Abstract
The elucidation of driver mutations involved in the molecular pathogenesis of cancer has led to a surge in the application of novel targeted therapeutics in lung cancer. Novel oncologic research continues to lead investigators towards targeting personalized tumor characteristics rather than applying targeted therapy to broad patient populations. Several driver genes, in particular epidermal growth factor receptor (EGFR) and ALK fusions, are the earliest to have made their way into clinical trials. The avant-garde role of genomic profiling has led to important clinical challenges when adapting current standard treatments to personalized oncologic care. This new frontier of medicine requires newer biomarkers for toxicity that will identify patients at risk, as well as, new molecular markers to predict and assess clinical outcomes. Thus far, several signature genes have been developed to predict outcome as well as genetic factors related to inflammation to predict toxicity.
KEYWORDS : Lung cancer, biomarkers, toxicity, novel therapies
Introduction
In 2013, an estimated 228,000 new cases of lung cancer will be diagnosed in the United States and more than 70,000 will die from the disease. The risk of developing lung cancer for all American men and woman during their lifetimes is between 6-7%. This risk increases with age, genetic susceptibility and toxic exposures (e.g., smoking) (1). Lung cancer is a heterogeneous group of carcinomas comprised of several histologic subtypes: adenocarcinoma, squamous cell carcinoma, and large cell and small cell neuroendocrine tumors. The vast majority of molecular research focuses on the most prevalent histologic subtypes: adenocarcinoma and squamous cell carcinomas.
Since the initial heralding in the last decade of “the six hallmarks of cancer”, advances in the study of molecular pathways, identification of biomarkers and novel targeted therapies have made their way to clinical applications and widened the scope of our understanding of the molecular pathogenesis of lung cancer (2,3). The appropriate introduction of targeted therapies into current standards of care remains an open area of clinical investigation.
The current understanding of the mechanisms of transformation from normal physiologic epithelial cells to malignant lung cancer has evolved alongside our increasing knowledge of many other cancer types and falls into a multi-step paradigm (4,5). A series of either chromosomal or nucleotide aberrations and epigenetic events in driver genes lead to immortality and the malignant phenotype of lung cancer (6). It is theorized that during this multi-step transformation, certain driver genes cause “addiction” and are required for tumor maintenance and targeting these biomarkers will lead to the eradication of selective cancer cells.
Various lung cancer biomarkers have been identified, including epidermal growth factor receptor (EGFR) mutations, EML4/ALK fusion genes, p53 mutations, RAS/MAP kinase mutations, Her-2 overexpression and PI3K/mTOR mutations.
A consequence of targeted radiotherapy in lung cancer is damage to the surrounding organs at risk which include the lung and heart. The majority of molecular biomarkers of toxicity in lung cancer focus on lung damage or pneumonitis. Attempts have been made to combine dosimetric parameters in lung radiotherapy with various lung biomarkers to define a group of patients most at risk for severe lung toxicity.
Lung cancer molecular markers
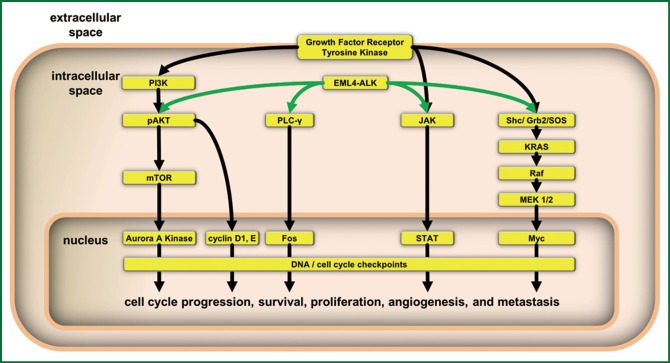
The search for a cancer biomarker or targetable genetic aberration requires years of preclinical studies in vitro and in vivo. Currently there are approximately a dozen biomarkers that have demonstrated clinical benefit and another dozen are currently under investigation (7). Of these, several are considered lung cancer driver genes by the NCI’s lung cancer mutation consortium. These include EGFR, KRAS, HER2, PI3K, BRAF and ALK fusions (4). Of these EGFR, KRAS, HER2 and ALK fusions are predictive of response to targeted therapies (5,8-11). These driver genes play an important role in lung cancer tumorigenesis involving alterations in their proliferative potential, apoptotic signaling, angiogenesis and invasion/extravasation. Clinically relevant pathways are depicted in Figure 1 and include the RAS/MAP kinase, PI3K/AKT/mTOR, JAK/STAT pathways and cell cycle checkpoints. It is known that, in varying degrees, these biomarkers are mutated, amplified or overexpressed in non-small cell lung cancers. Table 1 outlines the relative frequency with which each driver gene occurs in lung cancer (5,8,12,13).
Figure 1.
Summary of intracellular signaling pathways containing the crucial driver genes in lung cancer which promote tumor cell proliferation, survival, angiogenesis and metastatic potential.
Table 1. Lung cancer genetic aberrations and associated targeted therapy.
| Biomarker gene | Aberration | Targeted therapeutic | Frequency of aberration [%] |
|---|---|---|---|
| EGFR | Mutation or amplification | Gefitinib, erlotinib, cetuximab | [10-25] (35% in Asian patients) |
| HER2 (ERBB2) | Mutation or amplification | Trastuzumab | [5-10] |
| BRAF | Mutation | Sorafenib | [2-3] |
| p53 | Mutation or deletion | Advexin a p53 adenoviral vector | [30-50] |
| VEGF | Overexpression | Bevacizumab, afibercept | |
| PI3K | Modified and activated | BEZ235, LY294002 | [1-3] |
| mTOR | Activated | Rapamycin, RAD001, CCL-779 | [70-75] |
| RAS | Mutation leading to activation | Tipifarnib, lonafarnib | [10-15] (20-30% in Adenocarcinoma) |
| MEK | Activated | Trametinib, salumetinib | [1-2] |
| c-KIT | Overexpressed | Imatinib | [1-2] |
| EML/ALK | Fusion | Crizotinib | [5-13] |
EGFR
This family of receptor tyrosine kinases (RTKs) include the EGFR or HER1 and HER2-4 (14). They are a group of RTKs with approximately 75% homology that once bound to an extracellular ligand form homo- and heterodimers which leads to their intracellular signaling (5). The vast majority of mutations in this family occurs within the tyrosine kinase domain and correlate with drug sensitivity (15). Therapeutic targets for this family are summarized in Table 1 and include small molecule inhibitors, gefitinib and erlotinib, and monoclonal antibodies, cetuximab and trastuzumab. Interestingly mutations in EGFR seem to occur more frequently in never-smokers, people of Asian descent, and women with adenocarcinomas (5,15). These groups also seem to be more sensitive to molecular inhibition. Several studies have found both EGFR amplifications and most mutations correlate with improve clinical outcomes (8). There are, however, mutations that predict a negative response to EGFR inhibition which include the T790M mutation, a concomitant KRAS mutation or MET amplification. More recent studies suggest a D761Y mutation in exon 19 and insertion within exon 20 leads to further resistance to targeted therapy (16). HER2 mutations occur much less frequently although mutations seem to correlate with those in EGFR mutated patients. Targeting Her2-4, however, has not led to improved outcomes in unselected patients and large groups of patients harboring these mutations have not been identified (8,9,17,18).
RAS/RAF/MAP kinase pathway
In lung cancer, nearly all clinically relevant mutations in the RAS family occur in KRAS. Once mutated RAS is activated and may lead to cellular transformation and sustained proliferation making this family an ideal candidate for targeting. Several drugs, among them tipifarnib and lonafarnib, are known as farnesyl transferase inhibitors and have been developed to target RAS modification. In order to perform intracellular cell signaling (8), RAS requires modification with a farnesyl group. This allows proper attachment to the cell membrane. Without proper modification and cell membrane localization, RAS becomes ineffective.
BRAF is a part of a family of serine/threonine kinases downstream of RAS. BRAF is mutated in lung cancer but this occurs much less frequently than with melanoma (Table 1). Because the mutations in BRAF differ substantially between lung and melanoma, the translational use of vemurafenib for treatment of lung cancer is unlikely. However, the use of oral RAF kinase inhibitors like sorafenib is being studied. Sorafenib is unique in that it is an inhibitor of the RAF/MAP kinase pathway and has activity on multiple tyrosine kinases (VEGF and PDGF) allowing for multiple pathways involved in lung tumorigenesis to be targeted (8,11,19).
Once activated BRAF signals MEK1/2 which goes on to activate the MAP kinase pathway through ERK1/2. These downstream effectors are known to be constitutively activated in human lung cancer cell lines. Oral inhibitors such as Cl-1040 and PD03244901 have been developed and studies are actively being pursued (8,20).
ALK translocations (ALK/EML4 and ROS1)
The echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase fusion gene (EML4/ALK) is the most common form of translocation. The fusion protein results in a constitutively active tyrosine kinase (21). This fusion product is more common in the young, low volume or never-smokers with adenocarcinoma histology with signet ring features. ALK rearrangements are clinically detected with fluorescence in situ hybridization. A dual ALK translocation inhibitor called crizotinib is available to suppress the effects. Both preclinical and clinical testing has demonstrated radiosensitivity and remarkable response rates of EML/ALK positive tumors to therapy with crizotinib (9,22). Several second site mutations L1152R, L1196M and C1156Y have been and confer resistance to crizotinib treatment. ROS1 rearrangements have also been identified recently to remain sensitive to crizotinib (8).
P53
The p53 protein is a transcription factor that is modified in various cellular stress situations. It functions to initiate apoptosis or to arrest the cell cycle. P53 is well known, as it is the most frequently mutated gene in human cancers (4). The majority of mutations in p53 are inactivating mutations, or deletions, although some missense mutations result in a gain-of-function phenotype that portends a poor prognosis in lung cancer (8). Classically, cigarette smoking is linked to transversion mutations in lung cancer. Clinical applications to subvert p53 have been made by using adenoviral gene replacement vectors to re-introduce wildtype p53 (4,8,21). This is based on the preclinical work demonstrating that tumors that harbor a mutant p53 undergo apoptosis if wildtype p53 is re-expressed within the cell. Early phase clinical trials have determined this vector to be safe and effective in lung cancer and continued studies are planned (23).
The PI3K/mTOR pathway
Phosphatidylinositide-3 kinase (PI3K) encoded from the oncogene PIK3CA belongs to a family of lipid kinases leading to mammalian target of rapamycin (mTOR) activation that is estimated to be activated in nearly 75% of lung cancers (8). PI3K leads to inhibition of apoptosis and a regulation of growth. PIK3CA is mutated in lung cancer (Table 1), leading to high levels of kinase activity and downstream signaling. When combined with radiotherapy, PI3K inhibitors such as LY294002 and wortmannin reduce downstream effects which stall the growth potential and cell killing of human cell lines. These drugs are, however, rather toxic as they are nonspecific and inhibit a broad range of this family of kinases. Most recently, pharmaceutical companies are attempting to isolate isoform specific inhibitors of PI3K for a variety of cancers, IC486068 and IC87114 (8,18,21).
mTOR is a serine/threonine protein kinase. This kinase is the main downstream effector of the pathway that leads to regulation of cell growth. Two complexes, mTORC1 and mTORC2, form a catalytic subunit allowing for both cellular activity and possible therapeutic targeting. Several available therapeutic drugs are available, including Sirolimus and derivatives such as CCI-779, RAD001 and AP23576. Both have shown activity in lung cancer and are under further current clinical study (8,21,22).
JAK/STAT
The Janus kinase (JAK) and Signal Transducer and Activator of Transcription (STAT) pathway has been implicated in preclinical study to increase cell proliferation and inhibit apoptosis through downstream effects like BCL, Cyclin and MYC in lung cancer. JAK localizes toward and is activated by ligand bound receptor tyrosine kinases leading to phosphorylated sites recognized by the SH2 domain of various STATs. They become phosphorylated by JAKs and form homo- and heterodimers which localize to the cell nucleus and regulate gene transcription. Interestingly, several STATs may be phosphorylated directly by EGFR and other kinases. Most notably, STAT3 has been linked to lung cancer oncogenesis within cell lines that carry a mutated EGFR. In fact, in EGFR mutants, STAT3 activation is necessary for cell growth and survival. Downstream of STAT3 is an inhibitor of apoptosis named survivin which functions to increase cell proliferation through the cell cycle and inhibition of apoptosis through caspases. This pathway of signaling is an attractive therapeutic target and preclinical work using TG101209 has demonstrated induced radiosensitivity, likely through inhibition of STAT3 (8,21,22).
TGF-B and angiogenesis
Transforming growth factor beta (TGF-β) is a cytokine that regulates multiple cellular processes, including cell survival, growth and immunomodulation. TGF-β activates downstream effectors in the SMAD family. TGF-β plays a dual role in lung cancer. During early tumorigenesis, TGF-β induces apoptosis and is responsible for growth inhibition. And, as we will see later, it also plays a role in inflammation. However, in late stage lung cancers, TGF-β induces angiogenesis (3,8,22).
Vascular density and angiogenesis correlate with advanced stage lung cancers and poor survival. A critical mediator in angiogenesis is the VEGF family. VEGF receptor inhibitors include the monoclonal antibody bevacizumab and the fusion protein aflibercept which bind circulating VEGF amongst others currently under investigation. Assessing response after treatment with bevacizumab has become a challenge. Pooling available anti-VEGF trials has allowed assessment of possible biomarkers to measure outcome. In fact, recent data suggests biomarkers such as circulating short VEGF-A, as well as modified expression of receptors neuropilin-1 and VEGF receptor 1, are potential candidates to predict outcome (8,24). A prospective biomarker study named MERiDiAN will stratify patients based on their short VEGF isoform and plans to address this issue.
Biomarkers of radioresistance
The development of radiation resistance relies on innate tumor characteristics. Classically, the most important features in the response of tumors and normal tissues to fractionated radiotherapy are referred to as the “4 Rs”: repair of DNA damage, redistribution of cells within the cell cycle, accelerated repopulation and reoxygenation of hypoxic tumor cells (25). During the accelerated repopulation phase, tumor cells begin to repair their damage and proliferate at a markedly faster rate. During this phase, several cellular mechanisms take place that lead to resistance to radiotherapy: cellular senescence, DNA repair and cell cycle checkpoints regulation. Unfortunately the pathways and mechanisms of resistance are complex, and to date, are poorly elucidated. However, several investigators have shed light on genes likely related to both innate and acquired radioresistance. Innate radioresistance refers to genes present prior to exposure to ionizing therapeutic radiation and the acquired genes are those whose expression is changed after exposure to ionizing radiation. Using various methods of gene expression profiling a series of pathways involved in hypoxia, DNA repair and apoptosis have been studied in human lung cancer cell lines. Eighteen key genes linked to radioresistance were identified but of these genes only three have been validated to date. The three validated genes were MDM2, Livin α and TP54I3 (18,26).
MDM2 involved in innate radiation resistance encodes a protein called E3 ubiquitin-protein ligase which is an important negative regulator of p53 both through ubiquitinylation leading to degradation and inhibition of transcriptional activation (27). It has been demonstrated that up-regulation of MDM2 expression leads to radioresistance and targeted down regulation with siRNA leads to a reversion back to radiosensitivity. The remaining two validated genes are associated with acquired radioresistance where Livin-α is up-regulated and TP53I3 is down-regulated. Livin is a novel inhibitor of apoptosis (IAP) which is normally not expressed at high levels. In 2011, it was found that levels of expression are highly up-regulated after exposure to radiation leading to acquired resistance, especially in isoform α. The tumor protein p53 inducible protein 3 (TP53I3) gene is nearly turned off subsequent to fractionated radiotherapy leading to a depression of p53 cell death signaling (18).
Other potential mechanisms of resistance to radiation include mutations in EGFR and RAS. Preclinical studies have shown low levels of apoptosis in human cell lines with KRAS mutations in codon 12 (12V). It is theorized that this low level of apoptosis is mediated through modification of ERK. This may explain the resistance to radiotherapy. Various investigators have demonstrated a link between high levels of survivin expression and radioresistance (28,29). Radioresistance through mutations in EGFR has been studied and linked to various intracellular pathways yet no clear mechanism has been discovered.
Immunotherapy in lung cancer
Over the past several years, the importance of immune responses in cancer stem from the update of “the hallmarks of cancer” which included several new mechanisms important to cancer cell proliferation and evasion of the body’s innate system of immunosurveillance (30). It was noted that cancer cells require the ability to thrive in a chronically inflamed environment and evade and suppress the immune system. With this knowledge researchers have begun to seek out mechanisms to effectively activate immune reactivity, counteract immune suppression and characterize cancer specific antigens that are present throughout the cell’s lineage.
The basis for immunotherapy lies in mounting an adaptive response to cancer specific antigens. This relies on the tumor microenvironment, myeloid suppressing cells like T-regulatory (Treg) cells and the discovery of conserved cancer cell antigens (30-33).
In fact, Suzuki et al. have begun to clarify the importance of the tumor microenvironment on the risk of recurrence (33). The tumor microenvironment was studied by separating eight tumors infiltrating immune cells from the tumor and surrounding stroma and studying the expression of several cytokines in nearly 1,000 early stage lung cancer patients. Several markers were found to be significantly strong predictors of the risk for a recurrence at five years. These markers included an elevated forkhead box P3 (FOXP3): CD3 ratio and high levels of interleukin-7 receptor. The interleukin-7 receptor was also linked to worse overall survival. It was also noted that high levels of interleukin-12 receptor β2 was associated with a lower risk of recurrence. It turns out that FOXP3 is a marker for Treg cells. The expression of FOXP3 was also noted in the tumor stroma emphasizing the necessity of the tumors microenvironment in the relapse potential. IL-12 and its associated receptor acts as a tumor suppressor that is associated with less aggressive tumors. On the other hand, IL-7R has been shown to enhance angiogenesis by upregulating VEGF-D and acts through the JAK/STAT pathway. Several therapeutic targets have been suggested to counteract these newly found prognosticators in early lung cancer cells including cyclophosphamide which may deplete Treg cells and alter the FOXP3:CD3 ratio, reintroducing IL-12 or stimulating the IL-12R and blocking angiogenesis and the STAT family (33-35).
Several other mechanisms have been thoroughly studied to manipulate the immune environment including cytotoxic T lymphocyte anigen-4 (CTLA-4), programmed death 1 (PD-1), PD-1 ligands and damage associated molecular-pattern molecules (DAMPs) (33). CTLA-4 is expressed on CD4 cells and inhibits cytotoxic T lymphocytes. Ipilimumab is an antibody which targets CTLA-4. A clinical response relies on nonspecific alterations in immunogenicity through changes in total lymphocyte number and dendritic cells as well as altering expression of indoleamine dioxygenase. Ipilimumab has demonstrated a progression free survival in advanced stage, metastatic lung cancer in combination with chemotherapy. Other inhibitors of T cells include the PD-1 receptor which is a co-inhibitor factor present on T cells that is activated by PD ligands 1 and 2 (PD-L1 and PD-L2). Both PD-1 and PD-L1 have been targeted clinically in metastatic lung cancer demonstrating an objective response in 10-33% of patients with squamous cell carcinoma. Much lower response rates have been noted in adenocarcinomas (34,36). DAMPS such as heat-shock proteins (HSP) and high-mobility group box 1 (HMGB1) enhance autophagy which is down regulated in cancer cells. It is theorized this may play a role in the abscopal effect and manipulation of DAMPS may increase the chances for systemic control of disease (34,35,37).
Lung cancer vaccines have been developed and demonstrated impressive results in several clinical trials. Targets range from conserved proteins, molecular biomarkers to nonspecific targets. Mucin 1 (MUC1) is a cellular adhesion molecule expressed on many epithelial cells and is largely conserved within malignant lung cancer cells. MUC1 targeting vaccines including BLP-25 and TG4010 have demonstrated improvements clinical outcomes in early phase trials. BLP-25 is the only MUC1 vaccine that has thus far demonstrated a significant improvement in overall survival. The phase IIB trial demonstrated a 31% 3-year overall survival compared to 17% with best supportive care (34,38). Although no benefit in survival was demonstrated in metastatic disease. Importantly, the administration of BLP-25 was administered with cyclophosphamide to inhibit T cell suppression. Several phase three trials including the START and INSPIRE trials are currently assessing BLP-25 in the phase III setting. The TG4010 vaccine acts by inducing MUC1 and IL-2 expression through transfection with a recombinant vaccine virus. There have been promising results in early phase studies yet no significant improvement in clinical outcomes. Clinical outcome with this technique relies on the expression and recognition of transfected targets and phase three studies are now excluding patients with increased NK cell activity as these patients tended to have worse outcomes and toxicity. The CIMAvax EGF vaccine has demonstrated an improved median survival through targeting the EGFR receptor but this effect is limited to those patients that produce a good antibody response to the vaccine. MAGE-A3 is another conserved protein that has been targeted for vaccine development which in phase II studies has led to a trend to improved overall survival. This has led to the MAGRIT phase III study. Belagenpumatucel-L is a vaccine targeting TGF-β. The high-dose arm had a significantly improved median survival of nearly one year without significant toxicity. This has led to a phase II trial (NCT00676507) (34,38).
Combining immunotherapy with radiotherapy has been postulated to improve clinical outcome. Commonly after standard fractionated radiotherapy most cells undergo apoptosis as their mechanism for cell death which is non-immunogenic. But it is theorized that with hypofractionated therapy cells in combination with immunomodulaters may make tumor cells more immunogenic. In fact, Shaue et al. demonstrated in a murine melanoma model a threshold where doses of 7.5 Gy were immunostimulatory yet less hypofractionated doses were not effective (39). The exact mechanism of enhancement of the innate and adaptive immune systems is unclear but there have been several reports demonstrating marked reduction in systemic disease after local radiotherapy (39,40).
Status of personalized care in lung cancer
Personalized medicine has become a hot topic due to the lower costs of genetic testing and the voluminous research each year that demonstrate new molecular biomarkers. Rather than treating tumors based on stage and anatomical location the ultimate goal of personalized oncology is to identify sub-classes of molecular tumor types, which will lead to improved treatment strategies and prognosis.
Biomarker driven clinical trials utilizing first generation EGFR tyrosine kinase inhibitors (erlotinib and gefitinib), as well as ALK inhibitors such as crizotinib have improved clinical outcomes with demonstrated response rates between 50-75% (16,41,42). In fact, these studies have led to a recent change in the National Comprehensive Cancer Network 2013 guidelines for non-small cell lung cancer which recommends molecular testing in the work-up of metastatic lung cancer patients. Now, many clinicians and several multi-disciplinary tumor boards are recommending molecular testing be done earlier and earlier in the clinical presentation of disease.
Although molecular testing is becoming a part of our clinical acumen in lung cancer serious limitations of our current targetable biomarkers exist. The largest limitation in applying these data to the general population lies in the fact that Americans only harbor between 10-30% of ALK and EGFR mutations and between 80-90% of all lung cancer patients do not harbor these mutations at all (8,16,43). In patients that harbor a targetable mutation between 25-50% of them do not respond to therapy. Efforts to determine the mechanisms of resistance amongst patient’s harboring these mutations as well as emerging ALK inhibitors and second generation EGFR inhibitors will hopefully address this key issue.
Our understanding of the molecular pathways of driver mutations and their mechanisms of resistance will continue to improve. Many of the aforementioned molecular biomarker subtypes will likely be a part of our growing clinical armamentarium as the fight continues to tailor therapy to each tumor.
Molecular markers: clinical applications and outcomes
The application of novel therapeutics to disrupt driver gene pathways has met with mixed results. Attempts to use these molecular biomarkers earlier in the pathogenesis of lung cancer are under active investigation.
Erlotinib, crizotinib and bevacizumab have played a role in improving clinical outcomes in metastatic lung cancer (11,44-47). Yet, the use of concurrent or adjuvant EGFR inhibitors has led to inferior or equivocal results compared to current standard therapy (47). Also, the use of concurrent bevacizumab remains perilous. Many clinicians believe that the unselected nature of these trials has led to unexpected results. Logically, patients that harbor these mutations should have improved clinical outcomes (45,46,48). This has been noted with the addition of crizotinib in patients harboring the fusion gene with metastatic disease (49). Researchers await the results of the cetuximab data from the RTOG 0617 trial to determine if the addition of targeted therapy will lead to improved clinical outcomes in combined modality therapy. Excitingly, personalized targeted therapy is being explored in an upcoming RTOG trial assessing the efficacy of induction targeted therapy followed by standard therapy. Of course, the drawbacks in this design are that induction therapy will delay local therapy. But the safety of combining these therapies with combined modality therapy remains unclear and adjuvant therapy has demonstrated poor results.
Further genetic testing has been explored to identify sub-groups of patients with improved outcomes. In fact, a 5-gene signature was identified and validated by researchers in Taiwan (50). Using gene expression profiling, risk scores and decision-tree analysis, the researchers found DUSP6, MMD, STAT1, ERBB3 and LCK were independent predictors of relapse free and overall survival. They performed a microarray analysis of 16 genes in 125 patients and grouped patients into high risk and low risk groups. Using their 5-gene signature, the median overall survival in the low risk group was 40 months while the rate for those in the high risk group was 30 months with a P<0.001. Relapse free survival was also significant; 29 months in low risk patients and 13 months in high risk patients. Importantly, these genes functions were observed in various realms of tumorigenesis, including apoptosis, cell differentiation and metastatic potential.
Preclinical studies have found other predictive biomarkers, including inhibitors of DNA binding ID1 and ID3. Immunohistochemical staining for ID1/3 was performed in 17 stage III lung cancer patient that received combined modality treatment. Interestingly, a dramatic improvement in progression free and overall survival was demonstrated. In patients without ID1/ID3 co-expression, the median progression free survival was 30 months compared to 1 month in those with co-expression. The median overall survival for patients without ID1/ID3 co-expression was 45 months and for those with co-expression was six months (51). It is theorized that these genes may correlate with the extent of hypoxia leading to resistance to radiotherapy (52).
Recently, there has been a remarkable uptrend of clinical trials addressing the use of targeted therapies earlier in the pathogenesis of disease (53). Importantly, the application of these novel therapeutics is being tailored to individual tumors which will hopefully improve clinical outcomes. The characterization of driver genes and prognostic biomarkers like the 5-gene signature and ID1/3 expression is an exciting revelation in lung cancer but we still require further study and validation in large randomized trials to determine if these biomarkers are clinically relevant.
Radiation pneumonitis and novel biomarkers for toxicity
Radiation pneumonitis is characterized by inflammation of the lung after delivering therapeutic doses of radiation to the thorax. Clinically significant pneumonitis is considered any toxicity that will require medical intervention. Clinically significant radiation pneumonitis occurs in approximately 5-50% of patients with lung cancer and is one of the most common clinical toxicities. It is also one of the most dangerous (54). Approximately 80% of clinically significant pneumonitis manifests in the first 10 months following therapy. The frequency of different clinical endpoints varies among patients with radiation pneumonitis: 20-80% will have a radiologic abnormality, 5-50% will have shortness of breath and <3% will develop a bronchial stricture.
Quantitative analysis of normal tissue effects in the clinic (QUANTEC) is the guide radiation oncologists use to interpret dose volume histograms. The recommended dose-volume limits generally used (many caveats exist) in clinical practice include: the volume of lung receiving over 20 Gy (V20) of less than 30-35% and a mean lung dose of less than 20-23 Gy (55). These constraints portend a risk of less than 20% risk of pneumonitis. In patients after a pneumonectomy, more stringent limits include a V5<60%, V20<10% and a mean lung dose of <8 Gy. There are also factors that affect risk for pneumonitis. Classically, young age groups (<60-70 years old) and active smokers have a lower risk of developing pneumonitis. The use of concurrent chemotherapy increases the risk of radiation pneumonitis.
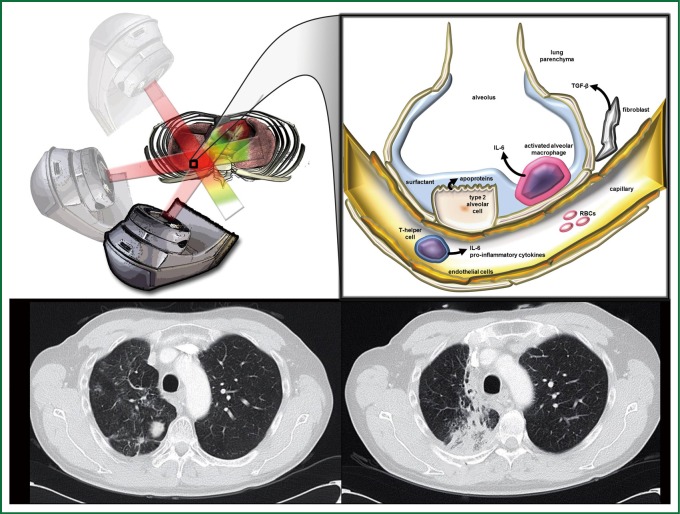
Acute radiation pneumonitis (within 12 weeks of radiotherapy) and subsequent pulmonary fibrosis which forms within the first 1-2 years results from a cascade of inflammatory cytokines and vasculature changes. Below is a depiction of several key markers of pneumonitis during the pathogenesis of fibrosis (Figure 2). The alveolar epithelium of the lung is made up of Type I (>90%) and Type II pneumocytes and upon exposure to radiotherapy there is a large loss of type I pneumocytes through apoptosis. The Type II alveolar cells begin to proliferate and produce surfactant apoproteins to repair the surrounding damage. Cells within the extracellular matrix including macrophages, fibroblasts along with circulating T helper cells begin secreting cytokines including IL-6 and TGF-β recruiting other inflammatory cells and beginning the cascade leading to collagen deposition and fibrosis within the lung parenchyma (56).
Figure 2.
Mechanism of Pulmonary Toxicity. Radiation therapy is targeted at a right lower lobe lung mass (upper left panel). The irradiation of normal tissue during radiotherapy (black box, inset) causes certain patients to develop radiation pneumonitis, which is associated with release of IL-6 from neutrophils, TGF-β from fibroblasts, and apoproteins in surfactant from type II alveolar cells (black box inset, magnification). Pre- and one year post- radiotherapy axial CT slices from a patient that developed radiation pneumonitis in the right lung is displayed (lower panel, left and right, respectively). Illustration created by Nicholas G. Zaorsky, M.D.
Recently, biomarkers and organ interactions have become important predictors of radiation pneumonitis. Inflammatory cytokines are known to participate in the pathogenesis of radiation pneumonitis and they pose a possible serum biomarker for toxicity. An early study linking serum markers to lung toxicity was the ROTG 91-03 trial studying stage II and III lung cancer patients undergoing 60-66 Gy of radiotherapy but were not surgical candidates (57). Some patients in this trial were able to receive concurrent or sequential chemotherapy but during the initial phases of the trial patients received radiotherapy alone. They found that after 10 Gy, elevated serum IL-6 (>0) predicted for acute grade 2 or higher radiation pneumonitis. At the same time, elevated levels of surfactant apoproteins (>797) after 20 Gy were correlated with late radiation pneumonitis. They also noted that a diffusion capacity of <54 and age >60 portends a higher risk of radiation pneumonitis. The remainder of the serum markers studied failed to correlate well with pneumonitis, including TNF and TGF-β.
TGF-β is the most heavily studied and scrutinized inflammatory biomarker for lung toxicity because it has conflicting data regarding its predictive ability for radiation pneumonitis (58,59). Several studies have linked elevations in TGF-β levels to radiation pneumonitis. They reported that levels of TGF-β differ significantly during radiotherapy and that sampling time determines the level of serum concentration. Other studies found that technical factors related to testing blood samples may explain the elevations in TGF-β levels. Still others found that normal tissue production of TGF-β during radiotherapy was influenced by the genetic background of the tumor and the patient (52,59).
Nonetheless, a combined analysis from Michigan and China found that elevation of serum TGF-β1 levels during radiotherapy (at four weeks) compared to pre-treatment TGF-B levels predicted for pneumonitis. The addition of mean lung dose helps stratify patients at the highest risk. Using a TGF-β ratio of >1 and mean lung dose of >20 Gy as risk factors, they categorized patients into three groups: no risk factors (low risk), one risk factor (intermediate risk) and both risk factors (high risk group). The risk of pneumonitis for each group was <5% for low risk, 50% for intermediate risk and 66% for the high risk group. A similar study was performed using TGF-β levels at the end of therapy and V30 (58). They were also able to adequately stratify each set of patients based on these two factors. Several investigators have found the combination of inflammatory markers with dose-volume characteristics seems to be the best predictor for pneumonitis, rather than being compared to any factor alone. Unfortunately, these studies found a marker that must be drawn during therapy and in some cases this was too late to make any significant change in the outcome.
A recent sophisticated study that searched for single nucleotide polymorphisms (SNPs) of TGFβ1 gene found genotypes at lower risk for radiation pneumonitis. This study randomly acquired DNA from 164 lung cancer patient’s resected tumor specimen and genotyped each sample to reveal SNPs in the TGF-β gene. The CT/CC genotypes in rs1982073:T869C TGFβ1 allele had a lower risk of developing radiation pneumonitis after radiotherapy independent of dosimetric factors such as mean lung dose and V20 (41). This may allow pre-treatment assessment of pneumonitis risk and further allow personalized radiotherapy treatment planning.
Strikingly, there is data linking parameters of radiation dose administered to the heart to lung toxicity. A single institutional review of hundreds of dose volume parameters found several variables, heart D10, lung D35 and maximum dose of the lung, were significant predictors for radiation pneumonitis in their cohort of patients (60). Due to the confounding variables within this type of analysis, further assessment and generalization to other patient populations are needed prior to using these variables in everyday practice. Additionally, heart toxicity has been linked to several biomarkers including pro-BNP and troponins (61). Though, no studies have linked these biomarkers to heart toxicity after completing radiotherapy to the lungs.
Other mechanism based biomarkers have been developed to determine improved outcomes in patients taking targeted therapies. These mechanism based biomarkers are well known side-effects, such as an acneiform rash with EGFR inhibitors, hypertension for VEGR inhibitors, hypothyroidism with multitargeted tyrosine kinase inhibitors and hyperglycemia with mTOR or PI3K inhibitors. Through analysis of the most recent targeted therapy trials in lung cancer, as well as analysis of other anatomic sites, trends were identified linking improved clinical outcomes in those patient’s that experienced mechanism based toxicities (62). Conversely, it is postulated that a lack of mechanism based toxicity is a surrogate for lack of effective tumor response. These data are interesting, yet they remain preliminary.
Lately researchers have begun combining targeted therapies in lung cancer with standard chemoradiotherapy. This raises a question: How will the addition of targeted therapies alter the therapeutic window?
Several early phase clinical trials assessing the safety and efficacy of adding bevacizumab to standard chemoradiotherapy in lung cancer have found an alarming rate of tracheoesophageal fistulas. Tracheoesophageal fistulas are normally an exceedingly rare occurrence in the treatment of lung cancer. However, in a small pooled analysis, investigators found more than 10% incidence of tracheoesophageal fistula formation prompting the early termination of these investigations (44,63,64). Another early phase trial assessed the incidence of clinically significant pneumonitis. When combined with chemoradiotherapy in advanced lung cancer, they found a clinically significant pneumonitis rate of 67% (44,63). Although these studies are relatively small, they demonstrate an alarmingly high rate of significant lung and esophageal toxicity occurs with the addition of bevacizumab in standard chemoradiotherapy. This finding has prompted many researchers to abandon the addition of current generation VEGF inhibitors in combined modality lung cancer treatment. Additional studies using next generation anti-angiogenic factors are needed to further characterize the safety and efficacy of this modality of treatment.
The controversial multi-institutional RTOG trial 0617 also assessed whether the addition of targeted therapy to combined modality therapy may improve outcomes. They used a 2×2 factorial design comparing standard dose (60 Gy) versus high dose radiotherapy (74 Gy), with and without the addition of cetuximab. Paradoxically, there were significantly more local failures in the high dose arm, 34% versus 25% in the standard dose arm. Also noted was a startling stratification in survival, with a median survival in the standard dose arm of 28.7 months and 19.5 months in the high dose arm. The only significant difference in toxicity was esophagitis was three times higher (65). Many questions about these results remain unanswered. Some postulate that overall treatment time plays a role. Using tighter treatment margins without using 4D CT scans to determine tumor motion or awaiting the additional dosimetric data.
The appropriate timing of targeted therapies to use in combined modality therapy remains unclear. To address this issue, a trial in the pre-activation stage RTOG 1306 will add targeted therapies as an induction therapy for advanced stage lung cancer. Patients with stage III non-squamous, non-small cell lung cancer with N2 or N3 disease will be enrolled. All patients will have surgical staging and tissue sent for molecular testing that searches for EGFR mutations and ALK translocations. Patients will be randomized based on their mutation analysis to receive either standard chemoradiotherapy or induction therapy with either erlotinib or crizotinib based on their mutation status.
The era of personalized medicine continues to bloom by allowing tailored treatments in addition to standard therapy. However, there are many unknown variables to consider when adding novel therapeutics to other cytotoxic therapies, as we have not completely defined the various therapeutic ratios. We have begun to define newer markers of toxicity. These latest findings will help next generation trials assess and prevent toxicity in lung cancer patients.
Hypofractionated radiotherapy and pneumonitis
Hypofractionated radiotherapy is employed as a means of either dose escalation or shortening overall treatment times for both early and late stage lung cancer (66). However, the optimal dose, fractionation and schedule remain under investigation. There are several early phase clinical trials with data maturing which have combined hypofractionated radiotherapy with targeted agents including erlotinib (NCT00983307) and ZD1839 (NCT00328562). As of November of 2013, there are no active clinical trials assessing targeted therapies and hypofractionated radiotherapy registered to clinicaltrials.gov, which highlights a need for continued investigation. Patient factors and dosimetric information related to pneumonitis in the setting of hypofractionated radiotherapy is derived from early phase clinical trials and large retrospective analysis. A recent phase I study assessing hypofractionated attempting to raise the biologic effective dose (BED) over 100 Gy for patients of all stages revealed 16% grade 2 and no grade 3 radiation pneumonitis. However, six patients experienced grade 4 or 5 radiation toxicity including hemoptysis, lung abscess and bronchocavitary fistula. Univariate analysis demonstrated a significant association of high grade toxicity and total irradiation dose over 75 Gy with a 2-year incidence of toxicity of 31% vs. 1.8%. The maximal tolerated dose in this trial was 63.25 Gy in 25 fractions. The dose parameters which significantly predicted for 5% toxicity at two years were a D3cc of 75 Gy and a Dmax of 83 Gy (66). The high grade toxicities were attributed, by the investigators, to high doses as mentioned above being delivered to central structures including the proximal bronchial tree. The rate of pneumonitis for stereotactic body radiotherapy (SBRT), a form of ultra-hypofractionated therapy which employs image guidance and smaller treatment margins, has demonstrated rates of pneumonitis between 5-21% (67).
As the use of these techniques has increased, more attention has been paid to the size of the tumor volume treated and the dose to the uninvolved lung. Several studies revealed larger primary tumor volume, mean lung dose, and maximum dose to the tumor predicted for higher rates of pneumonitis (67,68). Reasonable dosimetric guidelines include a mean lung dose less than 6 Gy, a contralateral mean lung dose less than 3.6 Gy, and a V20 <10%. Factors which may predict for increased risk for pneumonitis include concurrent systemic therapy, active smoker, advanced age (>65), central location, and size of treatment volume (>145 cc) (66-69). Since the available toxicity data is more robust in the setting of hypofractionated or SBRT alone, it is prudent that combination targeted therapy and hypofractionated or SBRT be conducted on prospective clinical trials to allow detailed assessment of possible toxicities as available dosimetric and patient factors may underestimate the rates of high-grade toxicity.
Conclusions
Lung cancer is a heterogeneous group of tumor sub-types. Each type carries individualized mutations in multiple driver gene pathways. Classically, cancer therapies have been applied based on anatomic site, stage and other limited prognostic information. With the explosion of data that demonstrates targetable biomarkers in cancer, we are faced with new challenges to balance toxicity with clinical outcomes.
Genetic signatures have been discovered that influence outcome and one day may identify groups of patients that benefit from more aggressive therapy. Novel organ specific toxicity-related biomarkers in combination with radiotherapy derived parameters will improve treatment decisions and allow real-time treatment modifications to prevent long-term toxicity.
New approaches based on tumor and normal tissue characteristics are necessary to continue improving clinical outcomes. New multi-disciplinary tumor boards should be formed based on genetic tumor characteristics rather than tumor sites. Medicine requires an ever-increasing level of sophistication to interpret studies and design clinical trials. Technology, data management and analysis and novel therapies will improve more rapidly than ever before impacting our ability to predict and change clinical outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer Statistics, 2013. CA Cancer J Clin 2013;63:11-30 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70 [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol 2005;23:3243-56 [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI’s lung cancer mutation consortium (LCMC). J Clin Oncol 2011;29:CRA7506 [Google Scholar]
- 5.Rowinsky EK. The erB family: targets for therapeutic development against cancer and therapeutic strategies sing monocloncal antibodies and tyrosine kinase inhibitors. Annu Rev Med 2004;55:433-57 [DOI] [PubMed] [Google Scholar]
- 6.Decker RH, Lynch TJ. Unmet challenges in the use of novel agents in locally advanced non-small-cell lung cancer. J Clin Oncol 2012;30:582-4 [DOI] [PubMed] [Google Scholar]
- 7.Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato M, Shames DS, Gasdar AF, et al. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol 2007;2:327-43 [DOI] [PubMed] [Google Scholar]
- 9.Oldenhuis CN, Oosting SF, Gietema JA, et al. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer 2008;44:946-53 [DOI] [PubMed] [Google Scholar]
- 10.Sidransky D.Emerging molecular markers of cancer. Nat Rev Cancer 2002;2:210-9 [DOI] [PubMed] [Google Scholar]
- 11.Shepherd FA, Domerg C, Hainaut P, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol 2013;31:2173-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxnard GR, Binder A, Jӓnne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol 2013;31:1097-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekido Y, Fong KM, Minna JD. Molecular genetics of lung cancer. Annu Rev Med 2003;54:73-87 [DOI] [PubMed] [Google Scholar]
- 14.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor resceptor signaling athway in lung cancers. Int J Cancer 2006; 118:257-62 [DOI] [PubMed] [Google Scholar]
- 15.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46 [DOI] [PubMed] [Google Scholar]
- 16.Wu K, House L, Liu W, et al. Personalized targeted therapy for lung cancer. Int J Mol Sci 2012;13:11471-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verweij J, de Jonge M.Multitarget tyrosine kinase inhibition: [and the winner is…] J Clin Oncol 2007;25:2340-2 [DOI] [PubMed] [Google Scholar]
- 18.Aréchaga-Ocampo E, Villegas-Sepulveda N, Lopez-Urrutia E, et al. (2013). Biomarkers in Lung Cancer: Integration with Radiogenomics Data, Oncogenomics and Cancer Proteomics - Novel Approaches in Biomarkers Discovery and Therapeutic Targets in Cancer, Dr. Cesar Lopez (Ed.), ISBN: 978-953-51-1041-5, InTech, DOI: 10.5772/53426 [DOI] [Google Scholar]
- 19.Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada clinical trials group study BR.21. J Clin Oncol 2008;26:4268-75 [DOI] [PubMed] [Google Scholar]
- 20.Roberts PJ, Stinchcombe TE, Der CJ, et al. Personalized medicine in non-small-cell lung cancer: is DRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol 2010;28:4769-77 [DOI] [PubMed] [Google Scholar]
- 21.Ausborn NL, Le QT, Bradley JD, et al. Molecular profiling to optimize treatment in non-small cell lung cancer: a review of potential molecular targets for radiation therapy by the translational research program of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys 2012;83:e453-64 [DOI] [PubMed] [Google Scholar]
- 22.Rose-James A, Sreelekha TT. Molecular markers with predictive and prognostic relevance in lung cancer. Lung Cancer Int 2012;2012:12. [DOI] [PMC free article] [PubMed]
- 23.Fujiwara T, Tanaka N, Kanazawa S, et al. Multicenter phase I study of repeated intratumoral delivery of adenoviral p53 in patients with advanced non-small-cell lung cancer. J Clin Oncol 2006;24:1689-99 [DOI] [PubMed] [Google Scholar]
- 24.Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol 2013;31:1219-30 [DOI] [PubMed] [Google Scholar]
- 25.Withers HR. The four R’s of radiotherapy. Adv Radiat Biol 1975;5:241-7 [Google Scholar]
- 26.Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heist RS, Zhou W, Chirieac LR, et al. MDM2 polymorphism, survival, and histology in early-stage non-small-cell lung cancer. J Clin Oncol 2007;25:2243-7 [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Moretti L, Giacalone NJ, et al. Inhibition ofJAK2 signaling by TG101209 enhances radiotherapy in lung cancer models. J Thorac Oncol 2011;6:699-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu B, Mu Y, Cao C, et al. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res 2004;64:2840-5 [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74 [DOI] [PubMed] [Google Scholar]
- 31.Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia 2010;15:411-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavallo F, De Giovanni C, Nanni P, et al. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother 2011;60:319-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki K, Kyuichi K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal foxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol 2013;31:490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control 2013;20:22-31 [DOI] [PubMed] [Google Scholar]
- 35.Rüttinger D, Winter H, van den Engel NK, et al. Immunotherapy of cancer: key findings and commentary on the third Tegernsee conference. Oncologist 2010;15:112-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi SF, Brahmer JR, et al. Safety, activity and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahara H, Sato M, Thurin M, et al. Emerging concepts in biomarker discovery; the US-Japan workshop on immunological molecular markers in oncology. J Transl Med 2009;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero P.Current state of vaccine therapies in non-small-cell lung cancer. Clin Lung Cancer 2008;9Suppl 1:S28-36 [DOI] [PubMed] [Google Scholar]
- 39.Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan X, Liao Z, Liu Z, et al. Single nucleotide polymorphism at rsl1982073: T869C of the TGFβ1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol 2009;27:3370-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol 2010;28:744-52 [DOI] [PubMed] [Google Scholar]
- 43.Keedy VL, Argeaga CL, Johnson DH. Does gefitinib shorten lung cancer survival? Chaos redux. J Clin Oncol 2008;26:2428-30 [DOI] [PubMed] [Google Scholar]
- 44.Sandler AB, Schiller JH, Gray R, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J Clin Oncol 2009;27:1405-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicenter, randomized, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9 [DOI] [PubMed] [Google Scholar]
- 46.Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line--is there a difference? J Clin Oncol 2013;31:1081-8 [DOI] [PubMed] [Google Scholar]
- 47.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer. J Clin Oncol 2008;326:2450-6 [DOI] [PubMed] [Google Scholar]
- 48.Fong T, Morgensztern D, Govindan R.EGFR inhibitors as first-line therapy in advanced non-small cell lung cancer. J Thorac Oncol 2008;3:303-10 [DOI] [PubMed] [Google Scholar]
- 49.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer N Engl J Med 2010;363:1693-703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med 2007;356:11-20 [DOI] [PubMed] [Google Scholar]
- 51.Castañon E, Bosch-Barrera J, López I, et al. Id1 and Id3 co-expression correlates with clinical outcome in stage III-N2 non-small cell lung cancer patients treated with definitive chemoradiotherapy. J Transl Med 2013;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raben D, Bunn PA. Biologically targeted therapies plus chemotherapy plus radiotherapy in stage III non-small-cell lung cancer: a case of the Icarus syndrome? J Clin Oncol 2012;30:3909-12 [DOI] [PubMed] [Google Scholar]
- 53.Buettner R, Wolf J, Thomas RK. Lessons learned from lung cancer genomics: the emerging concept of individualized diagnostics and treatment. J Clin Oncol 2013;31:1858-65 [DOI] [PubMed] [Google Scholar]
- 54.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281-93 [DOI] [PubMed] [Google Scholar]
- 57.Hartsell WF, Scott CB, Dundas GS, et al. Can serum markers be used to predict acute and late toxicity in patients with lung cancer? Am J Clin Oncol 2007;30:368-76 [DOI] [PubMed] [Google Scholar]
- 58.Zhao L, Wang L, Ji W, et al. Elevation of plasma TGF-β1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: a combined analysis from Beijing and Michigan. Int J Radiat Oncol Biol Phys 2009;74:1385-90 [DOI] [PubMed] [Google Scholar]
- 59.Fu XL, Hong H, Bentel G, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V30 and transforming growth factor β. Int J Radiat Oncol Biol Phys 2001;50:899-908 [DOI] [PubMed] [Google Scholar]
- 60.Huang EX, Hope AJ, Lindsay PE, et al. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol 2011;50:51-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Errico MP, Grimaldi L, Petruzzelli MF, et al. N-terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys 2012;82:e239-46 [DOI] [PubMed] [Google Scholar]
- 62.Dienstmann R, Braña I, Rodon J, et al. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 2011;16:1729-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lind JS, Senan S, Smit EF. Pulmonary toxicity after bevacizumab and concurrent thoracic radiotherapy observed in a phase I study for inoperable stage III non-small-cell lung cancer. J Clin Oncol 2012;30:e104-8 [DOI] [PubMed] [Google Scholar]
- 64.Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-48 [DOI] [PubMed] [Google Scholar]
- 65.Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy ± cetuximab for stage III non-small cell lung cancer: results on radiation dose in RTOG 0617. J Clin Oncol 2013;31:abst 7501.
- 66.Cannon DM, Mehta MP, Adkison JB, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 2013;31:4343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker R, Han G, Saranqkasin S, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys 2013;85:190-5 [DOI] [PubMed] [Google Scholar]
- 68.Bongers EM, Botticella A, Palma DA, et al. Predictive parameters of symptomatic radiation pneumonitis following stereotactic or hypofractionated radiotherapy delivered using volumetric modulated arcs. Radiother Oncol 2013;109:95-9 [DOI] [PubMed] [Google Scholar]
- 69.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444-50 [DOI] [PMC free article] [PubMed] [Google Scholar]