Abstract
Background
Myocarditis is a life-threatening heart disease characterized by myocardial inflammation, necrosis and chronic fibrosis. While mast cell inhibition has been suggested to prevents fibrosis in rat myocarditis, little is known about its effectiveness in attenuating cardiac remodeling and dysfunction in myocarditis. Thus, we sought to test the hypothesis that mast cell inhibition will attenuate the inflammatory reaction and associated left ventricular (LV) remodeling and dysfunction after fulminant autoimmune myocarditis.
Methods and Results
To induce experimental autoimmune myocarditis, we immunized 30 rats with porcine cardiac myosin twice at a 7-day interval. On day 8 animals were randomized into treatment either with an intraperitoneal (IP) injection of 25mg/kg of cromolyn sodium (n=13), or an equivalent volume (~0.5ml IP) of normal saline (n=11). All animals were scanned by serial echocardiography studies before treatment (baseline echocardiogram) and after 20 days of cromolyn sodium (28 days after immunization). Furthermore, serial cardiac magnetic resonance was performed in a subgroup of 12 animals. After 20 days of treatment (28 days from first immunization), hearts were harvested for histopathological analysis. By echocardiography, cromolyn sodium prevented LV dilatation and attenuated LV dysfunction, compared with controls. Postmortem analysis of hearts showed that cromolyn sodium reduced myocardial fibrosis, as well as the number and size of cardiac mast cells in the inflamed myocardium, compared with controls.
Conclusions
Our study suggests that mast cell inhibition with cromolyn sodium attenuates adverse LV remodeling and dysfunction in myocarditis. This mechanism-based therapy is clinically relevant and could improve the outcome of patients at risk for inflammatory cardiomyopathy and heart failure.
Keywords: myocarditis, cardiac remodeling, mast cells, fibrosis
Introduction
Myocarditis, a serious and potentially fatal cardiac disease,1,2 is an important and under-diagnosed cause of both acute heart failure and the late development of dilated cardiomyopathy.3,4 Despite clear evidence of immune system involvement in the pathogenesis of myocarditis, 5–7 studies have failed to show that the use of immunosuppressive treatment clearly benefits acute myocarditis patients.8,9 Thus, treatment of myocarditis remains non-specific and supportive, demonstrating the need to develop effective, mechanism-based therapy for fulminant myocarditis and its subsequent complications.
Targeting mast cells could provide a mechanism-based therapy to attenuate inflammation and cardiac remodeling in myocarditis. Mast cells are granulocytes that develop in bone marrow and migrate with the blood stream to different tissues where they differentiate and mature. Although found mainly in the skin, gastrointestinal tract and airways, they are normally known to reside in cardiac tissue.10 Cardiac mast cells degranulate in response to infectious and inflammatory stimuli, producing and releasing many mediators such as histamine, leukotrienes, growth factors, proteases and several cytokines, including IL-1 and TNF-α which are main participants in the pathogenesis of myocarditis and dilated cardiomyopathy.11
During the last decade, increased evidence has suggested that mast cells play an important role in the pathological processes which are part of a variety of cardiac diseases and which lead to the development of dilated cardiomyopathy and heart failure.12 Mast cells have been recognized as a potential target for the development of cardioprotective agents in ischemia-reperfusion injury,13 and have been identified as key-factors in the process of myocardial collagen degradation and fibrosis in the stressed, injured or diseased heart.14–16
Mast cell stabilization compounds have shown promising results in the treatment of various cardiac diseases in rats. For instance, nedocromil sodium effectively prevented left ventricular (LV) remodeling, as measured by Millar conductance catheter in hypertensive rats,17 and reduced morbidity and mortality from heart failure in volume overload rats.18 Cromolyn sodium improved cardiac contractility following hemorrhagic shock and resuscitation of rats,19 significantly attenuated pathological cardiac hypertrophy,20 and reduced myocardial fibrosis, as measured by histopathology, in rats with post-myocarditis dilated cardiomyopathy.21
However, previous studies on mast cell inhibition in myocarditis did not use up-to-date imaging modalities, such as echocardiography or cardiac magnetic resonance (CMR) and it is unclear whether mast cell inhibition can improve cardiac remodeling and function. Therefore, we aimed to determine whether mast cell stabilization, using cromolyn sodium, would not only attenuate the inflammatory reaction and subsequent myocardial fibrosis, but also adverse remodeling and dysfunction, during autoimmune myocarditis in rat. The findings of the present study could be relevant to the treatment of patients with autoimmune fulminant myocarditis.
Materials and Methods
The study was performed in accordance with the guidelines of The Animal Care and Use Committee of Sheba Medical Center, Tel-Aviv University, which conforms to the policies of the American Heart Association and the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, NIH Publication no.85-23).
Experimental autoimmune myocarditis (EAM) Model
To induce EAM we anesthetized rats with a combination of Ketamine-Xylazine (40mg/10mg/kg), and subjected them to porcine cardiac myosin (PCM) immunization (PCM; M0531, Sigma-Aldrich, Israel). In brief, we emulsified PCM with an equal volume of complete Freund’s adjuvant (CFA; Difco, Detroit, MI, USA) mixed with desiccated Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI, USA). On days 1 and 8, 0.2ml PCM-CFA emulsion (containing 1mg PCM) was subcutaneously injected into the foot pad of rats.
Treatment
Thirty male Lewis rats were used to determine the effect of mast cell stabilization on histological and functional outcome in myocarditis. After exclusion of animals that died during EAM induction or anesthesia, the final analysis included 24 rats.
Rats were randomized into two groups: first, a treatment group (n=13) to which an intraperitoneal (IP) injection of 25 mg/kg cromolyn sodium (Sigma) was administered starting at day 9 for a period of 20 days. This dose was based on similar studies showing prevention of infiltration and degranulation of mast cells in rats.21 The second group was a control group (n=11) which received an equivalent volume of normal saline IP for the same time period.
Echocardiography to evaluate remodeling and function
Transthoracic echocardiography was performed in all animals before day 1 (baseline echocardiogram) and after 20 days of cromolyn sodium treatment (28 days after 1st PCM injection). Echocardiograms were performed with a commercially available echocardiography system (Sonos 7500, Philips, Andover, MA, USA) equipped with a 12-MHz phased-array transducer. All measurements were averaged for 3 consecutive cardiac cycles and reviewed by a cardiologist and an echocardiography expert blinded to study protocol.22
Cardiac magnetic resonance (CMR)
CMR examinations were performed under isoflurane anesthesia (1.5%, Abbot Laboratories Ltd., UK), at a constant temperature of 37°C using home-built hot water circulation. Serial CMR scans were performed in a subgroup of animals (n=12) as a baseline evaluation before day 1, and after 20 days of treatment (28 days after 1st PCM injection) to evaluate LV function, scar formation and pericardial effusion. A 3T MRI system was used (3T HDxt Ver.15 M4A, GE Medical Systems) using a custom-built quadrature cylindrical radiofrequency volume coil, I.D. 77mm × 178mm length (Doty Litzcage Coil-Doty Scientific, Inc., Columbia, SC, USA). All scans were performed using electrocardiogram gating. For cine CMR, sequence parameters were as follows: ECG-gated multiphase gradient-echo pulse sequence with TR=11.8ms, TE=4.3ms, flip angle=20°, field of view (FOV) = 8cm, matrix = 384×192 (in plane resolution of 208μm), receiver bandwidth (BW) = 31.25kHz, slice thickness = 1.5–2mm, 2 averages, and 1 view per segment.
We analyzed CMR images using a dedicated post-processing CMR Workstation (Medis Medical Imaging Systems BV, QMass MR 7.4, Leiden, The Netherlands). Cine images acquired in the short-axis plane with multiple slices were used for functional analysis. Epicardial and endocardial LV borders at end diastolic and systolic phases were manually contoured. End diastolic and systolic volumes, ejection fraction, LV wall thickness, wall thickening and wall motion were calculated and evaluated accordingly.
Postmortem morphometric and histological analysis
After treatment and functional evaluation, animals were sacrificed with an overdose of pentobarbital and hearts were arrested with 15% KCl. Hearts were then harvested and processed for histological examination after perfusion with 4% formaldehyde (15 mmHg) for 20 minutes. Adjacent blocks were embedded in paraffin, sectioned into 5-μm slices and stained with hematoxylin-eosin and Masson-Trichrome (Sigma). The slides stained with Masson-Trichrome were used to evaluate LV remodeling by morphometric analysis including parameters like LV area, average wall thickness, fibrotic tissue area, and percentage of fibrosis out of the surface LV area.
To identify mast cells in the heart tissue, serial sections were immuno-labeled with a monoclonal antibody against mast-cell tryptase (Imgenex, San Diego, CA, USA). Mast cell density was quantified by counting the number of tryptase-positive cells per field (X200) in at least 11 fields from each slide. Average mast cell size was calculated by measuring the area of at least 12 cells per slide in different fields (X400).
To assess vessel density, serial sections were immuno-labeled with antibodies against α-smooth muscle actin isoform (Sigma-Aldrich, St. Louis, MO, USA). Angiogenesis was quantified by counting the number of small-medium sized blood vessels per field (X100) in at least 5 fields from each slide.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.00. All values were expressed as mean ±SEM. Differences between baseline and 28 days were assessed with 2-tailed paired t tests. The difference between means of groups was compared by a 2-tailed unpaired t test. In addition, a general linear model, two-way repeated-measures ANOVA was used in order to assess the variation over time of functional measures among the experimental groups. The model included the effects of treatment, time, and treatment-by-time interaction. Bonferroni correction was used to assess the significance of predefined comparisons at different time points.
Results
Cromolyn sodium attenuates LV remodeling and dysfunction
Cromolyn sodium attenuated significant adverse LV dilatation and dysfunction 28 days after immunization (Table 1). While control rats developed a marked increase from baseline in LV diastolic and systolic dimensions and marked LV dysfunction (p<0.001, Table 1), cromolyn-treated rats experienced a non-significant increase in LV dimensions and a modest deterioration in LV function (Table 1). For instance, LV end diastolic area and LV end systolic area increased from baseline by 61±14 and 154±44% in controls, and only by 8±8 and 63±29% in treated animals. Fractional shortening and fractional area change deteriorated by 27±6 and 26±6% in controls, and by nearly half (16±5 and 13±6%) in treated animals. Thus, cromolyn sodium reduced the adverse changes of LV remodeling and dysfunction in myocarditis.
Table 1.
Comparison of LV remodeling and function by 2-dimensional echocardiography, before (Baseline) and 28 days after induction of experimental autoimmune myocarditis, following 20 days of daily IP injection of cromolyn sodium or saline
| Cromolyn sodium (n=13) | Control (n=11) | P for treatment effect | |
|---|---|---|---|
| LVDD, mm | |||
| Baseline | 7.1±0.2 | 6.7±0.2 | |
| 28 days | 7.1±0.3 | 7.7±0.2 | 0.51 |
| P | 0.91 | 0.005 | |
| LVSD, mm | |||
| Baseline | 3.4±0.1 | 3.4±0.2 | |
| 28 days | 4.1±0.3 | 4.9±0.3 | 0.15 |
| P | 0.15 | 0.001 | |
| LVDA, mm2 | |||
| Baseline | 36±1 | 31±3 | |
| 28 days | 38±2 | 46±4 | 0.067 |
| P | 0.46 | <0.001 | |
| LVSA, mm2 | |||
| Baseline | 11±1 | 11±1 | |
| 28 days | 16±2 | 24±3 | |
| P | 0.077 | 0.004 | |
| FS, % | |||
| Baseline | 51±1 | 50±2 | |
| 28 days | 42±2 | 35±2 | 0.087 |
| P | 0.01 | 0.001 | |
| FAC, % | |||
| Baseline | 68±1 | 68±2 | |
| 28 days | 58±3 | 49±3 | 0.046 |
| P | 0.03 | 0.001 | |
Values are means ± SE.
P values in the left columns are for the differences between baseline and 28-days measurements. P values in the right column reflect comparison of the differences between treatment and control groups over time (see Methods).
LVDD =LV end diastolic dimension; LVSD = LV end systolic dimension; LVDA = LV end diastolic area; LVSA = LV end systolic area; FS = fractional shortening = [(LVDD (−) LVSD)/LVDD]×100; FAC = Fractional area change = [(LVDA (−) LVSA)/LVDA]×100.
CMR was performed in a subgroup of animals (n=12) (Table 2). Although both groups experienced a significant increase in LV end diastolic volume and systolic volume, the cromolyn-treated group experienced a smaller increase of these variables (Fig. 1). Cromolyn-treated rats presented a smaller decline in ejection fraction and maintained a better stroke volume compared with controls.
Table 2.
Comparison of LV remodeling and function by CMR, before (Baseline) and 28 days after induction of experimental autoimmune myocarditis, following 20 days of daily IP injection of cromolyn sodium or saline
| Cromolyn (n=7) | Control (n=5) | P for treatment effect | |
|---|---|---|---|
| LV wall thickness, mm | |||
| Baseline | 2.5±0.08 | 2.5±0.08 | 0.63 |
| 28 days | 2.7±0.16 | 2.8±0.22 | |
| P | 0.32 | 0.46 | |
| LVDA, mm2 | |||
| Baseline | 36±2 | 34±3 | |
| 28 days | 43±3 | 45±3 | 0.97 |
| P | 0.02 | 0.01 | |
| LVSA, mm2 | |||
| Baseline | 9±1 | 9±0.9 | |
| 28 days | 13±2 | 18±2 | 0.12 |
| P | 0.03 | 0.005 | |
| LV mass, mg | |||
| Baseline | 437±24 | 453±38 | |
| 28 days | 501±34 | 480±43 | 0.95 |
| P | 0.19 | 0.65 | |
| EDV, μL | |||
| Baseline | 306±22 | 301±34 | |
| 28 days | 417±29 | 451±32 | 0.68 |
| P | <0.001 | 0.04 | |
| ESV, μL | |||
| Baseline | 116±9 | 114±10 | |
| 28 days | 226±20 | 277±29 | |
| P | 0.001 | 0.007 | |
| SV, μL | |||
| Baseline | 190±17 | 187±25 | |
| 28 days | 191±27 | 174±6 | 0.67 |
| P | 0.96 | 0.61 | |
| EF, % | |||
| Baseline | 62±2 | 62±2 | |
| 28 days | 45±5 | 39±3 | 0.35 |
| P | 0.03 | 0.001 | |
Values are means ± SE.
P values in the left columns are for the differences between baseline and 28-days measurements. P values in the right column reflect comparison of the differences between treatment and control groups over time (see Methods).
LV = left ventricular; LVDA = LV end diastolic area; LVSA = LV end systolic area; EDV = end diastolic volume; ESV= end systolic volume; SV= stroke volume = EDV – ESV; EF = ejection fraction =[(EDV (−) ESV)/EDV]×100.
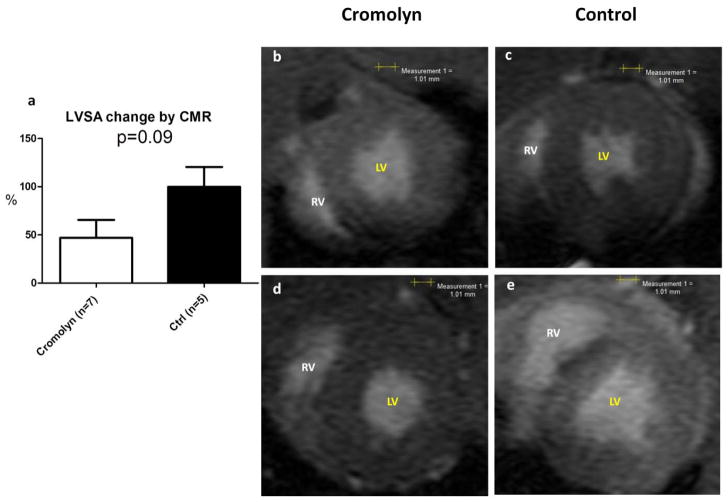
Figure 1. Cromolyn sodium attenuates LV dilatation compared to controls, 28 days after induction of EAM by CMR analysis.
(a) LV dilatation attenuated in rats treated with cromolyn sodium compared with control group - lower % of change in LVSA (LV end systolic area). (b–e) Representative end-systolic short-axis images from CMR function movies at baseline (b–c) and after 28 days (d–e). Notice the extensive dilatation of both LV and RV (right ventricle) in the control rat (e), which is attenuated in the cromolyn-treated rat (d).
Cromolyn sodium preserves LV wall thickness and mass, and reduces fibrosis
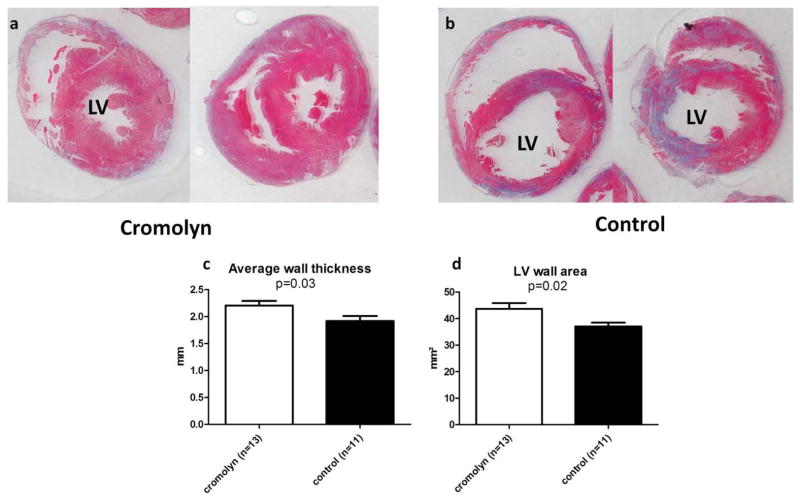
Postmortem morphometric analysis of the heart sections was performed 28 days after induction of EAM (Table 3). LV wall thickness and mass were relatively preserved in cromolyn-treated hearts compared with controls (Fig. 2). However, LV cavity area was 37% larger in the controls than in the treatment group (22.2±3.4 vs.16.3±2.6 mm2, p=0.18). Furthermore, the proportion of myocardial fibrosis was 39% smaller in the cromolyn sodium-treated hearts (p=0.065, Fig. 3).
Table 3.
Results of postmortem morphometric analysis.
| Cromolyn (n=13) | Control (n=11) | P | |
|---|---|---|---|
| LV Average wall thickness (mm) | 2.2±0.1 | 1.9±0.1 | 0.03 |
| Septal wall thickness (mm) | 1.9±0.1 | 1.6±0.1 | 0.11 |
| Whole LV area (mm2) | 60±0.5 | 59.3±3.9 | 0.89 |
| LV Wall area (mm2) | 43.7±2.2 | 37.1±1.4 | 0.02 |
| LV cavity (mm2) | 16.3±2.6 | 22.2±3.4 | 0.18 |
| Fibrotic area (mm2) | 3.9±0.5 | 4.6±0.6 | 0.38 |
| Percentage of fibrosis out of LV wall area (%) | 8.9±1 | 12.4±1.5 | 0.065 |
Values are means ± SE.
LV = left ventricular
Figure 2. Cromolyn sodium preserves LV wall thickness and mass by postmortem morphometry, 28 days after EAM induction.
Representative sections of heart treated with cromolyn sodium (a) or saline (b) in Masson Trichrome staining. Morphometry shows that cromolyn treatment preserves LV wall thickness (c) and area (d) compared to control.
Figure 3. Cromolyn sodium reduces fibrosis by postmortem morphometry, 28 days after EAM induction.
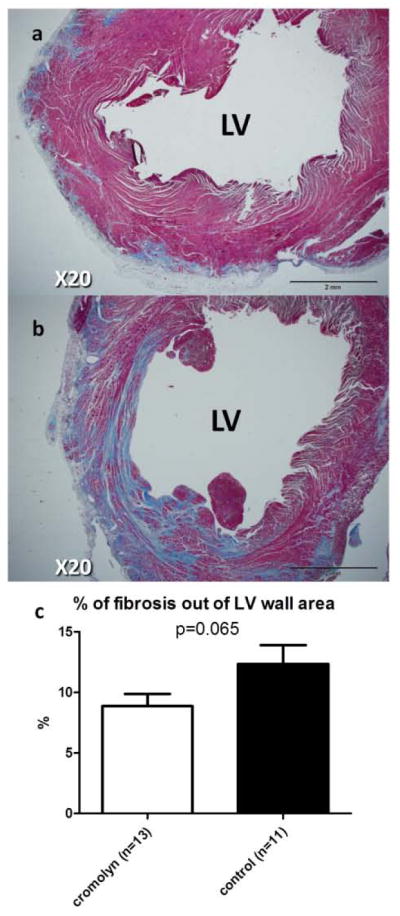
Representative sections of the heart treated with cromolyn sodium (a) or saline (b) in Masson Trichrome staining. Morphometry shows that cromolyn treatment reduces proportion of fibrosis (c).
Cromolyn sodium reduces cardiac mast cell density and size, and improves angiogenesis
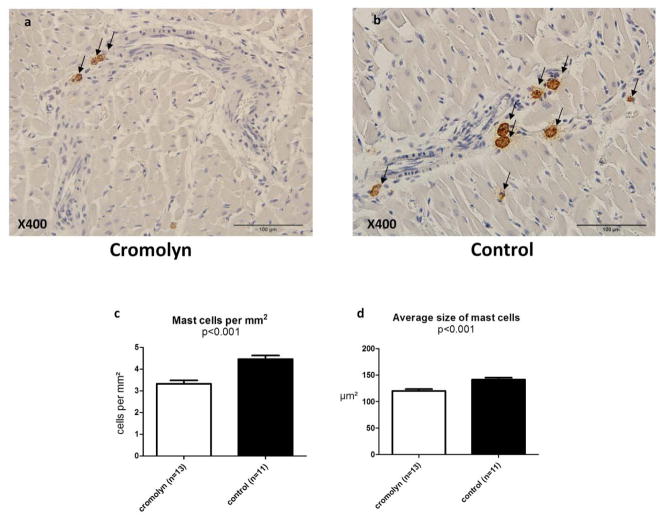
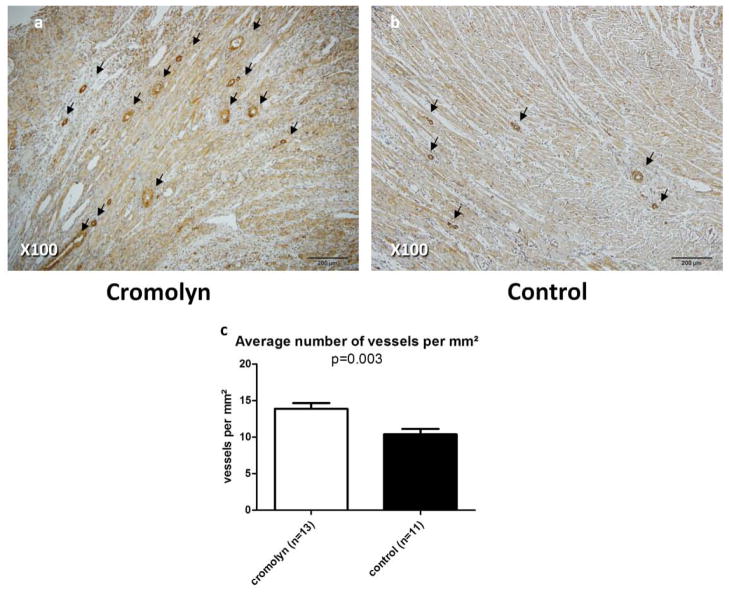
Administration of cromolyn sodium significantly reduced mast cell density in the myocardium 28 days after induction of EAM, compared with control rats (Fig. 4). Moreover, average mast cell size was smaller in the myocardium of cromolyn-treated rats compared with controls (Fig. 4). Angiogenesis was improved in cromolyn-treated hearts compared with controls (Fig. 5).
Figure 4. Cromolyn sodium reduces cardiac mast cell density and size, 28 days after EAM induction.
Representative sections of the heart treated with cromolyn sodium (a) or saline (b) using a monoclonal antibody to mast-cell tryptase (brown). Arrows indicate mast cells containing tryptase. Analysis based on average density and size shows that cromolyn sodium treatment significantly reduced cardiac mast cell density (c) and size (d).
Figure 5. Cromolyn sodium improves angiogenesis 28 days after EAM induction.
Representative sections of the heart treated with cromolyn sodium (a) or saline (b) using antibodies to α-smooth muscle actin. Arrows indicate positively stained small-medium sized blood vessels. Analysis based on average density of blood vessels (c) shows that cromolyn treatment significantly improved angiogenesis.
Discussion
The major new findings of the present study suggest a protective effect of mast cell inhibition in the acute phase of fulminant myocarditis. While this effect was evident from the reduction of fibrosis and the preservation of LV wall mass, it was also supported, for the first time, by functional evaluation using serial echocardiography and CMR studies. These non-invasive modalities allowed us to perform a more comprehensive investigation of the effect of mast cell inhibition, and to create a more solid basis for further clinical research regarding the use of cromolyn sodium in myocarditis.
Mast cell and myocarditis
Mast cell enzymes have been shown to mediate the extracellular matrix remodeling in the heart through activation of matrix metalloproteinases.23 Degranulating cardiac mast cells are observed as early as 6 hours following coxsackievirus B3 infection,24 and increased numbers of cardiac mast cells have been reported in human patients with end-stage cardiomyopathy.25 More specifically, the survival of mast-cell deficient mice was significantly higher compared with controls in a model of acute viral myocarditis,26 and the anti-inflammatory effect of eplerenone in viral myocarditis in mice was shown to be related to inhibition of mast cell-derived proteinases, which resulted in improved myocardial remodeling by suppressing fibrosis.27 Moreover, endomyocardial biopsies of patients with acute myocarditis have shown that cardiac mast cells increase significantly in these patients, compared with controls.28
Mast cell inhibition in myocarditis
Although significant advances in understanding the pathophysiological mechanisms of myocarditis have been achieved during the last years, standard treatment strategies remain limited to non-specific heart failure therapy in most cases.9 Immunosuppressive therapy has been evaluated in several clinical trials but has not become a standard for myocarditis therapy.9 Thus, mast cell modulation could be an alternative therapeutic approach. Previous studies have suggested that mast cells play a role in the pathogenesis of myocarditis and dilated cardiomyopathy24,26,29,30 and that their inhibition under these circumstances results in decreased fibrosis and remodeling in rats and mice.21,31,32 However, except for the protective effect of IL-10 gene transfer in EAM, that was related to prevention of mast cell degranulation32, little is known regarding the effect of mast cell inhibition in the early phases of myocarditis, and the functional consequences of this intervention.
We sought to initiate cromolyn sodium treatment before the progression phase of EAM. This phase is known as the autoimmune response phase, between 10 and 21 days post-immunization, in which inflammation is significantly increased in the heart.33 We also chose to evaluate not only histological but also the functional effects of treatment 28 days from immunization, to assess the mast cell stabilization effect in fulminant myocarditis. Cromolyn sodium was administered IP and had a systemic effect as a mast cell stabilizer, preventing degranulation of mast cells by stabilizing the mast cell membrane in all tissues, including resident cardiac mast cells, thereby also reducing cytokine secretion, as shown in previous studies.19,34 Twenty-eight days later, we found that cardiac mast cell density and size were reduced compared with controls, providing possible evidence for the inhibition of cytokine release, which led to attenuated chemotaxis, replication and differentiation of mast cells. The role of mast cells in promoting local tissue damage and extracellular matrix degradation is strongly related to the ability of their products, such as chymase and tryptase, to provide alternative matrix metaloproteinase activation.23,35,36 In addition, among many cytokine-releasing cells, mast cells are the only ones known to store pre-formed TNF-α in their cytoplasmic granules and release it upon activation.37 Thus, stabilization of mast cells prevented cytokine storm and local damage to heart tissue. This environment was less appropriate for the inflammatory processes involved in fibrosis and remodeling of cardiac tissue to occur, and indeed we found that cromolyn sodium reduced the proportion of myocardial fibrosis. This result was expected, since cardiac mast cell density has been demonstrated to strongly correlate with collagen volume fraction21 and to increase coincidently with the pattern of heart fibrosis.29 Mast cells have been linked to the process of angiogenesis and especially tumor angiogenesis,38 and the injection of mast cell granules after myocardial infarction in rats resulted in improved angiogenesis.39 Thus, inhibition of mast cells would have been expected to prevent angiogenesis in the injured cardiac tissue. Surprisingly, however, we found that cromolyn sodium increased angiogenesis compared with controls, and we propose that this result could reflect less fibrosis, thereby facilitating improved tissue healing and angiogenesis.
We were specifically interested in using CMR due to its increasingly more frequent use for the non-invasive assessment of patients with suspected myocarditis,40,41 and its effectiveness in assessing LV dimensions and global LV function in pre-clinical and clinical research. In our study, we used a clinical 3T CMR scanner with a specially designed rat coil. We believe that the small number of animals in the subgroup undergoing CMR prevented the results from being as significant as the results of the echocardiography analysis. Nonetheless, we conclude that LV dilatation and increased end-systolic and end-diastolic volumes, hallmarks of the late phase of myocarditis,42 were inhibited in cromolyn-treated rats, while LV function was relatively preserved. Based on the functional results by CMR and echocardiography, together with the prevention of mast cell degranulation and the reduction in mast cell density, it is reasonable to assume that inhibition of mast cells has resulted in improved cardiac function in rats with fulminant myocarditis.
Limitations
We chose to stop treatment after 20 days and to assess treatment effects 28 days from the first immunization. Prolonged treatment or follow-up may be needed for further analysis and assessment of treatment effect through a longer period of chronic myocarditis and dilated cardiomyopathy. In addition, we did not provide mechanistic data to demonstrate the effect of mast cell inhibition at the molecular level, but rather relied on previous studies which showed these effects.
Some differences between groups were not statistically significant (p<0.05). The lack or borderline statistical significance might be related to small animal numbers, particularly in the CMR tested animals. The high sensitivity of rats with myocarditis to prolonged anesthesia during CMR study restricted the use of CMR in the sick animals. However, the extent of many changes between the groups was robust, and p values of 0.067, 0.087, and even 0.15 do not exclude the possibility of significant differences as strongly as a p value of 0.51. Nevertheless, the treatment effect on variables that have clinical prognostic significance, such as LV systolic area and fractional area change, was statistically significant. Notably, with CMR there was no change in LV wall thickness, whereas postmortem analysis did show a difference. These inconsistencies might be related to technical and location differences between the methods: the morphometric and CMR analyses did not necessarily measure exactly the same heart slice. Finally, CMR analysis did not include contrast-enhancement imaging and analysis used to evaluate fibrosis.
Conclusion
Our study supports the possible use of cromolyn sodium or other mast cell stabilizers in preventing cardiac remodeling and LV dysfunction in experimental myocarditis. Future research should concentrate on evaluating the optimal time for therapy initiation and the required duration of treatment. Moreover, it may lead to further investigations into the efficacy of this method in humans, in an effort to reduce complications and mortality resulting from myocarditis.
Acknowledgments
Funding Sources:
This work was supported by the US-Israel Binational Science Foundation (FHE, JL), and the Schlezak Foundation (JL), Tel Aviv University, Israel.
The authors thank Yair Rubinstein (CMR), Patricia Benjamin (echocardiography) and Lena Shuval (echocardiography) for their excellent technical assistance and Mrs. Vivienne York for the skillful English language editing of the paper. This work was performed in partial fulfillment of the MD thesis requirements of the Sackler Faculty of Medicine, Tel Aviv University.
Footnotes
Conflict of Interest Statement
- Yair Mina - None
- Shunit Rinkevich-Shop – None
- Eli Konen - None
- Orly Goitein – None
- Tammar Kushnir – None
- Frederick H. Epstein – None
- Micha S. Feinberg – None
- Jonathan Leor- None
- Natalie Landa-Rouben - None
Declaration of Conflicting interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119(8):1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 2.Grun S, Schumm J, Greulich S, et al. Long-Term Follow-Up of Biopsy-Proven Viral Myocarditis: Predictors of Mortality and Incomplete Recovery. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 19.1091–1100(8):99. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- 4.Sagar S, Liu PP, Cooper LT., Jr Myocarditis. Lancet. 2011 doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4(4):411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 6.Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev Med Virol. 2005;15(1):17–27. doi: 10.1002/rmv.445. [DOI] [PubMed] [Google Scholar]
- 7.Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68(8):5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333(5):269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 9.Kindermann I, Barth C, Mahfoud F, et al. Update on Myocarditis. Journal of the American College of Cardiology. 2012;59(9):779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 10.Rakusan K, Sarkar K, Turek Z, Wicker P. Mast cells in the rat heart during normal growth and in cardiac hypertrophy. Circ Res. 1990;66(2):511–516. doi: 10.1161/01.res.66.2.511. [DOI] [PubMed] [Google Scholar]
- 11.Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. 1994;72(6):561–566. doi: 10.1136/hrt.72.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011;89(1):12–19. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh M, Saini HK. Resident cardiac mast cells and ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2003;8(2):135–148. doi: 10.1177/107424840300800207. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Chancey AL, Tzeng HP, et al. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation. 2011;124(19):2106–2116. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palaniyandi SS, Inagaki K, Mochly-Rosen D. Mast cells and epsilonPKC: a role in cardiac remodeling in hypertension-induced heart failure. J Mol Cell Cardiol. 2008;45(6):779–786. doi: 10.1016/j.yjmcc.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol. 2008;45(1):56–61. doi: 10.1016/j.yjmcc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Card Fail. 2005;11(7):548–556. doi: 10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53(6):1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 19.Santone DJ, Shahani R, Rubin BB, Romaschin AD, Lindsay TF. Mast cell stabilization improves cardiac contractile function following hemorrhagic shock and resuscitation. Am J Physiol Heart Circ Physiol. 2008;294(6):H2456–2464. doi: 10.1152/ajpheart.00925.2007. [DOI] [PubMed] [Google Scholar]
- 20.Singh AP, Singh M, Balakumar P. Effect of mast cell stabilizers in hyperhomocysteinemia-induced cardiac hypertrophy in rats. J Cardiovasc Pharmacol. 2008;51(6):596–604. doi: 10.1097/FJC.0b013e31817ae60f. [DOI] [PubMed] [Google Scholar]
- 21.Palaniyandi Selvaraj S, Watanabe K, Ma M, Tachikawa H, Kodama M, Aizawa Y. Involvement of mast cells in the development of fibrosis in rats with postmyocarditis dilated cardiomyopathy. Biol Pharm Bull. 2005;28(11):2128–2132. doi: 10.1248/bpb.28.2128. [DOI] [PubMed] [Google Scholar]
- 22.Landa N, Miller L, Feinberg MS, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117(11):1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 23.Janicki JS, Brower GL, Gardner JD, et al. Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovasc Res. 2006;69(3):657–665. doi: 10.1016/j.cardiores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Fairweather D, Frisancho-Kiss S, Gatewood S, et al. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37(2):131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 25.Patella V, de Crescenzo G, Lamparter-Schummert B, De Rosa G, Adt M, Marone G. Increased cardiac mast cell density and mediator release in patients with dilated cardiomyopathy. Inflamm Res. 1997;46(Suppl 1):S31–3.2. [PubMed] [Google Scholar]
- 26.Higuchi H, Hara M, Yamamoto K, et al. Mast cells play a critical role in the pathogenesis of viral myocarditis. Circulation. 2008;118(4):363–372. doi: 10.1161/CIRCULATIONAHA.107.741595. [DOI] [PubMed] [Google Scholar]
- 27.Xiao J, Shimada M, Liu W, Hu D, Matsumori A. Anti-inflammatory effects of eplerenone on viral myocarditis. Eur J Heart Fail. 2009;11(4):349–353. doi: 10.1093/eurjhf/hfp023. [DOI] [PubMed] [Google Scholar]
- 28.Hiruta Y, Adachi K, Okamoto T, Fujiura Y, Toshima H. Cardiac mast cells in myocardial diseases. Kokyu To Junkan. 1991;39(11):1133–1138. [PubMed] [Google Scholar]
- 29.Kitaura-Inenaga K, Hara M, Higuchi K, et al. Gene expression of cardiac mast cell chymase and tryptase in a murine model of heart failure caused by viral myocarditis. Circ J. 2003;67(10):881–884. doi: 10.1253/circj.67.881. [DOI] [PubMed] [Google Scholar]
- 30.Postan M, Correa R, Ferrans VJ, Tarleton RL. In vitro culture of cardiac mast cells from mice experimentally infected with Trypanosoma cruzi. Int Arch Allergy Immunol. 1994;105(3):251–257. doi: 10.1159/000236765. [DOI] [PubMed] [Google Scholar]
- 31.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165(6):1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palaniyandi SS, Watanabe K, Ma M, Tachikawa H, Kodama M, Aizawa Y. Inhibition of mast cells by interleukin-10 gene transfer contributes to protection against acute myocarditis in rats. Eur J Immunol. 2004;34(12):3508–3515. doi: 10.1002/eji.200425147. [DOI] [PubMed] [Google Scholar]
- 33.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 34.Shiota N, Rysa J, Kovanen PT, Ruskoaho H, Kokkonen JO, Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21(10):1935–1944. doi: 10.1097/00004872-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97(17):1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 36.Fang KC, Raymond WW, Lazarus SC, Caughey GH. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J Clin Invest. 1996;97(7):1589–1596. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beil WJ, Login GR, Aoki M, et al. Tumor necrosis factor alpha immunoreactivity of rat peritoneal mast cell granules decreases during early secretion induced by compound 48/80: an ultrastructural immunogold morphometric analysis. Int Arch Allergy Immunol. 1996;109(4):383–389. doi: 10.1159/000237267. [DOI] [PubMed] [Google Scholar]
- 38.Crivellato E, Nico B, Ribatti D. Mast cell contribution to tumor angiogenesis: a clinical approach. Eur Cytokine Netw. 2009;20(4):197–206. doi: 10.1684/ecn.2009.0167. [DOI] [PubMed] [Google Scholar]
- 39.Kwon JS, Kim YS, Cho AS, et al. The novel role of mast cells in the microenvironment of acute myocardial infarction. J Mol Cell Cardiol. 2011;50(5):814–825. doi: 10.1016/j.yjmcc.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Zagrosek A, Abdel-Aty H, Boye P, et al. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging. 2009;2(2):131–138. doi: 10.1016/j.jcmg.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. Journal of the American College of Cardiology. 2009;1475–1487(17):53. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutschow S, Leschka S, Westermann D, et al. Left ventricular enlargement in coxsackievirus-B3 induced chronic myocarditis--ongoing inflammation and an imbalance of the matrix degrading system. Eur J Pharmacol. 2010;630(1–3):145–151. doi: 10.1016/j.ejphar.2009.12.019. [DOI] [PubMed] [Google Scholar]