Endocrinology. 1991;128:2457–2468. doi: 10.1210/endo-128-5-2457. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Walker CL. Fetal and early postnatal environmental exposures and reproductive health effects in the female. Fertility and Sterility 2008. 2008;89(2 Suppl):e47–e51. doi: 10.1016/j.fertnstert.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biology of Reproduction. 2007;77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biology of Reproduction. 2006;75:924–932. doi: 10.1095/biolreprod.106.051813. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13498–134503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CY, Johnson MP, Prescott JL, Tindall DJ. The androgen receptor of the testicular-feminized (Tfm) mutant mouse is smaller than the wild-type receptor. Endocrinology. 1989;124:771–775. doi: 10.1210/endo-124-2-771. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Hardy MP, Inigo IV, Huhtaniemi I, Bardin CW, Moo-Young AJ. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: a quantitative immunohistochemical study. Biology of Reproduction. 2000;63:368–376. doi: 10.1095/biolreprod63.2.368. [DOI] [PubMed] [Google Scholar]