Abstract
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) is the treatment most likely to achieve prolonged survival for peritoneal surface disease from various primaries, yet management of peritoneal sarcomatosis is controversial as a result of the propensity of sarcomas for hematogenous spread and the paucity of effective chemotherapy. Therefore, we reviewed our experience in patients with sarcomatosis. A retrospective analysis of a prospective database of 990 procedures was performed. Eastern Cooperative Oncology Group, age, type of primary, resection status, morbidity, mortality, and outcomes were reviewed. Over 20 years, 17 cytoreductions for sarcomatosis were performed. After excluding patients with gastrointestinal stromal tumor or uterine leiomyosarcoma, 10 procedures performed in seven patients remained. Median follow-up was 84.8 months. R0/1 resection was achieved in 60 per cent. The 30-day morbidity was 50 per cent; no operative mortality rate was observed. R2 resection had no long-term survivors. The reason for death was peritoneal recurrence in 57 per cent. Median survival was 21.6 months and five-year survival was 43 per cent. Median survival for patients with peritoneal sarcomatosis treated with CRS-HIPEC is similar with the historical reported survival before introducing chemoperfusion. Although a complete cytoreduction is related to improved survival, the role of HIPEC in these patients is unknown. A multi-institutional review will help define the role of CRS-HIPEC in this population.
Soft tissue sarcomas represent 1 per cent of adult malignancies or 11,280 new cases annually in the United States.1 Approximately 36 per cent of sarcomas originate in the abdominal viscera or retroperitoneum and typically spread hematogenously to the lungs and the liver or directly to peritoneal surfaces and adjacent organs.2 For those amenable to resection, local recurrence for abdominal sarcoma ranges from 35 to 82 per cent.3, 4 Sarcomas that spread or recur by seeding nearby peritoneal surfaces represent a particularly ominous moiety.5 Sarcomatosis is defined as the intra-abdominal dissemination of sarcoma and may be present at initial diagnosis but is more frequently observed at recurrence presumably as a result of tumor spillage during the initial resection.6, 7 Once encountered, the prognosis has been historically dismal, highlighting the need for better treatment options.7
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) is the treatment most likely to achieve prolonged survival in peritoneal surface disease from a variety of epithelial tumors. Large reviews of patients with treated peritoneal surface disease have shown improvements in long-term survival predominantly for appendiceal, colorectal, and mesothelioma primaries.8–11 When CRS-HIPEC is applied to sarcomatosis, outcomes have been less clearly beneficial.4, 5, 12–17 The lack of effective chemotherapeutic agents coupled with the fact that sarcomas tend to spread hematogenously has produced controversy regarding the use of CRS-HIPEC in this patient population. Furthermore, a consensus statement regarding the locoregional treatment of sarcomatosis concluded that a better comparison is needed between CRS-HIPEC and CRS alone.16 Therefore, we chose to review our experience with CRS-HIPEC for patients with sarcomatosis in an attempt to define expected outcomes.
Methods
A prospective database containing 990 procedures for peritoneal surface disease preformed from 1992 to 2012 was retrospectively analyzed. Institutional Review Board approval was obtained to support this study. Patients included in the study had a primary diagnosis of abdominal sarcoma with intra-abdominal dissemination. Gastrointestinal stromal tumors (GISTs) and uterine leiomyosarcomas were excluded as a result of introduction of imatinib and usually moderate biologic behavior, respectively. The remaining patients were analyzed based on Eastern Cooperative Oncology Group (ECOG) performance status, age, type of primary, and resection status. Morbidity, mortality, and survival were also reviewed.
All patients identified in the database received CRS-HIPEC according to our previously published protocol.8 The aim of surgery was to remove all gross disease before perfusion with hyperthermic intraperitoneal chemotherapy (HIPEC). The greater omentum was routinely removed as well as any involved peritoneum, tissue, or organs not vital to the patient. Tumors or implants not amenable to resection were reduced as much as possible without compromising vital structures. After resection, inflow and outflow catheters were placed through the skin, and the laparotomy incision was closed with a running suture at the skin level to create a watertight seal. Crystalloid solutions were infused through the inflow until a circuit was established among the abdominal cavity, a pump, and a heat exchanger. Once good flow was established, 40 mg mitomycin C (in divided doses) was added to the perfusate. Some patients also received 20 to 30 mg of mitoxantrone or 125 mg/m2 cisplatin. The perfusate temperature was titrated to achieve an outflow temperature of 40°C. The perfusion circuit was run for 60 to 120 minutes after which the perfusate was drained, the skin reopened, and the abdomen inspected. The abdomen was closed in standard fashion and the procedure concluded. Sarcomatosis was confirmed with final pathology in all patients.
The degree of resection was judged by the surgeon and classified as follows: R0 for complete macroscopic resection with no evidence of involved margins on final pathology and R1 for complete macroscopic resection of gross tumor with positive microscopic margins on final pathology. Cytoreductions with residual macroscopic disease were characterized as R2 and subdivided based on the size of residual disease (R2a 5 mm or less, R2b 2 cm or less, R2c greater than 2 cm). Only resections classified as R2b or less received HIPEC.
All data were collected prospectively and analyzed retrospectively. Patients were typically followed with examinations and computed tomography imaging every 6 months. There was no missing data, and no patients were lost to follow-up. Median overall survival (OS) was estimated from the date of the CRS-HIPEC to the date of death or last follow-up using the Kaplan-Meier survival analysis. Overall survival analyses were performed using SAS 9.3 (Cary, NC). For those patients who received more than one CRSHIPEC, survival was determined from the initial procedure.
Results
Over 20 years, 17 cytoreductions for sarcomatosis were identified. Seven patients with a primary diagnosis of GIST or uterine leiomyosarcoma were excluded leaving 10 CRS-HIPECs performed in seven patients. Median follow-up was 84.8 months. There were four males and three females with ages ranging from 27 to 77 years (mean, 53.7 years). Diagnoses included leiomyosarcoma, spindle cell sarcoma, desmoplastic small round blue cell sarcoma, fibrosarcoma, and hemangiopericytoma. ECOG performance status was 0 or 1 for 90 per cent of the patients. Average preoperative albumin was 4.2 g/dL (Table 1). R0/1 resection was achieved in 60 per cent of all CRS. When excluding repeat CRS-HIPEC, R0/1 resection was achieved in 71 per cent of patients.
Table 1.
Patient and Tumor Characteristics
| Characteristic | Count |
|---|---|
| Sex | |
| Male | 4 |
| Female | 3 |
| Age, mean (range), years | 53.7 (27–77) |
| Type of primary | |
| Hemangiopericytoma | 2 |
| Spindle cell sarcoma | 2 |
| Leiomyosarcoma | 1 |
| Desmoplastic small round blue cell sarcoma | 1 |
| Fibrosarcoma | 1 |
| ECOG before each CRS | |
| 0 | 5 |
| 1 | 4 |
| 2 | 0 |
| 3 | 1 |
| Albumin, mean (range), g/dL | 4.2 (3.5–4.7) |
| Resection status | |
| R0/1 | 6 |
| R2a | 4 |
| R2b–c | 0 |
ECOG, Eastern Cooperative Oncology Group; CRS, cytoreductive surgery.
The 30-day morbidity was 50 per cent and included anemia, neutropenia, obstructive uropathy, thrombophlebitis, and readmission. Nausea, vomiting, and dehydration led to a 30-day readmission rate of 20 per cent. Hospitalization ranged from five to 17 days with a mean of 10. Both operative mortality and reoperation rates were 0.
The local recurrence (intraperitoneal) rate after complete CRS-HIPEC was 67 per cent, and the average time to recurrence was 15.3 months. One patient recurred twice, both after R0/1 resections, and eventually received a third and final CRS-HIPEC where an R2a resection was achieved. Progression of disease after an R2 resection was documented in all cases with an average time to progression of 4.8 months. One of these patients progressed locally as well as distantly in both the lung and liver.
The two patients with a hemangiopericytoma were alive at the conclusion of the study with no evidence of recurrent disease and represent the only patients in this series without known recurrence. Two patients with spindle cell sarcoma died from recurrent disease after an R0 resection. One died 4 months after surgery; the other received two repeat CRS-HIPECs and survived 5 years before dying of his disease. The patient with a leiomyosarcoma recurred both locally and with distant disease.
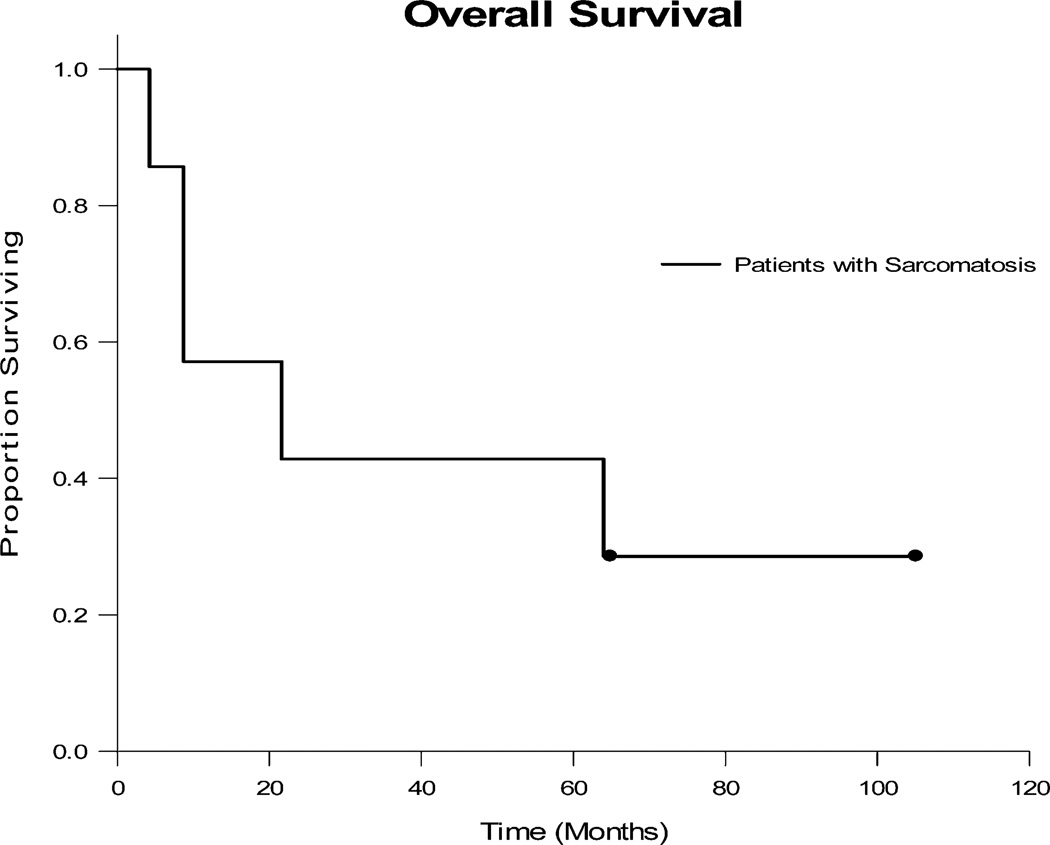
Median OS was 21.6 months (Fig. 1). One- and five-year survival rates were 57 and 43 per cent, respectively. Of all patients who received CRS-HIPEC, four (57%) died from peritoneal recurrence, one (14%) died from unknown causes with recurrence, and two (29%) were alive five years after surgery with no evidence of disease. Median OS in the cohort of patients initially receiving an R0/1 resection was 64 months. Conversely, there were no long-term survivors after an incomplete resection. Although complete resection was associated with improved survival, sample sizes were too small for a meaningful statistical comparison.
Fig. 1.
Overall Survival of patients with sarcomatosis treated with cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Survival was measured from the date of CRS-HIPEC.
Discussion
Morbidity after CRS-HIPEC for sarcomatosis is far from insignificant, ranging from 16 to 50 per cent, whereas operative mortality has been reported as high as 7 to 11 per cent.4, 5, 13, 18 HIPEC-specific toxicity ranges from 11 to 90 per cent and has been associated with gastrointestinal, hepatic, hematologic, renal, and metabolic manifestations.5, 13 As a result of a combination of advanced stage of disease and morbidity, postoperative quality of life usually returns to age-specific baseline within three and six months in most patients.5, 19–21
A potential disadvantage surrounding the use of CRS-HIPEC for sarcomatosis stems from the affinity of sarcomas for hematogenous spread. In one series, 11 per cent of patients with sarcomatosis had distant disease at presentation, yet this percentage increased to 28 per cent during the course of treatment.6 Another series reported distant recurrence in 16 per cent to the lungs, 11 per cent to the liver, 8 per cent to bone, and 5 per cent to lymph nodes.13 It is noteworthy that one patient in the current series developed distant metastasis after CRS-HIPEC. This is consistent with the results of other series. One study reported similar survival in patients with sarcomatosis with or without liver metastasis suggesting that survival is limited by sarcomatosis not hematogenous spread of disease.18 Aggressive locoregional therapy such as CRS-HIPEC, however, leaves distant disease unaddressed. We consider distant metastatic disease a strong contraindication for a cytoreduction; therefore, we make stringent efforts to rule out distant metastasis before offering CRS-HIPEC. In addition, we attempt to identify those tumors that, for unknown reasons, have less likelihood for hematogenous spread and tend to recur locally.
Interestingly, median OS in our series was similar to survival before the introduction of HIPEC. Karakousis et al. reported median OS of 15 months in all patients treated with CRS alone.6 In the only randomized controlled study to date, Bonvalot et al. found no difference between patients treated with and without intraperitoneal chemotherapy after complete cytoreduction. Although this trial was underpowered to make definite conclusions on survival, it reported a median OS of 29 months.12 Median survival in our series fell between that reported by Karakousis and Bonvalot as did the proportion of patients receiving a complete resection, thereby suggesting that resection status is more influential on survival than the use of HIPEC. Additional studies for comparison between outcomes achieved with CRS alone and CRS-HIPEC for sarcomatosis are listed in Table 2. Overall, no dramatic differences exist in survival outcomes with the addition of HIPEC in the small series published to date. Direct comparisons, however, are difficult because included histologies and rates of complete resection vary between the series.
Table 2.
Survival Outcomes for Patients with Sarcomatosis after Cytoreductive Surgery (CRS) Alone or Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS + HIPEC)
| Author | Year | Excluded Histologies |
Procedure | No. | R1/0 (%) | Median Overall Survival |
5-year Survival (%) |
|---|---|---|---|---|---|---|---|
| Karakousis6 | 1992 | None | CRS alone | 72 | 64 | 15 | — |
| None | CRS alone | 46* | 100 | 23 | 4 | ||
| Rossi4 | 2004 | None | CRS + HIPEC | 60 | 68 | 36 | 38 |
| Bonvolat12 | 2005 | None | CRS alone | 19 | 100 | 29 | 37 |
| Lim5 | 2006 | None | CRS + HIPEC | 19/9† | — | 16.9/5.5y | — |
| GIST | CRS + HIPEC | * | — | 19.6/3.2† | — | ||
| Gusani9 | 2008 | None | CRS + HIPEC | 6 | — | 39.6 | — |
| Baratti13 | 2010 | None | CRS + HIPEC | 37 | 75.7 | 26.2 | 24.3 |
| Salti22 | 2011 | GIST, ULS | CRS + HIPEC | 13 | 70 | 12 | — |
| Current series | GIST, ULS | CRS + HIPEC | 7 | 71 | 21.6 | 43 |
Cohort is a subset of the data listed immediately above.
The addition of mitoxantrone to HIPEC.
GIST, gastrointestinal stromal tumor; ULS, uterine leiomyosarcoma.
Not surprisingly, several series support the finding that a complete resection is associated with improved survival regardless of the presence or type of intraperitoneal chemotherapy used.4, 6, 13, 18, 22, 23 Salti et al. found that resection status was the only significant variable influencing survival after CRS-HIPEC.22 Furthermore, Ng et al. found that if resection of all gross disease was achieved, survival was not significantly different from patients with complete resection of localized sarcomas.23 This does support the potential role of CRS for sarcomatosis.
Similar to Karakousis, we observed no long-term survivors after an incomplete resection.6 Our data suggest that the administration of HIPEC to this cohort of patients offers no survival benefit while exposing the patient to all of its associated morbidity and toxicity. Therefore, incomplete resection has only a palliative role in controlling symptoms and should not be followed by chemoperfusion.18 We no longer offer HIPEC in this situation.
Because sarcomatosis is rare, a small sample size was an unavoidable limitation of this study as well as other studies in the literature. This small number did prevent us from a subgroup analysis, yet in general, our results are similar to other reports on the use of HIPEC for sarcomatosis.
In conclusion, although complete cytoreduction is related to improved survival, adding HIPEC in sarcomatosis patients is controversial given the potential toxicity, significant recovery time, and lack of a documented benefit. We currently treat highly selected sarcomatosis patients with cytoreduction and no longer offer HIPEC. A multi-institutional review would be helpful in further defining the role of CRS-HIPEC in patients with sarcomatosis, because the rarity of the problem makes prospective trials impractical.
Footnotes
Presented at the Annual Scientific Meeting and Postgraduate Course Program, Southeastern Surgical Congress, Jacksonville, FL, February 9–12, 2013.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Singer S, Maki RG, O’Sullivan B. Soft tissue sarcoma. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, et al., editors. Cancer: Principles & Practice of Oncology. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. pp. 1533–1577. [Google Scholar]
- 3.Mudan SS, Conlon KC, Woodruff JM, et al. Salvage surgery for patients with recurrent gastrointestinal sarcoma: prognostic factors to guide patient selection. Cancer. 2000;88:66–74. doi: 10.1002/(sici)1097-0142(20000101)88:1<66::aid-cncr10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Rossi CR, Deraco M, De SM, et al. Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis: clinical outcome and prognostic factors in 60 consecutive patients. Cancer. 2004;100:1943–1950. doi: 10.1002/cncr.20192. [DOI] [PubMed] [Google Scholar]
- 5.Lim SJ, Cormier JN, Feig BW, et al. Toxicity and outcomes associated with surgical cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with sarcomatosis. Ann Surg Oncol. 2007;14:2309–2318. doi: 10.1245/s10434-007-9463-z. [DOI] [PubMed] [Google Scholar]
- 6.Karakousis CP, Blumenson LE, Canavese G, et al. Surgery for disseminated abdominal sarcoma. Am J Surg. 1992;163:560–564. doi: 10.1016/0002-9610(92)90556-7. [DOI] [PubMed] [Google Scholar]
- 7.Bilimoria MM, Holtz DJ, Mirza NQ, et al. Tumor volume as a prognostic factor for sarcomatosis. Cancer. 2002;94:2441–2446. doi: 10.1002/cncr.10504. [DOI] [PubMed] [Google Scholar]
- 8.Levine EA, Stewart JH, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–953. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Gusani NJ, Cho SW, Colovos C, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15:754–763. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 10.Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 11.Chua TC, Liauw W, Saxena A, et al. Evolution of locoregional treatment for peritoneal carcinomatosis: single-center experience of 308 procedures of cytoreductive surgery and perioperative intraperitoneal chemotherapy. Am J Surg. 2011;201:149–156. doi: 10.1016/j.amjsurg.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Bonvalot S, Cavalcanti A, Le PC, et al. Randomized trial of cytoreduction followed by intraperitoneal chemotherapy versus cytoreduction alone in patients with peritoneal sarcomatosis. Eur J Surg Oncol. 2005;31:917–923. doi: 10.1016/j.ejso.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Baratti D, Pennacchioli E, Kusamura S, et al. Peritoneal sarcomatosis: is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann Surg Oncol. 2010;17:3220–3228. doi: 10.1245/s10434-010-1178-x. [DOI] [PubMed] [Google Scholar]
- 14.Huh WW, Fitzgerald NE, Mahajan A, et al. Peritoneal sarcomatosis in pediatric malignancies. Pediatr Blood Cancer. 2013;60:12–17. doi: 10.1002/pbc.24293. [DOI] [PubMed] [Google Scholar]
- 15.Munene G, Mack LA, Temple WJ. Systematic review on the efficacy of multimodal treatment of sarcomatosis with cytoreduction and intraperitoneal chemotherapy. Ann Surg Oncol. 2011;18:207–213. doi: 10.1245/s10434-010-1229-3. [DOI] [PubMed] [Google Scholar]
- 16.Rossi CR, Casali P, Kusamura S, et al. The consensus statement on the locoregional treatment of abdominal sarcomatosis. J Surg Oncol. 2008;98:291–294. doi: 10.1002/jso.21067. [DOI] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Review of a personal experience in the management of carcinomatosis and sarcomatosis. Jpn J Clin Oncol. 2001;31:573–583. doi: 10.1093/jjco/hye088. [DOI] [PubMed] [Google Scholar]
- 18.Berthet B, Sugarbaker TA, Chang D, et al. Quantitative methodologies for selection of patients with recurrent abdominopelvic sarcoma for treatment. Eur J Cancer. 1999;35:413–419. doi: 10.1016/s0959-8049(98)00375-x. [DOI] [PubMed] [Google Scholar]
- 19.Hill AR, McQuellon RP, Russell GB, et al. Survival and quality of life following cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colonic origin. Ann Surg Oncol. 2011;18:3673–3679. doi: 10.1245/s10434-011-1793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuellon RP, Danhauer SC, Russell GB, et al. Monitoring health outcomes following cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2007;14:1105–1113. doi: 10.1245/s10434-006-9304-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsilimparis N, Bockelmann C, Raue W, et al. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Ann Surg Oncol. 2013;20:226–232. doi: 10.1245/s10434-012-2579-9. [DOI] [PubMed] [Google Scholar]
- 22.Salti GI, Ailabouni L, Undevia S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal sarcomatosis. Ann Surg Oncol. 2012;19:1410–1415. doi: 10.1245/s10434-012-2240-7. [DOI] [PubMed] [Google Scholar]
- 23.Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215:68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]