Abstract
The adult fungiform taste papilla is a complex of specialized cell types residing in the stratified squamous tongue epithelium. This unique sensory organ includes taste buds, papilla epithelium and lateral walls that extend into underlying connective tissue to surround a core of lamina propria cells. Fungiform papillae must contain long-lived, sustaining or stem cells and short-lived, maintaining or transit amplifying cells that support the papilla and specialized taste buds. Shh signaling has established roles in supporting fungiform induction, development and patterning. However, for a full understanding of how Shh transduced signals act in tongue, papilla and taste bud formation and maintenance, it is necessary to know where and when the Shh ligand and pathway components are positioned. We used immunostaining, in situ hybridization and mouse reporter strains for Shh, Ptch1, Gli1 and Gli2-expression and proliferation markers to identify cells that participate in hedgehog signaling. Whereas there is a progressive restriction in location of Shh ligand-expressing cells, from placode and apical papilla cells to taste bud cells only, a surrounding population of Ptch1 and Gli1 responding cells is maintained in signaling centers throughout papilla and taste bud development and differentiation. The Shh signaling targets are in regions of active cell proliferation. Using genetic-inducible lineage tracing for Gli1-expression, we found that Shh-responding cells contribute not only to maintenance of filiform and fungiform papillae, but also to taste buds. A requirement for normal Shh signaling in fungiform papilla, taste bud and filiform papilla maintenance was shown by Gli2 constitutive activation. We identified proliferation niches where Shh signaling is active and suggest that epithelial and mesenchymal compartments harbor potential stem and/or progenitor cell zones. In all, we report a set of hedgehog signaling centers that regulate development and maintenance of taste organs, the fungiform papilla and taste bud, and surrounding lingual cells. Shh signaling has roles in forming and maintaining fungiform papillae and taste buds, most likely via stage-specific autocrine and/or paracrine mechanisms, and by engaging epithelial/mesenchymal interactions.
Keywords: fungiform papilla, papilla placode, paracrine signaling, stem cells, taste cell progenitors, Ptch, Gli1, Gli2
INTRODUCTION
The adult fungiform papilla is a complex set of tissues and cell types that reside in the specialized multilayered epithelium of the tongue. This unique sensory organ includes: taste buds; a layered and keratinized surface epithelium; and, lateral epithelial walls that extend into the underlying connective tissue to surround a core of lamina propria cells (Figure 1). The core is engorged with blood vessels and bundles of nerve fibers from geniculate and trigeminal sensory ganglia. Furthermore, the core itself is specialized into apical and more basal regions. Surrounding the fungiform papillae are other specializations of the lingual epithelium, the filiform papillae. The filiform structures do not contain taste buds, but have sharp, heavily keratinized, apical spines. Thus, to develop and maintain the fungiform papillae, many regulatory steps must be orchestrated that require close interactions between the tongue epithelium and connective tissue core.
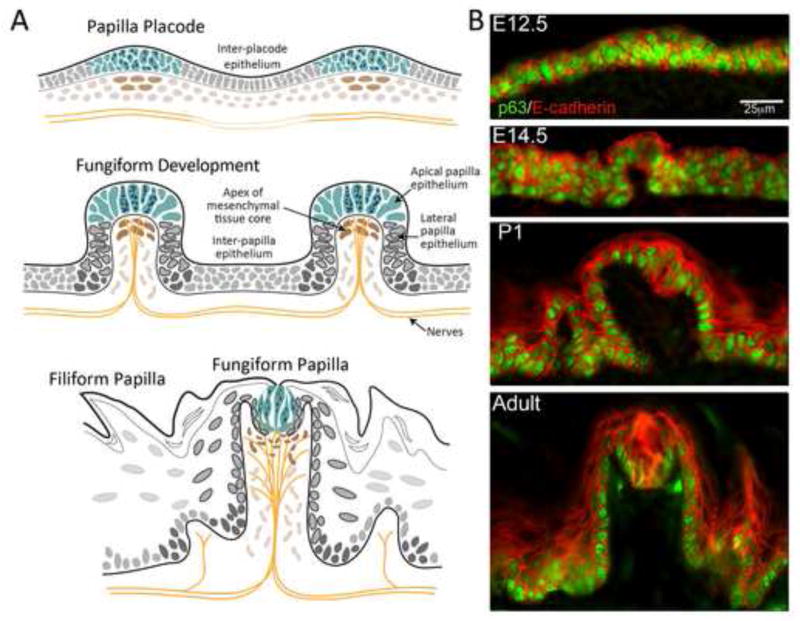
Figure 1. Papillae and taste buds develop in a complex epithelium.
A. Schema for fungiform papilla development. The Papilla Placode (about E12.5 to E13.5) develops as a collection of epithelial cells over mesenchymal cells, with an inter-placode epithelium that is a single layer of cells. Nerves are within the tongue but not yet approaching the epithelium. With Papilla Development (about E14.5 to E17.5) cell compartments become distinctive. The fungiform papilla epithelium is differentiated at the apex and forms lateral papilla walls that delimit the papilla and extend around a papilla core of mesenchymal cells. From within the core, nerve fiber bundles distribute to the epithelium. The inter-papilla epithelium brackets fungiform papillae. In the adult, the Fungiform Papilla has a differentiated, apical collection of cells, the taste bud, in the apical epithelium. The inter-papilla epithelium has differentiated into non-gustatory, Filiform Papilla structures. In the papilla core a set of apical connective tissue, lamina propria cells is distinguished from other cells in the core. Nerves within the papilla branch to taste buds and surround, and innervate the taste bud cells. B. Cell layers and basal cells are apparent with immunoreactions for p63 and E-cadherin. The papilla placode at E12.5 is a collection of cells within an epithelium between placodes that is essentially a single layer. Proliferating cells (p63-positive) are throughout the epithelium. At E14.5 the developing papilla has basal (p63-positive) and apical (E-cadherin-positive) cell layers. Apical papilla cells are distinguished from lateral walls. The P1 papilla has multiple cell layers (E-cadherin) over a basal cell layer (p63) and a distinct apical cell collection that is the early taste bud. Filiform papillae are seen in inter-papilla epithelium. The Adult papilla has a taste bud in the apex and interruptions in the basal cell layer under the taste bud are apparent, where nerves enter to innervate the taste bud cells. Filiform papillae surround the fungiform papillae.
Placement of fungiform papillae also is tightly regulated, on the anterior two thirds of the tongue (Mistretta, 1991; Mbiene et al., 1997). These patterned papillae, accessible for observation and manipulation, are an important but often over-looked model for the development of ectodermal specializations, and for understanding fundamentals of sensory cell differentiation.
After embryonic formation and development, the taste papillae and nongustatory filiform papillae in the postnatal tongue grow, differentiate and turn over in cell renewal cycles (Mistretta, 1991; Mistretta and Hill, 1995, 2003). Importantly, the taste buds make a perinatal emergence and continue to differentiate and turn over postnatally, as taste bud cells are renewed throughout the life span (Beidler and Smallman, 1965; Hendricks et al., 2004; Krimm and Hill, 2000). Increased extent and complexity of taste bud innervation coincides with development of taste function and papilla and taste bud cell turn over.
Among the growing cast of identified molecules that regulate the fungiform papillae, Shh is a key player (reviewed in Mistretta and Liu, 2006). Shh signaling has roles in fungiform induction, development and patterning (Hall et al., 2003; Liu et al., 2004; Mistretta et al., 2003) and is proposed to function in taste bud formation (Thirumangalathu et al., 2009). However, there is no deep grasp of where the Shh signal originates and where signaling targets are positioned in the embryonic, postnatal and adult papilla and taste bud. Whereas there is demonstrated tight control of hedgehog signals that talk across epithelium and mesenchyme in formation of hair follicle, tooth and intestinal villus (Chuong et al., 2000; Dassule et al., 2000; St Jacques et al., 1998; Walton et al., 2012), we have scarce data about locales and timing for expression of Hh signaling components in papilla development.
The Hh pathway is well defined. When Hh ligands bind to the Patched (Ptch) transmembrane receptor, Ptch repression of a second transmembrane protein, Smoothened (Smo), is relieved (Robbins et al., 2012). Smo then initiates intracellular signaling, ultimately activating Gli transcription factors. This leads to induction of Shh target genes. Effectors of Hh signaling in vertebrates are Gli proteins, Gli1, Gli2, Gli3. Diffusible morphogens such as Shh can be strong activators at short range and continue activation at longer range, of 200 μm or more (Saha and Schaffer, 2006). At the same time, a surrounding zone of lateral inhibition can act to pattern tissues in coordination with other pathways (Liu et al., 2004; Zhou et al, 2006).
To understand how Shh signals in tongue, papilla and taste bud formation and maintenance, it is necessary to know where and when Shh ligand and pathway components are positioned. We identified Shh signaling centers in the context of defined cell and tissue compartments in fungiform papillae with reporter mouse lines. By mapping expression of the hedgehog targets Ptch1 and Gli1, which provide a direct indication of Shh responsiveness (Ahn and Joyner, 2004; Marigo et al., 1996), spatial and temporal changes in signaling centers were demonstrated and responding cells shown to bracket the restricted location of Shh protein and message. With lineage tracing for Gli1 we found that Shh-responding cells contribute progeny not only for maintenance of filiform and fungiform papillae, but also for taste buds. A requirement for normal Shh signaling in fungiform papilla, taste bud and filiform papilla maintenance was shown by Gli2 constitutive activation. We identified proliferation niches where Shh signaling is active and suggest that epithelial and connective tissue compartments harbor proposed stem and/or progenitor cell zones. In all, we report a set of hedgehog signaling centers that regulate development and maintenance of the taste organ, fungiform papilla and taste bud, and the lingual surround.
METHODS
Animals
Animal maintenance and use were in compliance with institutional animal care protocols and in accordance with National Institutes of Health Guidelines for care and use of animals in research. All dissections of E12.5–18.5 embryos were between 9:00 AM and 12:00 PM for consistency across litters (Mbiene et al., 1997). Noon of the day of vaginal plug detection was designated embryonic day 0.5 (E0.5). Embryos were staged by vaginal plug detection and confirmed by Thieler staging for development of multiple organs. P1 was the day when pups were born.
Mouse lines and tissue collection
Timed, pregnant C57BL/6 mice (E12.5, E14.5 and E18.5), postnatal mice (1–12 months) were from Charles River breeders.
Mouse lines that carry the bacterial β-galactosidase (lacZ) gene driven by Sonic hedgehog (Shh-lacZ, Jeong et al., 2004), Patched 1 (Ptch-lacZ, Goodrich et al., 1997; available Jackson Laboratory Strain 008211), Gli1 (Gli1-lacZ, Bai et al., 2002; available Jackson Laboratory Strain 003081), or Gli2 (Gli2-lacZ, Bai and Joyner, 2001; Jackson Laboratory Strain 007922) were used to monitor the expression of hedgehog signaling components. The reporter gene positive litters were genotyped by PCR and X-Gal staining, and wild type littermates were used for comparison.
Gli1-CreERT2;R26R bitransgenic mice were used for lineage tracing with conditional activation of lacZ reporter upon inducible Cre activation driven by the Gli1 promoter. Tamoxifen chow (0.4 mg per g diet) was fed for 4 weeks to induce lacZ gene expression in Gli1-expressing cells, which is maintained in their progeny. Tongues were analyzed from 1 day to 3 months after the termination of tamoxifen treatment, with X-Gal staining in whole tongue followed by cryostat sectioning.
K5-rtTA;TRE-GLI2* mice (Diamond et al., 2000; Grachtchouk et al., 2011) were used for functional analysis with a doxycycline-inducible, constitutively active truncation mutant of human GLI2 controlled by a Keratin 5 (K5) promoter-driven reverse tet transactivator (rtTA), so that the hedgehog pathway was activated in a ligand-independent manner. Doxycycline was administered at a dose of 200 μg/ml in 5% sucrose in drinking water, and doxycycline-containing chow (1000 mg/kg, Bio-Serve, Frenchtown NJ). Mice received doxycycline in chow and drinking water ad lib for 3 days, after which the mice were maintained on doxycycline chow for up to 7 or 12 days.
Mice were euthanized by an intraperitoneal injection of urethane (60 mg/g body weight) or a slow stream of CO2. Embryonic or postnatal tongue on mandibles were removed into cold, sterile phosphate buffered saline (PBS) and tissues were dissected and processed for different analyses.
Scanning electron microscopy
Tongues were fixed in 2.5% glutaraldehyde and 4% paraformaldehyde (PFA) in 0.1 M cacodylate buffer (pH 7.3) at 4°C, post-fixed in a sequence of aqueous 1% OSO4, 1% tannic acid, 1% OSO4, for 1 hr each on ice, and processed as described (Mbiene et al., 1997). Tissues were mounted, sputter coated with gold/palladium, and analyzed with SEM. Digital images were acquired at ×25, ×40, ×100, ×1000 original magnification and assembled using Photoshop (Adobe Systems, Mountain View, CA).
Histology
Mouse tongues were dissected and fixed in 4% PFA in 0.1 M PBS, pH 7.4, at 4°C for overnight, then dissected into pieces. Anterior half tongues were transferred to 70% ethanol, embedded in paraffin and serially sectioned in sagittal plane at 6–8 μm for hematoxylin and eosin staining. To compare lingual tissues between control and transgenic tongues, serial sections were examined to ensure evaluation of the complete anterior tongue. Photomicroscopy presented comparative tongue and papilla phenotypes.
Immunohistochemistry
Antibodies
Primary antibodies and dilutions were: βIII-tubulin (T2200, 1:1000, Sigma Aldrich, St Louis MO); BrdU (G3G4, 1:400, Developmental Studies Hybridoma Bank, University of Iowa); E-cadherin (AF748, 1:500, R&D Systems, Minneapolis MN); Gli2 (AF3635, 1:1000, R&D Systems); Keratin 5 (PVB-160P, 1:5000, Covance, Emeryville CA); Keratin 8 (TROMA-1, 1:200–1000, Developmental Studies Hybridoma Bank); Ki67 (M7249, 1:400, DakoCytomation, DK); Laminin (L9393, 1:500, Sigma Aldrich, St Louis MO,); Neurofillament-L, M, H (NB300-131, -133, -135, 1:3000, Novus Biologicals, Littleton CO); p63 (sc-8431, 1:100, Santa Cruz Biotechnology, Santa Cruz CA); Shh (AF464, 0.1 μg/ml, R&D Systems).
Tissue section immunohistochemistry
Dissected embryonic and postnatal tongues were fixed in 4% PFA in 0.1 M PBS, pH 7.4, at 4°C for 2–5 hr, then transferred to 30% sucrose in PBS at 4°C for 24 hr. Tissues were frozen in O.C.T. Serial sagittal sections were cut at 10 μm, thaw-mounted onto Superfrost®Plus slides (12-550-15, Fisher Scientific, Pittsburgh PA) and reacted as described (Liu et al., 2004; Mistretta et al., 2003). Sections were blocked with 10% donkey serum for 1 hour and incubated with primary antibody at 4°C for overnight. Following incubation with Alexa fluor® 488 or 568 conjugated secondary antibodies for 1–2 hr (1:500, Invitrogen/Molecular Probes, Grand Island NY). Slides treated with no primary antibody or with the same concentration of normal IgG were used as controls. For Shh, Ki67 and p63 immunoreactions, sections underwent antigen retrieval in citrate buffer (pH 6.0) at 90–95°C for 5 minutes.
X-Gal Staining
Tongue or tongue sections from lacZ reporter mice were fixed in 4% PFA on ice for 15 min, washed in PBS containing 2.0 mM MgCl2, transferred into freshly prepared X-Gal solution (1 mg/ml X-Gal in 0.1 M PBS with 2.0 mM MgCl2/0.01% sodium deoxycholate/0.2% Nonidet P-40/5 mM potassium ferricyanide/5 mM potassium ferrocyanide), and incubated at 37°C for 1–5 hours. Sections were counterstained with neutral red, dehydrated in serial ethanol solution, cleared in xylenes and mounted with Permount medium (SP-15, Fisher Scientific, Pittsburgh PA). Stained tissues were photographed as whole mounts and then cryo-sectioned for light microscopy.
In Situ Hybridization
The following probe templates were used for RT-PCR: Shh (NM_009170 nucleotides 1–1700), Ptch1 (NM_008957 nucleotides 300–1489), Gli1 (NM_010296.2 nucleotides 1072–3058). The PCR products were cloned into pCR4 TOPO vectors (Invitrogen/Molecular Probes, Grand Island NY). Probes were labeled using a DIG RNA labeling kit (SPR/P7, 11 175 025 910, Roche Applied Science, Indianapolis IN ). The sense probes were used as controls in parallel at every stage.
All tissues were fixed in 4% PFA at 4°C overnight and equilibrated in 30% sucrose for at least 24 hr at 4°C. Cryostat sections (10 μm) were treated with 10–50 μg/ml proteinase K in PBS for 15 minutes. The slides were post-fixed in 4% PFA for 10 minutes, followed by acetylation in acetic anhydride (pH 8.0) for 10 minutes. Sections were hybridized at 65°C overnight with the denatured probes in 50% deionized formamide, 1x SSC, 1x Denhardt’s solution, 10% dextran sulfate, 0.2 mg/ml yeast tRNA and 0.5 mg/ml herring sperm DNA. After rinses, the slides were incubated with anti-digoxigenin-AP, Fab fragments (11 093 274 910, 1:1000, Roche Science) for overnight at 4°C. Following rinses, the slides were equilibrated in the staining buffer (100 mM Tris·HCl pH 9.5, 100 mM NaCl, 50 mM MgCl2) at room temperature for 30 minutes. Color detection was performed in the dark in staining buffer supplemented with 100 mg/ml NBT (11 383 213 001, Roche Applied Science), 100 mg/ml BCIP (11 383 221 001, Roche Applied Science), 0.3% Tween-20 for 5–24 hours. After thorough rinses, slides were mounted with 70% glycerol in PBS and photographed for light microscopy.
Tissue analysis
Quantification for Shh-positive cells in placode and papilla development
Numbers of Shh-positive cells in placodes or papillae, were counted at E12.5, E14.5 and P1 stages. Two or three tongues were studied per stage, in serial sagittal sections of the anterior one third of the tongue, immunostained for Shh expression. Two or three non-consecutive slides were selected, excluding the median furrow, and in 8 to 10 sections per slide, numbers of Shh-positive cells was counted in 11 placodes or papillae. Cell numbers were quantified at 40× with light microscopy. Data are presented as range and median values.
At E14.5 using the same procedures, Ptch1-expressing cells were counted in one Ptch1 reporter tongue that had been immunostained for Shh. Eleven papillae were analyzed.
Papilla and taste bud quantification in transgenic mice
Fungiform papillae and taste buds were quantified in one Gli1-CreERT2;R26R mouse tongue at 3 months after termination of tamoxifen administration and in one K5-rtTA;TRE-GLI2* tongue after 12 days of doxycycline treatment. Serial sagittal sections from half of the tongue were reacted with X-Gal staining (Gli1-CreERT2;R26R ) or hematoxylin and eosin staining (K5-rtTA;TRE-GLI2*) and sections in about 1.0 mm tissue width (100 sections, from the median furrow toward lateral border) were examined with light microscopy. For reference, in the control mouse, each fungiform papilla contains one taste bud in the papilla apex. Each taste bud has a taste pore where microvilli from taste bud cells extend, in an opening (pore) in the taste bud surface. The taste pores can be identified with SEM. Within a single taste bud there are about 60 to 80 cells.
In Gli1-CreERT2;R26R mouse tongue sections, taste buds and papillae were divided into four groups or patterns (described in Results) based on distribution of intensely (most cells are X-Gal positive) or sparsely (most cells are X-Gal negative) labeled cells in the taste bud, and labeled cells in the perigemmal region, lateral papilla walls, and underlying mesenchyme. The percentage of patterns for each group was calculated as papilla number with the particular pattern divided by the total number of examined papillae in the half tongue (n = 37).
In the K5-rtTA;TRE-GLI2* mouse, serial tongue sections, 50 fungiform papillae were examined in the half tongue. Papillae were scored for presence or absence of a taste bud, and, taste buds were counted as typical or aberrant (thin and without a taste pore). The numbers of fungiform papillae without a taste bud, with a typical taste bud, or with an abnormal bud were quantified.
RESULTS
Cell compartments of the developing and adult fungiform papilla, taste bud and surrounding epithelium, and mesenchyme or lamina propria
Fungiform placode and papilla have defined epithelial and connective tissue compartments
Specialized cell compartments can be identified in developmental stages from papilla placode to embryonic papilla, through the adult stage when a single taste bud is differentiated in each rodent fungiform papilla (Figure 1). These compartments provide the context for demonstrating Shh signaling centers.
The papilla placode is a differentiated cluster of epithelial cells and underlying mesenchyme. Within the placode, a central set of cells is further differentiated (Figure 1A, Papilla Placode). Inter-papilla lingual tissue is less differentiated. Nerves in the mesenchyme are near but not yet within the placode epithelium. As the embryonic fungiform papilla develops (Figure 1A, Fungiform Development), epithelial layers extend into the mesenchyme and early lamina propria to encompass a core with gustatory innervation (Mbiene and Mistretta, 1997). With further growth, as epithelial layers increase and grow into the tongue, the core expands with some extension of the papilla above the lingual epithelium. The apical papilla epithelium is molecularly and cellularly distinct from lateral papilla walls.
At later postnatal and adult stages the fungiform papilla contains a well-developed, apical taste bud, in a stratified squamous epithelium with proliferation niches at the base of the papilla (Figure 1A, Fungiform Papilla). The papilla core has an extensive innervation and contains an apically specialized lamina propria that is rather dense under the taste bud. Well-developed filiform papillae surround gustatory fungiform organs (Figure 1A, Filiform Papilla).
These defined compartments provide a basis for interpreting Shh signaling centers in papilla development and maintenance.
Acquisition of a stratified epithelium and basal cells
We used immunoreactions for p63 to label early basal cells and for the transmembrane protein, E-cadherin to mark cell membranes. There is progressive acquisition of a multi- layered lingual and papilla epithelium. At placode formation, clusters of about 25 bilayered cells, all positive for p63 and E-cadherin, are in the lingual epithelium whereas the inter-placode epithelium remains as an essentially single layer of columnar cells with a periderm covering (Figure 1B, E12.5). The embryonic papilla already has distinctive apical epithelial cells that are not p63-positive (Figure 1B, E14.5) and the suprabasal inter-papilla epithelium is multilayered.
The postnatal day 1 (P1) papilla and lingual epithelium have a defined basal cell layer, positive for p63, and the large fungiform papilla encompasses a core of lamina propria, connective tissue cells (Figure 1B, P1). Layers of cells, outlined by E-cadherin positive membranes, are in fungiform papilla and surround epithelium. Incipient filiform papillae are found throughout the tongue. The adult mouse fungiform papilla is distinctive in its apical taste bud, composed of dozens of E-cadherin-positive cells with surrounding p63-positive cells (Figure 1B, Adult). p63-positive basal cells also delimit the fungiform papilla and taste bud and there are multiple cell layers on the papilla apex and inter-papilla tissue. Thus, differentiation of taste papillae, taste buds and non-gustatory papillae, progress in the context of stratification of the tongue epithelium.
The Shh ligand is temporally and spatially restricted in developing and mature papillae and taste buds
Progressive restriction of Shh ligand location
To determine with spatial and temporal accuracy where Shh is localized during fungiform and lingual epithelial development and maintenance, we used independent assays: immunostaining, in situ hybridization and LacZ reporter mice. The emerging placodes and developing papillae are strongly positive for the hedgehog signaling ligand, Shh.
At E12.5 Shh protein and message are intensely expressed in cells of the fungiform papilla placode, with some evidence of graded mRNA expression (Figure 2, E12.5). There is a range of 7–11 (median = 9) Shh-positive cells per central placode region. Notably, Shh protein also is throughout the basal lamina zone of papilla and in the between-papilla tongue regions (Figure 2, E12.5, Shh-ir, arrow). The intense distribution of Shh in the basal lamina of E12.5 lingual epithelium is important because components of the basal lamina are in a natural position to concentrate or sequester molecular regulators of epithelial development. Some specialization of mesenchyme cells just under the placodes is apparent with in situ hybridization (Figure 2, E12.5, in situ, arrows).
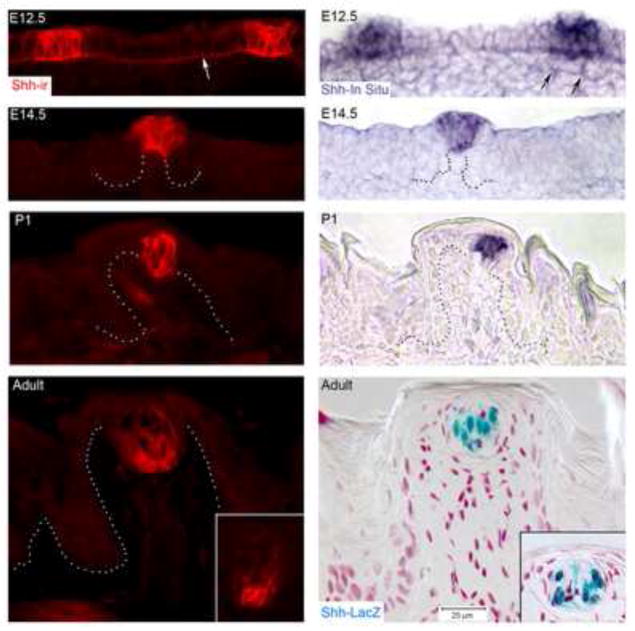
Figure 2. Shh ligand and message is spatially and temporally restricted in papilla development and maintained in adult taste bud.
With Shh immunoreaction (Shh-ir) and in situ hybridization (Shh-in situ), and in reporter mice (Shh-LacZ), an early and continuing restriction of Shh is apparent. At E12.5, Shh is within a cluster of about 9 epithelial cells within the papilla placode, and some specialization of mesenchymal cells is apparent (arrows, in situ). Notably Shh is located in the basal lamina of the placode and between placode epithelium (white arrow, Shh-ir). By E14.5 the emerging fungiform papilla can be distinguished (dotted outline) and the central apical epithelium is positive for Shh (ir and in situ). Shh, tightly restricted in the early postnatal, structurally differentiated papilla, P1, is confined to the early taste bud. Some Shh-positive, presumed endothelial cells are seen in the papilla core. In the Adult papilla, Shh positive cells are within the taste bud, and in serial sections a few basally placed cells are apparent (see insets).
As the fungiform papilla epithelium extends into the tongue, to surround a core of mesenchyme and developing lamina propria, the Shh protein and message are restricted in cells of the apex of the developing papilla (Figure 2, E14.5). A range of 6–7 (median = 7) Shh-positive cells is confined in the central, apical epithelium of each papilla. This matches the distinctive apical papilla region seen in Figure 1B, E14.5. Association of Shh with the basal lamina of the inter-papilla epithelium no longer is apparent.
The postnatal fungiform papilla has differentiated into a large structure, with basal cell and suprabasal epithelial layers at the papilla apex and in lateral walls (Figure 2, P1). Well-developed filiform papillae bracket the fungiform. However, Shh protein and mRNA are strictly confined to a subset of apical cells in the fungiform apical epithelium (range = 5–7; median = 6), within the cells of the early taste bud.
In the adult mouse tongue, Shh-positive cells are apparent only within the well differentiated taste bud, seen in immunoreactions (range = 3–7 cells; median = 5) and LacZ reporter mice (Figure 2, Adult). Intensely immunoreactive, basally positioned, Shh-positive cells are frequently observed in the adult taste bud (Figure 2, Adult, insets). Numbers of Shh-positive cells are variable and it is important to view expressing cells within the center of the bud if comparing among papillae. In sections at the taste bud center, intense Shh immunostaining or gene expression using Shh-lacZ reporter mouse tongues is generally detected in a few cells. Differences in numbers of Shh-expressing cells in taste buds detected by immunostaining and LacZ reporter mice could relate to differences in detection sensitivity, retention of β-Gal activity, or post-transcriptional regulation of Shh expression.
Thus, Shh ligand and mRNA are strong distinctive markers of the developing fungiform placode, papilla and eventually, taste bud. The restricted, intensely staining Shh protein and mRNA expression from placode through adult taste bud stages indicate areas of potential high hedgehog activity.
We also demonstrated Shh in a restricted taste bud cell localization in mice at 12 months of age (Supplemental Figure 1). Shh-positive cells are in basal locations of the taste bud. As positive controls for Shh immunoreaction reliability, data from hair follicle illustrate Shh-positive cells in the follicle matrix (Supplemental Figure 1).
Shh positive cells are epithelial only and partially overlap with cytokeratin 8, a taste bud cell marker in adult
To confirm that the Shh-positive cells are epithelial only in embryonic papilla development, we used double labeling with Shh and laminin antibody to delineate the basal lamina. A strict epithelial localization of Shh-positive cells is obvious in placodes, embryonic and postnatal papillae (Figure 3, Shh and Laminin, E12.5; E14.5; P1). In adult fungiform papilla, the taste bud is circumscribed by a laminin-positive basal lamina and there is a close association of some Shh-positive taste bud cells with the basal lamina (Figure 3, Shh and Laminin, Adult). Laminin-positive reactivity also labels vascular, endothelial cell lining in the papilla core.
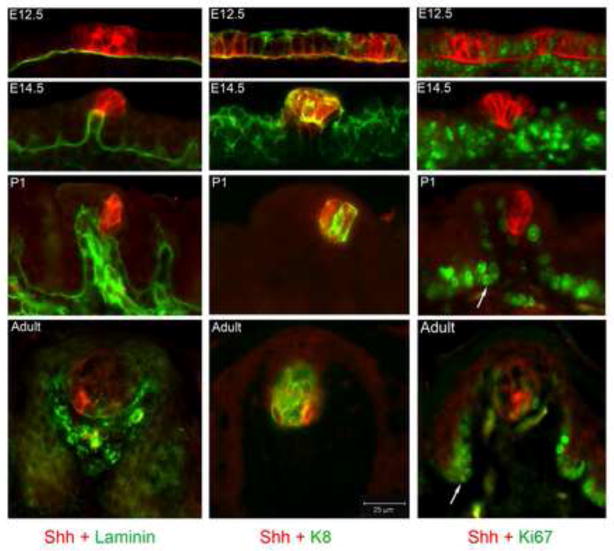
Figure 3. Shh positive cells are epithelial only, have progressive overlap with taste bud cells, and are surrounded by proliferating cells.
In immunoreactions for Shh and Laminin, the basal lamina is delineated from placode, E12.5, though Adult papilla stages, and demonstrates that Shh is epithelial only. Laminin also marks basal lamina in endothelial cells of the papilla core at P1 and Adult. With immunostaining for Shh and K8, the taste bud cell marker, K8 is apparent as an early marker of the single layered epithelium at E12.5. By E14.5, K8 still marks the lingual epithelium and also some cells within the apical papilla epithelium. At P1 Shh-positive cells predominate within the early taste bud whereas in the Adult several cells of the taste bud are K8-positive and fewer are Shh-positive or overlapping with Shh. In Shh and Ki67 immunoreactions, proliferating cells, Ki67-positive, surround the placodal cells in epithelium and mesenchyme at E12.5, although a few remain within the placode epithelium. At E14.5 proliferating cells surround Shh-positive, apical cells of the early papilla, and are within papilla walls and inter-papilla epithelium. In the P1 papilla, proliferating cells surround the early taste bud, are evident as basal cells of the papilla wall, and there are clusters of proliferation at the papilla base (arrow). Clusters of Ki67-positive cells surround the adult taste bud and also are seen at the papilla base in the Adult fungiform (arrow). Ki67-positive cells in the papilla core are most probably marking basal endothelial cells.
To determine timing for potential differentiation of taste bud precursor cells, in association with Shh protein restriction, we used an antibody to cytokeratin 8 (K8), a taste bud cell marker (Knapp et al., 1995), in double immunoreactions with Shh (Figure 3, Shh and K8). In epithelial differentiation, K8 appears initially as a marker of ‘simple’ epithelium (Bagutti et al., 1996). At E12.5 the Shh-positive papilla placodes are within an epithelium characterized by K8 immunostaining that is distributed along the apex of periderm cells of the entire lingual epithelium, with no co-localization with Shh (Figure 3, Shh and K8, E12.5). Shh label is seen in the between-papilla basal lamina at E12.5. In the embryonic papilla, K8 is detected throughout the epithelium (Figure 3, Shh and K8, E14.5). There is some double labeling for K8 and Shh in apical epithelial cells of the early fungiform papilla, but there is a clear predominance of Shh-positive cells in the papilla apex.
At P1 there is considerable overlap in Shh-positive and K8-positive cells, demonstrating gustatory differentiation in the early taste bud cells and loss of K8 expression in the lingual surround (Figure 3, Shh and K8, P1). Thus any simple epithelial cell signature of tongue epithelium is lost whereas K8 now exclusively labels some taste bud cells. In the adult taste bud, molecular markers have shifted in proportion and numerous taste bud cells are positive for the K8 marker, whereas far fewer cells are positive for Shh (Figure 3, Shh and K8, Adult; and, illustrated in confocal images, Supplemental Figure 2). Furthermore, Shh-positive only cells remain in the taste bud, demonstrating that not all Shh-expressing intrabud cells have differentiated to K8-positive cells.
Overall, these data demonstrate that Shh expressing cells of the papilla placode and early papilla are not sustained in large proportion in the adult taste organ, during the multiple cell turnover cycles of postnatal taste bud differentiation and maintenance in the adult. In all, the Shh signaling ligand is intense in about 6–10 epithelial cells throughout placode and papilla development but with a progressive tight restriction to cells of the taste bud only. This restriction to some intensely reactive taste bud cells is maintained in older mice, at 12 months. Within the taste bud, there is an emerging predominance of K8- positive cells from P1 to Adult, with proportionately fewer Shh-positive cells retained. In addition, there is an early intense association of Shh with basal lamina components of the lingual epithelium, and then papilla and taste bud epithelium, which demonstrates potential for signaling regulation between epithelium and connective tissue elements.
Shh positive cells are surrounded by proliferating cells from placode to taste bud stages
Shh is a known mitogen with roles in promoting cell proliferation in a range of tissue and organ types (Agathocleous et al., 2007; Mao et al., 2010; Rowitch et al., 1999). We used double labeling of Shh and Ki67, a mitotic activity marker for proliferating cells, to determine proliferation compartments in tongue and papilla and relation to Shh ligand. During papilla placode formation, Ki67-positive cells bracket the Shh-positive cells in placodes and are in the inter-placode epithelium, and in clusters of mesenchyme cells (Figure 3, E12.5, Shh and Ki67). Shh-positive cells, differentiated and well within the placode center, are apparently no longer dividing. In the early papilla there are dividing cells that bracket Shh-positive apical papilla cells (Figure 3, E14.5, Shh and Ki67). Proliferating cells surround the taste bud at P1 and in the adult (Figure 3, P1, Shh and Ki67).
Proliferating cells are also clustered in a particular basal region of the fungifrom papilla. The early papilla has epithelial clusters of Ki67 positive cells at the papilla base (Figure 3, E14.5, Shh and Ki67). In the well developed, postnatal and adult fungiform papilla, clusters of proliferating cells remain at the fungiform papilla base (Figure 3, Shh and Ki67, P1; Adult, arrows).
Thus proliferation zones bracket Shh ligand during apical papilla and taste bud development and maintenance. Proliferating cell clusters also characterize the base of the papilla.
Location of Shh responding cells
We located Shh responding cells with immunostaining, in situ hybridization and LacZ reporter mice to identify hedgehog targets, the Ptch receptor and Gli1 transcription factor. Ptch1 and Gli1 are transcriptional targets of Shh signaling and thus reflect Shh activity (Ahn and Joyner, 2004; Marigo et al., 1996).
In the embryonic papilla (E14.5) the Ptch1 receptor is in cells that bracket or surround Shh-positive cells, in the early papilla apex (Figure 4, Ptch, E14.5, three images and inset). From 8–12 cells (median = 10) are in the cell cluster that includes Shh-positive centrally positioned cells, and Ptch1-positive surround cells. An overlap of labeled Shh and Ptch1 cells is seen in some cells of the papilla apex. Ptch1 protein and mRNA also are in mesenchyme cells of the developing papilla core, positioned to receive Shh signals from the epithelium.
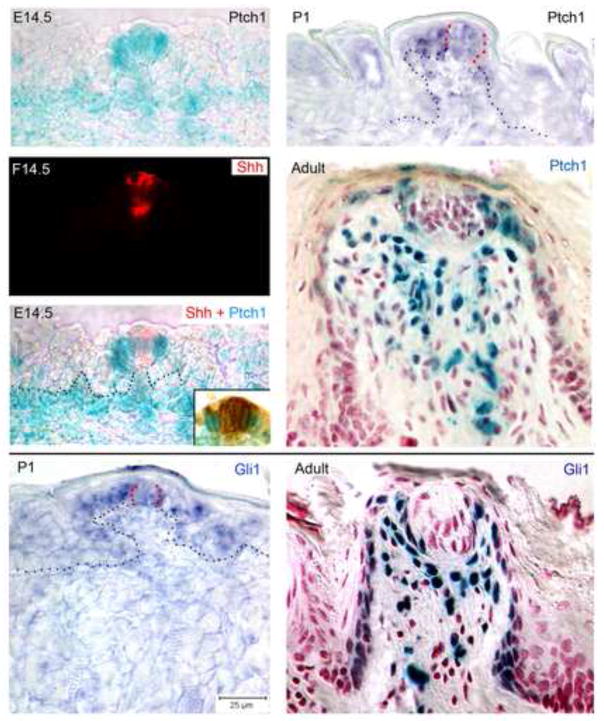
Figure 4. Shh responding cells, Ptch1- and Gli1-positive, bracket the Shh ligand, in epithelium and mesenchyme.
Locations of Shh signaling were identified with reporter mice and in situ hybridization. At E14.5, in reporter mice Ptch1 is intense in cells that surround Shh-positive cells of the early papilla apex. Ptch1 also is within mesenchyme just under the epithelium. At P1, Ptch1 surrounds the early taste bud cells (red outline; black outline for papilla), and in the Adult papilla, Ptch1 positive cells are bracketing the taste bud, in cells of the lateral papilla wall and in lamina propria under the taste bud. Gli1 is similarly located, in taste bud surround at P1 (in situ, red dots outlining taste bud cells) and in Adult, surrounding taste bud cells, in lateral papilla walls and in lamina propria cells around and under the taste bud, but not within the taste bud.
In the P1 fungiform papilla, Ptch1 mRNA surrounds a region of weak expression in the apical epithelium (Figure 4, Ptch1, P1). In lacZ reporter mice, Ptch1 expression surrounds the adult taste bud cells, is expressed in basal cells of the papilla wall, in lamina propria just under the taste bud, and is in possible co-localization with papilla innervation (Figure 4, Ptch1, Adult).
Gli1 mRNA in the P1 papilla brackets cells of the early taste bud and is within basal cells of the lateral papilla epithelium (Figure 4, Gli1, P1), similar to the Ptch1 P1 expression. In Gli1 reporter mice, the adult papilla has Shh signaling activity, or Gli1 expression, in: cells surrounding the taste bud; lamina propria cells collected just under the taste bud; and, in basal cells of the fungiform papilla wall (Figure 4, Gli1, Adult).
These results place the Hh responding cells, expressing Ptch1 and Gli1, in positions that bracket Shh-positive cells, that is: in the apex of early fungiform papillae; intensely in cells surrounding the early taste bud; intensely in cells around the adult taste bud; and in the lamina propria just under taste buds. Thus, responding cells for Shh signaling activity are well positioned to participate in a paracrine signaling process in placode and papilla formation, as well as in papilla and taste bud differentiation and maintenance. Responding cells and ligand define signaling centers of Shh activity in development of placodes and the apex of fungiform papillae; in maintaining fungiform papilla lateral walls and apical epithelium; and, in interactions with underlying mesenchyme or lamina propria. The similar Ptch1 and Gli1 labeling in lamina propria cells of the apical papilla core, in a basket-like distribution under the taste bud cells suggests co-localization with neural elements. Furthermore, the Shh signaling targets are positioned in regions of active cell proliferation shown in Figure 3, located to receive Shh signals, trigger proliferation and contribute cells to the developing and maintained fungiform papilla and taste buds.
Shh signaling cells maintain multiple lineages of the fungiform papillae and taste bud, and filiform papillae
In the postnatal tongue and fungiform papilla, organ development continues and the taste bud continues to differentiate, to acquire a full cell complement and mature innervation (Krimm and Hill, 2000; Hendricks et al., 2004). In the adult tongue, papilla and mature taste bud, cell turnover maintains a stratified squamous papilla epithelium and specialized sensory cells. We used lineage tracing to test the hypothesis that Shh signaling cells contribute to sustained maintenance of papillae and taste bud cells. Because Gli1 is positioned in key surround locations of the Shh ligand and provides a known indicator of Shh signaling (Ahn and Joyner, 2004), we used a Gli1-CreER mouse line with conditional activation of a lacZ reporter to identify the specific tongue and papilla zones where Shh signaling is active in adult Gli1-CreER;R26R mice. The reporter irreversibly activates the lacZ gene in Shh-responding, Gli1-expressing cells at the time of tamoxifen induction through genetic recombination, and thereby permits tracing to determine subsequent locations of progeny of these cells. Thus, we could identify genetically labeled Gli1 expressing cells and analyze the distribution of progeny in tissues of the tongue, fungiform papillae and taste buds at different times after gene activation.
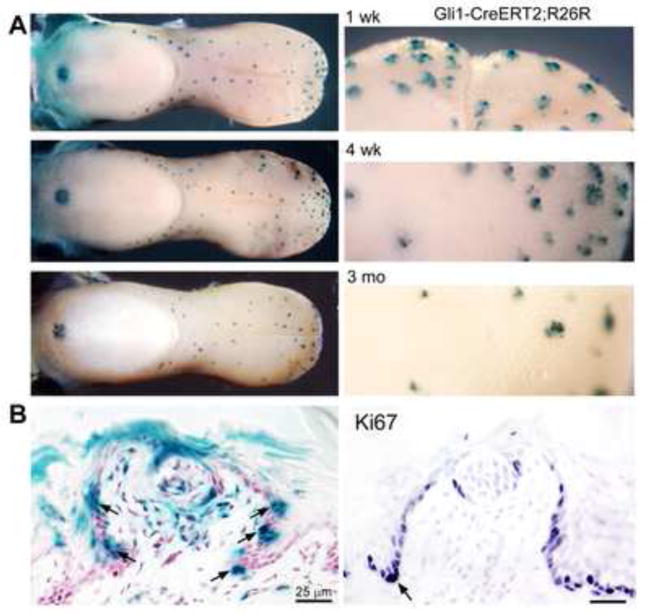
Gli1 expressing cells are in fungiform papillae throughout anterior tongue locations at 1 week to 3 months after administration of tamoxifen for 4 weeks (Figure 5A, whole tongues). Within one week after concluding the tamoxifen induction of LacZ gene expression, large clusters or clones of labeled cells are seen at the papilla base and in lateral papilla walls (Figure 5B, arrows). Gli1 expressing cells also are within the taste bud and in the lamina propria, just under the taste bud and in the papilla core. The Gli1 progeny are in papilla epithelium sites where proliferating cells are identified with Ki67 immunoreactions (Figure 5B, Ki67, arrow; and compare Figure 3, Shh and Ki67, Adult).
Figure 5. Gli1 expressing, derived cells are within epithelium and lamina propria of the adult fungiform papilla and in taste buds.
With lineage tracing in Gli1-CreERT2;R26R mice, we identified Gli1-expressing, derived cell progeny in fungiform papilla locations. A. In whole tongues at 1 and 4 weeks and 3 months after tamoxifen induction, fungiform papilla labeling is obvious, and in the single circumvallate papilla on posterior tongue. B. Tongue sections within the first week show large clusters of Gli1-CreER labeled cells, at papilla base and lateral walls (arrows, section at left). With Ki67 label, it is apparent that Gli1-expressing progeny are in proliferation zones of the papilla (for example, arrow at papilla base, section at right).
In tongue sections at various stages post tamoxifen treatment, we confirmed the presence of Gli1 expressing cells in: the basal cells at the fungiform papilla base and lateral papilla wall; in cells surrounding the taste bud; in cells within taste buds; and in lamina propria cells of the papilla core (Supplemental Figure 3, A, B). To demonstrate that Gli1 labeled cells in the taste bud indeed are taste bud cells, we used the K8 pan taste cell marker to compare with Gli1 progeny (Supplemental Figure 3, C, D).
To learn if Gli1 labeled cells are long-lived progenitors for fungiform papilla and taste bud cells over several weeks of multiple taste cell turnover cycles, we studied the Gli1 lineage-labeled mouse tongues at 3 months post activation in detail. We analyzed serial sections that included 37 taste buds and fungiform papillae and characterized four “patterns” of Gli1 lineage distributions (Figure 6A: 1,2,3,4). The patterns and proportion of pattern representation were: (1) with extensive label of the taste bud, perigemmal cells, basal cells at papilla base and lateral walls, and cells in the lamina propria core (22%); (2) with extensive label of the taste bud, perigemmal label, little or no label of papilla basal cells, and extensive label in the lamina propria core (11%); (3) no taste bud label, extensive label of lateral papilla basal cells and base of papilla, label in basal cells of filiform papilla, and extensive label of lamina propria cells (24%); (4) little or no taste bud label, perigemmal cell label, label of basal cells at papilla base only, extensive label in the core (43%). These patterns have similar characteristics to those reported in K14 lineage tracing (Okubo et al., 2009). In sum, the progeny of Gli1-lineage marked cells persist in the taste bud, perigemmal cells and cells of the papilla wall and base through at least 3 months or at least 9 taste cell renewal cycles. Thus some of the cells that expressed Gli1 during the tamoxifen labeling period function as taste bud progenitor cells. Other papilla examples illustrate variations of the patterns and the apparent ‘clonal’ nature of Gli1-marked cell distribution within taste buds (Figure 6B).
Figure 6. Gli1 labeled progenitors are retained in fungiform papilla, epithelium and connective tissue, and in taste buds over several weeks of multiple cell turnover cycles.
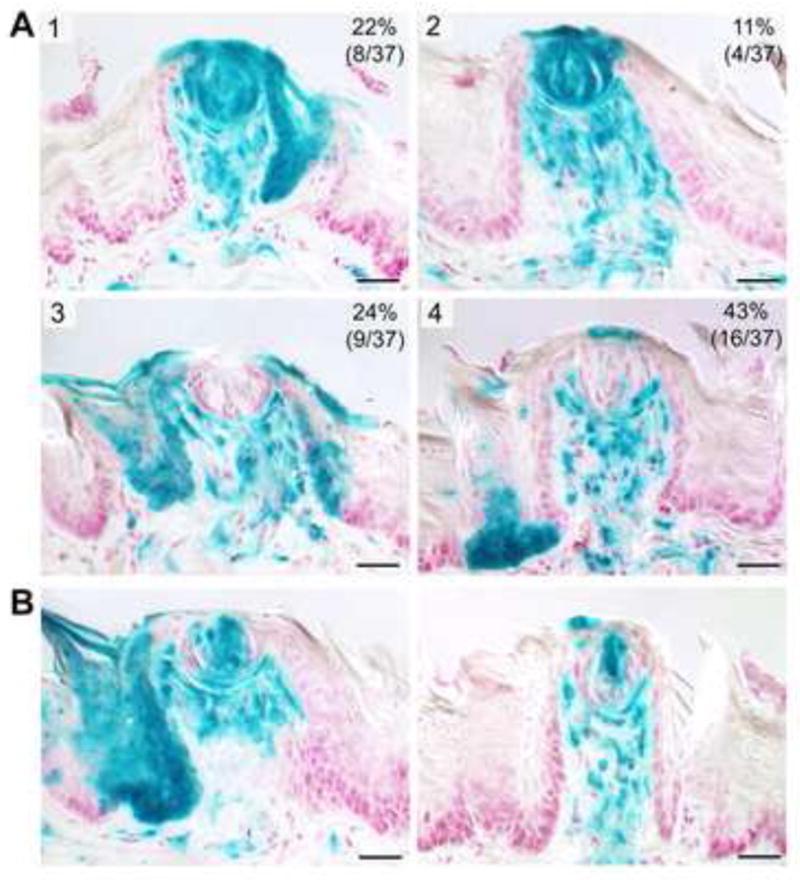
A. At three months post-activation, Gli1-expressing, derived cell progenitors were traced in serial sections and four patterns of lineage distributions were identified (numbered 1,2,3,4 and described in text). The patterns vary in overall representation (the % and number of papillae, out of 37 traced, is at top right in each pattern), and in the extent of Gli1 progeny within papilla walls, perigemmal cells, taste buds, and papilla lamina propria cells. B. Additional examples of Patterns 3 and 4 illustrate the apparent clonal nature of Gli1 progeny distribution within taste buds.
Through identification of descendants of cells that originally express Gli1, the patterns demonstrate that Shh-responsive cells and their progeny contribute to: fungiform papilla maintenance in the epithelial cell base, walls and apex, and in the connective tissue, lamina propria core; cells of the taste bud and perigemmal cells; and, filiform papilla maintenance. Thus, cells that transduce Shh signals contribute to multiple lineages of the epithelium and mesenchyme of filiform and fungiform papillae and to the taste buds, over many cycles of papilla and taste bud maintenance and renewal.
Sustained postnatal activation of the Shh signaling transcription factor, Gli2, alters fungiform papillae and taste bud maintenance, and integrity of filiform papillae and lingual epithelium
We have shown that Gli1 labeled cells or progeny of Gli1 expressing cells, indicators of Shh signaling activity, contribute to multiple life span cycles of cells of the fungiform papilla and taste bud cells, and of filiform papillae. Gli2 is the principal transcriptional activator of hedgehog signaling (Bai et al., 2002) and we proposed that Gli2 acts in maintaining taste organs and tongue epithelium.
Shh, Gli1 and Gli2 --- distinct ligand and transcription factor locations compared in adult tongue
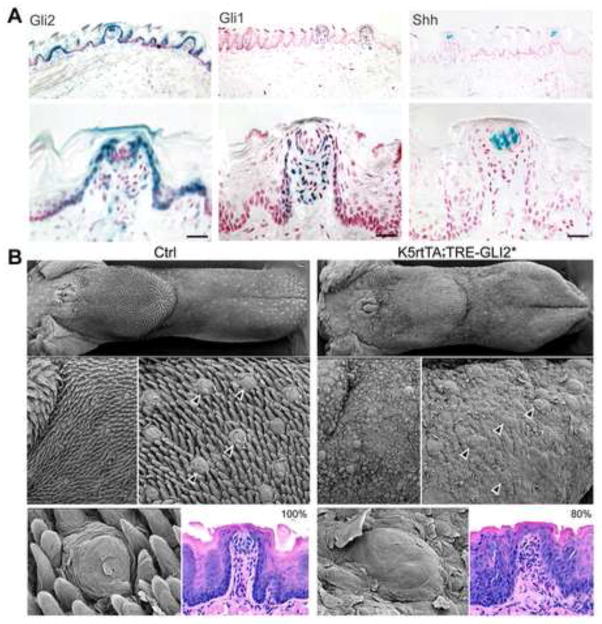
With Gli2 LacZ reporter mice, we found that Gli2 expressing cells are in the basal cells throughout the lingual epithelium (Figure 7A, Gli2). This contrasts with location of Gli1 expressing cells, restricted to cells surrounding the taste bud and the basal cells of fungiform papillae only, not inter-papilla epithelium, and with Shh expressing cells, restricted to the taste buds in adult tongue (Figure 7A, Gli1, Shh). Similar to Gli1, the Gli2 expressing cells are in epithelium surrounding the taste bud, in basal cells of the fungiform papillae, and in apical papilla lamina propria cells (Figure 7A), placing hedgehog transcription factors in positions to receive Shh signals from the taste bud.
Figure 7. Gli2 is in basal cells of papilla walls and lamina propria, surrounds Shh expression in the taste bud, and overlaps with, and extends beyond, Gli1 expression; activation of Gli2 for 7–12 days disrupts integrity of fungiform and filiform papillae and taste buds.
A. In reporter mice, for Gli2, Gli1 and Shh expression, Gli2 expressing cells are throughout basal cells of the tongue epithelium and fungiform papilla, in perigemmal cells around the Shh-positive taste bud, and in apical papilla lamina propria. This overlaps Gli1 expression, but Gli2 extends further, in inter-papilla basal cells. Low power tongue tissue and select fungiform papillae at higher power are shown. B. In the bottom panel, scanning electron micrographs (SEM) illustrate surface topography for Control (Ctrl) and GLI2* activated tongues (K5-rtTA;TRE-GLI2*). Fungiform papillae are seen in Gli2* tongues but distinct taste pores are not obvious compared to Control (arrowheads). Characteristic, spinous, filiform papillae are not seen. In hematoxylin and eosin sections, 80% of fungiform papillae in GLI2* tongues did not have an observed taste bud.
Activating GLI2 in adult tongue: tongue, papilla and taste bud effects
In a functional test of Shh signaling actions we asked whether activation of the pathway, by autonomously over-expressing the Gli2 transcription factor in postnatal mice, would alter papilla and/or taste bud maintenance. We used K5rtTA;TRE-GLI2* mice, with doxycycline-inducible activation of GLI2* in tongue epithelium basal cells. After 7 or 12 days postnatal doxycycline treatment, transgene activation was confirmed by immunostaining for MYC-tagged GLI2* in the tongue epithelium (Supplemental Figure 4).
In hematoxylin and eosin stained sections at 24–32 hours after doxycycline administration to activate GLI2*, fungiform papillae with taste buds are observed across anterior tongue of bitransgenic mice, similar to control tongues (data not shown). Therefore, one day of GLI2* activation is not sufficient to alter papilla and taste bud morphology. However, scanning electron micrographs (SEM) of control littermate or GLI2* mice demonstrate a profound effect after 7 or 12 days of GLI2* expression (Figure 7B). Although size of the tongue is not substantially different, the surface architecture is much altered (Figure 7B). In SEM, fungiform papillae do not have an obvious taste pore (Figure 7B, arrowheads). Filiform papillae have not retained spinous keratin extensions. Quantification from serial histological sections after 12 days activation indicates that taste buds are not maintained in 80% of papillae (Figure 7B, H&E). In the remaining 20% of papillae, taste buds are “aberrant”, with size reduction and disoriented cells (shown in Figure 8). Filiform papilla spines are lost although the keratinized surface remains.
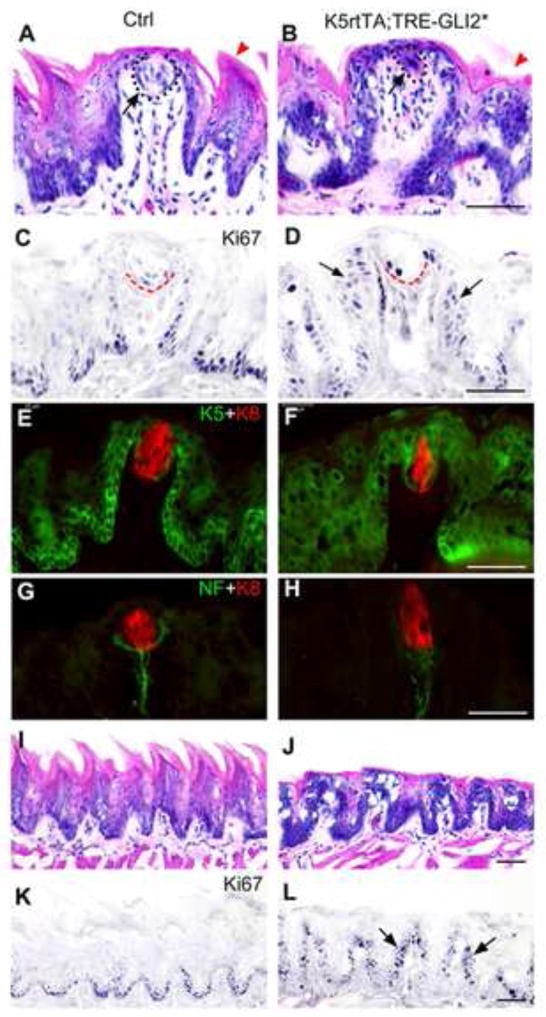
Figure 8. With Gli2* activation, fungiform and filiform papilla epithelium is hyperproliferative, remaining taste buds are thin, but K8-positive and innervated.
A, B. Hematoxylin and eosin sections illustrate that compared to the Control (Ctrl) papilla, fungiform papillae in the GLI2* tongue (K5rtTA;TRE-GLI2*) are misshapen. Clusters of cells are observed in some papillae that presumably represent thin, taste bud remnants (arrow points to dotted cell outline in B; compare to A). Red arrowheads in A and B point to filiform papillae. C, D. With Ki67 immunoreactions, cell proliferation is observed in suprabasal cell layers, where not seen normally, in the GLI2* tongue (arrows in D). A red outline indicates a taste bud (C) or cell cluster (D). E, F. K8 is used as a taste bud cell marker and demonstrates a much reduced set of taste bud cells in the GLI2* tongue. Also with K5, a basal cell cytokeratin, the suprabasal proliferation is obvious. G. H. Nerve fibers innervate the taste bud in Control and GLI2* tongues (neurofilament immunoreaction, NF). I, J. Hematoxylin and eosin stain, and K, L, Ki67 immunoreactions, demonstrate loss of spinous filiform papillae (J) and suprabasal proliferation in GLI2* tongue epithelium (arrows in L). Scale bars = 50 μm.
In hematoxylin and eosin sections, the typical fungiform papilla characteristics of a highly structured, single basal cell layer outlining the papilla and multilayered epithelium, with surrounding, spinous filiform papillae, are compromised in the GLI2* tongue (Figure 8, A, B). Furthermore, taste buds that remain in fungiform papillae do not retain typical size and structure in the GLI2* tongue (Figure 8 A, B, taste buds are outlined and indicated with an arrow). Ki67 immunostaining demonstrates that the basal cell layer is disrupted with GLI2* activation and suprabasal proliferation is seen throughout the epithelium (Figure 8, C, D, arrows in D). Intense Ki67 label within the taste bud suggests possible increased intra-bud cell proliferation (Figure 8, D, taste buds are outlined). However, based on immunostaining for the taste bud cell marker, K8, remaining taste buds are thinner but do contain labeled taste cells (Figure 8, E, F). We quantified diameter at the widest point for 9 taste buds in Control and 6 clearly identified taste buds in GLI2* tongue after 7 days transgene activation. Mean diameters (and standard deviations) were 36.6 ± 3.5 μm in control taste buds compared to 20.2 ± 5.3 μm in the GLI2* tongue. The thin, remaining taste buds retain innervation, demonstrated with neurofilament immunoreactions (Figure 8, G, H).
Not only is fungiform papilla and taste bud maintenance disrupted in the GLI2* mouse tongue, but also the filiform papillae have lost their characteristic, sharp, spinous extensions (Figure 8 I, J). Some surface keratin and a general orientation of the papillae are retained. From Ki67 immunoreactions, suprabasal proliferation in filiform papilla regions is apparent (Figure 8 K, L arrows).
With GLI2* activation, therefore, there is a rapid loss of fungiform papillae and taste buds; taste buds that remain are thinner but retain taste cells and innervation; spines are eliminated from filiform papillae; and, there is atypical cell proliferation in suprabasal layers of the epithelium. Thus, when the balance of hedgehog signaling is disrupted, lingual epithelial integrity is lost and differentiation of fungiform papillae and taste buds, and filiform papillae, is no longer sustained. The basal cell compartment is expanded and suprabasal cell proliferation is observed. These effects match predicted outcomes from localization of Shh, Gli1 and Gli2 expression, and from Gli1 lineage tracing in lingual epithelium, lamina propria and taste organs.
DISCUSSION
We have located Shh ligand in progressively restricted placode, papilla and ultimately taste bud cell locations, surrounded by responding cells in developing and adult gustatory organs. Because Shh can signal up to a few hundred microns in distance, not only cells in the immediate vicinity but also responding cells further afield can participate in Shh signaling centers. The responding cells overlap with zones of active epithelial cell proliferation and lamina propria regions of stromal cell clusters where nerve nets and vasculature abutt the basal lamina.
For development and organ maintenance, therefore, the fungiform papilla is defined by signaling compartments, centers or niches, within: the placode; the forming papilla apex and base; and, the adult papilla with taste bud. The multiple, stage-specific centers render the taste organ vulnerable to imbalance of the powerful Shh morphogen and mitogen, in several cell compartments. Indeed progeny of Gli1-expressing, Shh-responding cells contribute to multiple cell types in taste buds and taste papilla epithelial and connective tissues. Signal disruption in epithelial or lamina propria cell compartments can alter stem and progenitor cell cycling and lead to hyperproliferation and accompanying loss of differentiation. Our data identify Shh signaling centers in fungiform placode and papilla development, and demonstrate an essential reliance of the adult fungiform papilla and taste bud on properly regulated Shh signaling homeostasis.
The developing taste placode and papilla incorporate Shh signaling centers
A prior report that taste placodes do not contribute to papillae, but rather to taste buds only, and with no mesenchymal contribution, relies on a definition of placode that includes Shh-expressing cells only (Thirumangalathu et al., 2009). We show that the entire placode is composed of the Shh-expressing centrally positioned cells and the Shh-responding cells in surrounding epithelium and mesenchyme, to thereby contribute to progressive formation of the full papilla and taste buds. In fact stage-specific, functional roles for Shh signaling in placode induction and papilla development have been demonstrated (Liu et al., 2004). In short, the placode is more than a Shh-positive cell region but is an early Shh signaling center.
Shh is positioned to signal in postnatal and adult tongue, papilla and taste bud maintenance
Fungiform papillae must contain long-lived, sustaining or stem cells and short-lived, maintaining or transit amplifying cells that support the lateral and apical papilla epithelium and the specialized taste buds. The continuously renewing tongue epithelium and taste organs are extremely complex and therefore require balanced molecular signaling to retain structure and function. In the postnatal and adult fungiform papilla, the highly restricted Shh expression in the taste bud is well positioned to signal to receptors and transcription factors in surrounding perigemmal epithelium, abutting stromal cells in lamina propria, and in cells of lateral papilla walls. In short, Shh is tightly localized but, because it is a diffusible molecule, can regulate progenitor cell formation, turnover and maintenance in surrounding regions to sustain papilla- and taste bud-contributing cells.
Shh lineage tracing in fungiform papillae demonstrated that progeny of Shh-expressing progenitors are in two taste bud cell types, basal taste bud cells and ‘edge’ cells in P0 mice (Thirumangalathu et al., 2009). However the Shh-expressing progeny began to disappear prior to weaning and were absent in adults at 4 months. Although it was proposed that the depletion and loss of Shh-expressing progenitors might relate to loss of lacZ transcription, it was concluded that Shh-expressing, placode-derived cells are lost from mature taste buds. If correct, loss of Shh-expressing progeny from adult taste bud cells would suggest that the Shh-positive cells that we observe in taste bud cells, through 12 months of age in mouse, have differentiated from Shh-negative surround cells that migrate into the taste bud. Rather, we propose that maintained Shh-positive taste bud cells could derive also from internal, long-lived progenitors.
Miura and colleagues also reported limited Shh expression, in basal cells of taste buds of the circumvallate papilla, and a Ptch-expressing region around each taste bud (Miura et al., 2001). Ptch expressing cells were not seen in connective tissue under the circumvallate epithelium. In our data, the clear distribution of Ptch, Gli1 and Gli2 expressing cells in apical papilla lamina propria suggests opportunity for direct interaction between epithelium and connective tissue in fungiform papilla and taste bud regulation, as seen in other organ systems of intestinal crypt and villus (Mao et al., 2010; Walton et al., 2012), hair follicle (St Jacques et al., 1998) and tooth (Dassule et al., 2000). In demonstrations of neural crest-derived cell contributions to taste buds, roles for surrounding epithelium and underlying stroma in taste bud maintenance also are indicated (Liu et al., 2012). Furthermore, there are demonstrated roles for Shh signaling in neural crest cells in the mesenchyme of facial primordia in facial patterning and growth, and mesenchyme cells in the developing tongue that express Ptch1 contain a high density of cranial neural crest cells (Jeong et al., 2004). Certainly, the lamina propria cannot be ignored in papilla and taste bud differentiation and maintenance.
Gli1 lineage tracing and Shh-responding papilla and taste cell progenitors
With Gli1 lineage tracing we have shown that Gli1 expressing, Shh responding, cells contribute daughters to epithelium and lamina propria of the fungiform papilla and to nearby filiform papillae. Whereas the taste bud cells themselves do not express Gli1, the progeny of Gli1 expressing, progenitor cells come to reside in the taste bud. This is the first demonstration that Shh-signaling cells contribute progeny to cells of the taste bud, fungiform and filiform papillae.
We find at least three regions of Gli1 expressing progenitor cells that contribute to taste bud and fungiform papilla maintenance: (1) a highly proliferative zone at the base of the fungiform papilla; (2) a highly proliferative zone around the taste bud (perigemmal); (3) a zone in the apical connective tissue core of the papilla. The observed patterns of Gli1 expressing cells are compatible with the idea from classic and current literature that self-renewing or “stem” cells are at the base of the fungiform papilla, and from there the transit amplifying progeny migrate along lateral papilla walls to contribute to perigemmal and taste bud cells, or along filiform papilla walls to contribute to maintenance of nongustatory papillae (Hume and Potten, 1976; Okubo et al., 2009). Contributions to taste bud cells both from progenitors at the papilla base and from perigemmal cells are shown in K14 lineage tracing experiments (Okubo et al., 2009). In small intestine development, stem cells reside at the base of crypts and proliferate continuously; transit amplifying cell progeny migrate from the base up along the crypt walls and divide as they migrate (Itzkovitz et al., 2012). In hair follicle lineage tracing, large clones of multi-potent progenitor, transit amplifying cells migrate within the renewing follicle during the hair cycle (Zhang et al., 2009). The large clones of Gli1 labeled basal cells that we observe at the papilla base and in lateral papilla walls suggest that these Gli1 progeny are clusters of rapidly cycling, transit amplifying cells.
The importance of lamina propria, connective tissue contributions is noted again in these experiments, where Gli1-expressing, stromal cells of the apical papilla core are positioned for epithelial/connective tissue interactions, papilla/taste bud and nerve interactions, and maintenance of papilla structure. Gli1-expressing cells maintain the dermal papilla of the hair follicle and these target cells receive Shh from surrounding cells in the papilla (Brownell et al., 2011). In hair follicle dermal papilla, Shh-expressing, innervating neurons from the dorsal root ganglion are an additional signaling source for responding cells in the follicle. We propose that some of the Ptch1 and Gli1 expressing cells in the region of the nerve “net” just under the taste bud could be on the perineural sheath and receive Shh signals from the taste bud cells.
Overall, our Gli1 lineage data provide a new demonstration of Shh-responding cell contribution to fungiform papillae and taste bud cells. The Gli1 progeny persist through several cycles of tongue epithelial and taste bud cell turnover. Therefore at least some of the cells that contribute to taste bud cell maintenance are Gli1 expressors. The Gli1-Cre label-retaining cells are maintained over three months in mosaic patterns, and show label of: potential “stem” like cells, transit amplifying progeny, and differentiated cells. The results place Shh-signaling in a central position for taste bud and papilla maintenance.
Shh expressing and responding cells and taste bud cell turnover
The proposal that Shh expressing cells within the taste bud also contribute to taste bud cell renewal (Miura et al., 2006) is not excluded by our data. Mitotically active, potential progenitor and stem cell compartments were identified in mouse taste buds (Sullivan et al., 2010). Basal taste bud cells included rapidly cycling, progenitor cells and label retaining, slow cycling, stem cell-like cells. The data suggested that slow-cycling, candidate stem-like cells are principally within basal cells of the taste bud. Although the average taste cell life span is about 10 days, taste cell life span reportedly can range from 2 days to more than three weeks and longer-lived, stem-like taste bud cells include those labeled with taste cell specific markers (Beidler and Smallman, 1965; Hamamichi et al., 2006).
We propose that the Shh-positive taste bud cells in fungiform papillae, in a basal position, may be slowly cycling cells retained in the taste bud whereas the large proportion of K8-positive cells presumably derive from perigemmal cells that migrate into the taste bud and differentiate. The concept of multiple taste cell progenitors is reinforced by our observation that within the taste bud a subset of Shh-positive cells do not co-express K8, which marks simple epithelium (Bagutti et al., 1996) and taste bud cells (Knapp et al., 1995; Okubo et al., 2009). Shh-positive cells might be activated to proliferate more actively in injury or regeneration and/or might contribute to one particular type of taste bud cell.
Functional effects for activated Shh signaling in adult tongue and taste organs
By autonomously activating the transcription factor GLI2, the principal effector for hedgehog signaling (Bai et al., 2002), we tested functional effects of over-activating Shh signaling. In this model the activated hedgehog signaling is continuous (Grachtchouk et al., 2011). Constitutive activation of hedgehog signaling contributes to various basal cell and hair follicle tumors in skin (Grachtchouk et al., 2011) and is a logical potential regulator of tongue epithelial integrity.
We found that deregulating GLI2 for 7 to 12 days resulted in lost or aberrant filiform and fungiform papillae and taste buds. Taste bud cells have a turnover cycle ranging from 2 to at least 21 days (Hamamichi et al., 2006) and turnover cycles for lingual epithelial cells are on average a few days (Cameron, 1966). Therefore, rapid effects of this profound disruption in a major signaling pathway are compatible with effects on cell turnover.
The filiform papillae, fungiform papillae and taste buds are maintained in the lingual epithelium in the adult tongue, and the fungiform papilla depends also on a connective core that houses nerves and vessels essential to epithelial and taste bud integrity. Disruption of any lingual tissues will in turn disturb papilla and taste bud maintenance. Thus, in tongue and taste organs, uncontrolled Shh signaling can drive proliferation of epithelial and connective tissue cells at the expense of sustained differentiation programs that regulate turnover in fungiform papilla, fungiform taste bud cells and filiform papillae. The Shh signaling centers of ligand and responding cells can function in a set of epithelial and strmal cell microenvironments that regulate papilla and taste bud development and maintenance; when out of balance, in uncontrolled activation, there is a dysplastic phenotype.
In GLI2* tongues, suprabasal proliferation was observed in fungiform and filiform papilla epithelium, and the basal cell compartment was hyperproliferative. Our results are similar to aspects of carcinoma in stratified squamous epithelia, for example basal cell carcinoma in which hedgehog signaling is continuously activated and independent of ligand binding (Grachtchouk et al., 2011; Saran, 2010). In skin, oncogenic Hh signaling can disrupt the hair follicle growth cycle and epithelial cell proliferation is sustained, in contrast to ligand-dependent, cyclic physiological Shh signaling. When Shh signaling is deregulated in tongue, also, cell proliferation via upregulated self-renewal of progenitors takes over at the expense of differentiation.
Niches in fungiform papilla and taste bud regulation
We suggest that the fungiform papilla and taste bud encompass a developmental series of stem cell niches and propose that the identified Shh signaling centers are potential niches. A stem cell niche is recognized in several organ systems as a specialized, restricted microenvironment with tissue, matrix, cell and molecular attributes to support the stem cells, cell fate commitment and differentiation (Greco and Guo, 2010; Chen et al., 2013; Lander et al., 2012). The concept of the stem cell niche has not been developed in the taste field but consideration of possible niches can contribute to identification of stem cells that sustain papillae and taste buds.
Possible niches include the early fungiform papilla apex where a bracketing set of proliferative epithelial cells and mesenchymal cells express Ptch and Gli1, responding targets for the Shh signal in the central papilla apex. The Shh-positive epithelial cells are seated on a basal lamina, part of the niche, to provide matrix support and a venue for epithelial and mesenchymal molecular sequestration and exchange (Chen et al., 2013). Within the taste bud of the adult fungiform papilla, there is a potential basal cell niche of a few Shh-positive cells that are bracketed by proliferative perigemmal cells that express Ptch, Gli1, Gli2 and p63. Furthermore, these cells are seated on a basal lamina; are in direct proximity to apical lamina propria cells that also express Ptch, Gli1 and Gli2; are surrounded by a neural net of finely branched axons; and, are near the vasculature of the papilla core.
Another potential stem cell niche is identified at the base of the fungiform papilla, the lowest extension of papilla epithelium into underlying connective tissue, where basal cells are highly proliferative and on the basal lamina over a lingual stroma environment. This niche might well be the origin for cells that migrate along the lateral papilla wall and eventually reach perigemmal regions, to contribute to taste bud cells, as proposed from Gli1 lineage tracing results.
Multiple centers for Shh signaling in a paracrine mode
Hh signaling is necessary for various cell processes in organ formation and maintenance, and regulates cell renewal and differentiation in a concentration-dependent manner (Robbins et al., 2012). Thus, deregulated Hh signaling can lead to cell cycle effects and uncontrolled proliferation. With knowledge about where the Shh ligand and responding cells are located throughout papilla and taste bud development, we can predict and explain effects of Hh signaling in fungiform papilla and taste bud formation and maintenance.
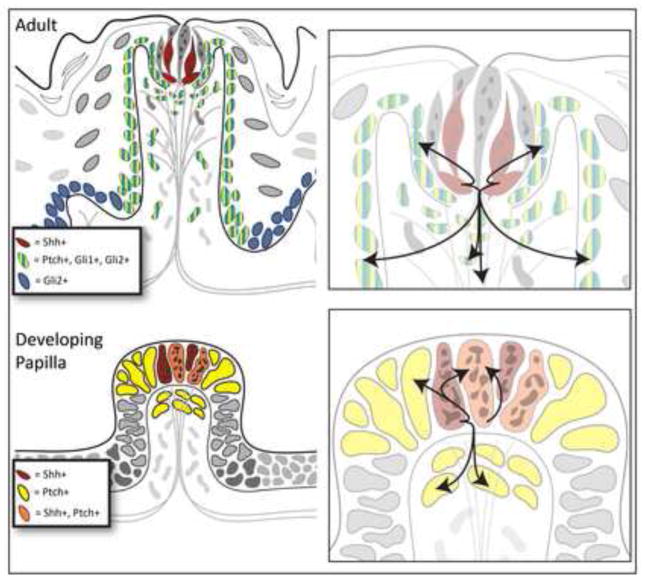
In developing and adult fungiform papilla and taste bud our data have positioned the Shh ligand to signal in various microenvironments in a paracrine mode (Figure 9). The Shh ligand, restricted to taste bud cells in the adult, can signal to responding and transcribing cells: in the perigemmal basal cell compartment, in the basal cell region of the lateral papilla walls, and in the apical connective tissue core of the papilla (Figure 9, Adult Papilla). During the early postnatal period there is an active increase in taste bud volume (Hendricks et al., 2004), in which taste bud cell number matches with innervation density (Krimm and Hill, 2000). An accelerated period of cell production is proposed as a potential mechanism for the increase in taste bud volume, shown to be associated with increased numbers of differentiated taste bud cells that have migrated into the taste bud (Hendricks et al., 2004). At this time the perigemmal and basal cell regions of lateral papilla walls are zones of high proliferation and paracrine Shh signaling to the taste bud surround can regulate proliferation.
Figure 9. Locations of Shh responding cells demonstrate potential microenvironments or niches for Shh signaling and a stage-specific autocrine and/or paracrine signaling mode.
Adult Papilla: Summary Diagram on the left places Shh only within the taste bud and Ptch1, Gli1 and Gli2 responding targets in perigemmal cells, in basal cells of the lateral papilla walls, and in lamina propria, connective tissue cells of the fungiform core. In addition, Gli2 expression extends to basal cells throughout the lingual epithelium. In the Expanded Diagram, arrows indicate paracrine signaling from taste bud cells to regulate perigemmal cells, lateral papilla basal cells, and lamina propria cells. Developing Papilla: Summary Diagram on the left places Shh within apical papilla epithelium and Ptch1 responding targets overlapping with some of the Shh-expressing cells, in epithelial cells surrounding the centrally placed Shh-positive cells, and in mesenchymal cells of the early papilla core and subepithelial tissue. In the Expanded Diagram on the right, arrows indicate autocrine signaling within the papilla apical epithelium and paracrine signaling to epithelial and mesenchymal cell surround.
We propose that Shh maintains the adult fungiform papilla and resident taste bud by regulating progenitor cells and, thereby, cell proliferation in papilla epithelium, stromal cells, and taste bud cells. With concentration-dependence, Shh signals nearby and at a distance to the papilla base and apex, to the lamina propria of the apical core, to the taste bud surrounding cells. Within each niche, responding cells in various cell and tissue contexts will interact with distinct levels of the Shh signal.
In the embryonic, early fungiform papilla, Shh-responding cells in epithelium and mesenchyme bracket a central Shh-expressing region in the epithelium, suggesting a paracrine mode of signaling as in the postnatal and adult papilla and taste bud (Figure 9, Developing Papilla). However, because Ptch1-expressing cells overlap with some of the Shh-expressing cells, an autocrine signaling mode also is likely during placode and papilla development (Figure 9, Developing Papilla).
CONCLUSIONS
In conclusion, our data define specific locations for Shh expression and Shh signaling; bring forward new demonstrations of Shh roles in tongue, papilla and taste bud development and maintenance; show for the first time a direct contribution of hedgehog responding, Gli1 labeled progeny to both taste bud cells and to cells of the papilla epithelium; and demonstrate a requirement for Shh signaling with the distinctive effects of activating Shh transcription factors in adult tongue. Shh signaling has roles in forming and maintaining fungiform papillae and taste buds, most likely via stage-specific autocrine and/or paracrine mechanisms, and by engaging epithelial/mesenchymal or connective tissue interactions.
Supplementary Material
A: Tongue tissue from 12 month mice, immunostained for Ki67 demonstrates maintained basal cell proliferation in lingual epithelium. B, C: Shh immunostaining demonstrates sustained Shh expression within taste bud cells at 12 months. Intensely labeled basal taste bud cells are seen. D, E: In hair follicles from 12 month mouse immunoreactions, Shh-positive cells are located within follicle matrix cells. Blue label is DAPI.
A, B, C: Confocal microscopy images illustrate Shh-positive cells in the basal taste bud region; they are not as numerous as K8-positive taste cells.
A, B: Gli1 progenitor cells are seen within taste buds. A basal cell region at the papilla base (A, arrow), that has Gli1-progenitor cells, is also a region of high cell proliferation. C, D: X-Gal labeled taste bud cells (C) are confirmed by subsequent labeling with the K8 pan taste cell marker on the same section (D). All scale bars = 25 μm.
Conditional activation of GLI2* is indicated with MYC immunostaining in basal cells in GLI2* tongue epithelium compared to Control. Scale bars = 50 μm.
Highlights.
Shh expression progressively restricted from apical papilla to taste bud cells only
Shh responding cells are in areas of active cell proliferation in fungiform papillae
Gli1-expressing cells contribute progeny to papilla and taste bud cells
Paracrine signaling from Shh-expressing cells required in adult papilla and taste bud
Shh signaling centers regulate development and maintenance of taste organs
Acknowledgments
We thank colleague Dr. James Corson for helping to conceptualize and for executing the diagrams in Figures 1 and 9. Support was from NIDCD NIH Grant R01 DC000456 (CMM), NIDDK NIH R01 DK065850 (DLG), and NIH R01 AR045973 and R01 CA118875 (AAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agathocleous M, Locker M, Harris WA, Perron M. A general role of hedgehog in the regulation of proliferation. Cell Cycle. 2007;6:156–159. doi: 10.4161/cc.6.2.3745. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. Dynamic changes in the response of cells to postitive hedgehog-signaling during mouse limb patterning. Cell. 2004;118:505–16. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Bagutti C, Wobus AM, Fassler R, Watt FM. Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and β1 integrin-deficient cells. Dev Biol. 1996;179:184–196. doi: 10.1006/dbio.1996.0250. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biology. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron IL. Cell proliferation, migration, and specialization in the epithelium of the mouse tongue. J Exp Zool. 1966;163:271–284. [Google Scholar]
- Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Patel N, Lin J, Jung HS, Widelitz RB. Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: perspectives in development and evolution. Cell Mol Life Sci. 2000;57:1672–1681. doi: 10.1007/PL00000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional geneexpression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linkd to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural stem cell fates and medulloblaststoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Gratchtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A, Wilbert D, Patel RM, Ferris J, Diener J, Allen M, Lim S, Syu L-J, Verhaegen M, Dlugosz AJ. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J Clin Invest. 2011;121:1768–1781. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1585–1593. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JMH, Bell ML, Finger TE. Disruptiion of Sonci hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263–277. doi: 10.1016/s0012-1606(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 2006;141:2129–2136. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Hendricks SJ, Brunjes PC, Hill DL. Taste bud cell dynamics during normal and sodium-restricted development. J Comp Neurol. 2004;472:173–182. doi: 10.1002/cne.20064. [DOI] [PubMed] [Google Scholar]
- Hume WJ, Potten CS. The ordered columnar structure of mouse filiform papillae. J Cell Science. 1976;22:149–160. doi: 10.1242/jcs.22.1.149. [DOI] [PubMed] [Google Scholar]
- Itzkovitz S, Blat IC, Jacks T, Clevers H, van Oudenaarden A. Optimality in development of intestinal crypts. Cell. 2012;148:608–619. doi: 10.1016/j.cell.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp L, Lawton A, Oakley B. Keratins as markers of differentiated taste cells of the rat. Differentiation. 1995;58:341–349. doi: 10.1046/j.1432-0436.1995.5850341.x. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Neuron/target matching between chorda tympani neurons and taste buds during postnatal rat development. J Neurobiol. 2000;43:98–106. [PubMed] [Google Scholar]
- Lander AD, Kimble J, Clevers H, Fuchs E, Montarras D, Buckingham M, Calof AL, Trumpp A, Oskarsson T. What does the concept of the stem cell niche really mean today? BMC Biology. 2012;10 (19):1–15. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, MacCallum DK, Edwards C, Gaffield W, Mistretta CM. Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev Biol. 2004;276:280–300. doi: 10.1016/j.ydbio.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Liu HX, Komatsu Y, Mishina Y, Mistretta CM. Neural crest contribution to lingual mesenchyme, epithelium and developing taste papillae and taste buds. Dev Biol. 2012;368:294–303. doi: 10.1016/j.ydbio.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Kim B-M, Rajurkar M, Shivdasani RA, McMahon AP. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137:1721–1729. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that Patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow distinctive and spatially restricted pathways. Acta Anat. 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, MacCallum DK, Mistretta CM. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J Comp Neurol. 1997;377:324–340. doi: 10.1002/(sici)1096-9861(19970120)377:3<324::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Mistretta CM. Developmental neurobiology of taste. In: Getchell T, Doty R, Bartoshuk L, Snow J, editors. Smell and Taste in Health and Disease. Raven Press; N.Y: 1991. pp. 35–64. [Google Scholar]
- Mistretta CM, Hill DL. Development of the taste system. Basic cell and neurobiology. In: Doty R, editor. Handbook of Olfaction and Gustation. Marcel Dekker Press; New York: 1995. pp. 635–668. [Google Scholar]
- Mistretta CM, Hill DL. Development of the taste system: Basic neurobiology. In: Doty RL, editor. Handbook of Olfaction and Gustation. 2. Chapter 36. Marcel Dekker; N.Y: 2003. pp. 759–782. [Google Scholar]
- Mistretta CM, Liu HX. Development of fungiform papillae: patterned lingual gustatory organs. Arch Histol Cytol. 2006;69:199–208. doi: 10.1679/aohc.69.199. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Liu HX, Gaffield W, MacCallum DK. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol. 2003;254:1–18. doi: 10.1016/s0012-1606(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 2006;69:209–225. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Sugiyama C, Kawamatsu M, Ninomiya Y, Motoyama J, Hino A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech of Devel. 2001;106:143–145. doi: 10.1016/s0925-4773(01)00414-2. [DOI] [PubMed] [Google Scholar]
- Okubo T, Clark C, Hogan BLM. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Science Signaling. 2012;5(246):re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH, St-Jacques B, Le SM, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K, Schaffer DV. Signal dynamics in Sonic hedgehog tissue patterning. Development. 2006;133:889–900. doi: 10.1242/dev.02254. [DOI] [PubMed] [Google Scholar]
- Saran A. Basal cell carcinoma and the carcinogenic role of aberrant Hedgehog signaling. Future Oncol. 2010;6:1003–1014. doi: 10.2217/fon.10.49. [DOI] [PubMed] [Google Scholar]
- St Jacques B, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Borecki AA, Oleskevich S. Stem and progenitor cell compartments within adult mouse taste buds. European J Neuroscience. 2010;31:1549–1560. doi: 10.1111/j.1460-9568.2010.07184.x. [DOI] [PubMed] [Google Scholar]
- Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009;136:1519–1528. doi: 10.1242/dev.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Kolterud A, Czerwinski MJ, Bell MJ, Prakash A, Kushwaha J, Grosse AS, Schnell S, Gumucio DL. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. PNAS. 2012;109:15817–15822. doi: 10.1073/pnas.1205669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu H-X, Mistretta CM. Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform papilla development. Dev Biol. 2006;297:198–213. doi: 10.1016/j.ydbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Tongue tissue from 12 month mice, immunostained for Ki67 demonstrates maintained basal cell proliferation in lingual epithelium. B, C: Shh immunostaining demonstrates sustained Shh expression within taste bud cells at 12 months. Intensely labeled basal taste bud cells are seen. D, E: In hair follicles from 12 month mouse immunoreactions, Shh-positive cells are located within follicle matrix cells. Blue label is DAPI.
A, B, C: Confocal microscopy images illustrate Shh-positive cells in the basal taste bud region; they are not as numerous as K8-positive taste cells.
A, B: Gli1 progenitor cells are seen within taste buds. A basal cell region at the papilla base (A, arrow), that has Gli1-progenitor cells, is also a region of high cell proliferation. C, D: X-Gal labeled taste bud cells (C) are confirmed by subsequent labeling with the K8 pan taste cell marker on the same section (D). All scale bars = 25 μm.
Conditional activation of GLI2* is indicated with MYC immunostaining in basal cells in GLI2* tongue epithelium compared to Control. Scale bars = 50 μm.