Abstract
The specific causes of prostate cancer are not known. However, multiple etiological factors, including genetic profile, metabolism of steroid hormones, nutrition, chronic inflammation, family history of prostate cancer, and environmental exposures are thought to play significant roles. Variations in exposure to these risk factors may explain inter-individual differences in prostate cancer risk. However, regardless of the precise mechanism(s), a robust DNA repair capacity may mitigate any risks conferred by mutations from these risk factors. Numerous single nucleotide polymorphisms (SNPs) in DNA repair genes have been found, and studies of these SNPs and prostate cancer risk are critical to understanding the response of prostate cells to DNA damage. A few SNPs in DNA repair genes cause significantly increased risk of prostate cancer, however, in most cases, the effects are moderate and often depend upon interactions among the risk alleles of several genes in a pathway or with other environmental risk factors. This report reviews the published epidemiologic literature on the association of SNPs in genes involved in DNA repair pathways and prostate cancer risk.
Keywords: Prostate cancer, polymorphism, DNA repair, cancer susceptibility
INTRODUCTION
Prostate is the most common site of cancer and the third leading cause of cancer mortality in men in the United States [1]. There is a large variation in prostate cancer incidence rates among ethnic groups. Incidence rates are the highest among African Americans (272 per 100,000 per year) and the lowest among Asians (2 per 100,000 per year) [1, 2]. Incidence of prostate cancer is increasing steadily in almost all countries, and the lifetime risk of prostate cancer for men in the United States is 18% [1, 3].
Although the specific causes of prostate cancer are not known, androgens, estrogens, inflammation and DNA repair capacity have been implicated. Androgens which play an important part in development and maintenance of the prostate can induce prostate cancer in rodents [4], and stimulate the in vitro proliferation of prostate cancer cells [5]. Carcinogenesis in prostate tissue involves multiple genetic events.
DNA is constantly damaged by endogenous oxygen free radicals and exogenous chemicals. DNA mutations are estimated to spontaneously occur 20,000–40,000 times everyday [6, 7]. The DNA repair process is important to the survival of the cell, therefore, different repair pathways are available to reverse the different types of DNA damage. In fact, over 150 DNA repair enzymes participate in this process [8]. Defects in these DNA repair pathways may increase persistent mutations in daughter cell generations, genomic instability, and ultimately a prostate cancer risk. These DNA repair genes can be classified into several distinct pathways: Direct reversal, base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), and double-strand break repair (DSBR). Depending upon the DNA damaging agents, different levels of contribution from different classes of DNA repair enzymes could be expected.
In this manuscript, we focused on single nucleotide polymorphisms (SNPs) and phenotypes in DNA repair genes that have been investigated in published epidemiological studies of prostate cancer.
METHODS
Numerous SNPs in different DNA repair genes have been identified, and many of them have been investigated in relation to human cancer susceptibility [9]. We identified studies relevant to prostate cancer using the search engine, Pubmed, (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi) in October 2007. The inclusion criteria for this manuscript were epidemiological studies of the association between polymorphisms in DNA repair genes and prostate cancer risk. Among 40 studies obtained from the search phrases "DNA repair” AND “prostate cancer" AND “polymorphism”, 11 epidemiological studies were included after review of the articles [10–20]. Among twelve additional epidemiological studies which were obtained after searching by single DNA repair gene name AND “prostate cancer”, five studies were excluded because they reported associations between phenotypes, such as expressions or activities of DNA enzymes and prostate cancer risk [21–25]. The remaining six studies also were included in this manuscript [26–31]. One article [19] was excluded from this review because the data of this article appears to be redundant with one published in Asian Journal of Andrology [31], thus a total of 16 published studies form the basis of this review.
The following notation is used to describe SNPs: uppercase letters represent amino acids with numbers indicating the codon and lowercase letters represent nucleotides with numbers indicating the sequence position.
RESULTS
By the end of October 2007, associations between SNPs in DNA repair genes and risk of prostate cancer have been reported in 16 published studies. Table 1 provides details on case-control studies of DNA repair gene polymorphisms and levels of association. Most studies were conducted in North America and five studies were conducted in China [12, 31], Taiwan [20], Japan [10], and UK [30]. Six studies were relatively large (438 – 996 cases) [13, 17, 18, 26, 28, 30], but Ten studies included 250 or fewer cases. Ten studies were hospital based case-control studies and four studies were population-based studies [12, 15, 27, 30]. Two studies used sibling and family based designs [13, 18].
Table 1.
Epidemiological studies of DNA repair gene SNPs and prostate cancer risk.
| Gene | Race | Study design | Case | Control | Genotype Distribution1 |
Comparisons | Adjustment2 | Odds ration (95%CI) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ADPRT V762A | Caucasian | Hospital | 438 | 427 | 70,26,4 73,25,2 |
VA vs. VV AA vs. VV |
Age, BPH, FH |
1.2 (0.9–1.6) 2.7 (1.1–6..5) |
[28] |
| African Americans |
Hospital | 50 | 97 | 90,10,0 91,9,0 |
VA vs. VV AA vs. VV |
1.0 (0.2–4.0) NA |
|||
| APE1 D148E | Caucasian | Hospital | 228 | 219 | 28,54,18 34,50,16 |
DE vs. DD EE vs. DD |
Age, S, FH | 1.2 (0.8–1.9) 1.2 (0.7–2.2) |
[11] |
| African Americans |
Population | 124 | 116 | 34,52,14 38,53,10 |
DE vs. DD EE vs. DD |
Age, S | 1.2 (0.7–2.2) 1.6 (0.6–3.8) |
||
| APE1 Q51H | Caucasian | Hospital | 228 | 219 | 89,10, 1 85,14, 1 |
QH vs. QQ HH vs. QQ |
Age, S, FH | 0.6 (0.3–1.2) 0.6 (0.03–14) |
[11] |
| African Americans |
Population | 124 | 116 | 79,19, 2 71,26, 3 |
QH vs. QQ HH vs. QQ |
Age, S | 0.8 (0.4–1.5) 0.7 (0.1–3.1) |
||
| ATM D1853N | Caucasians | Population | 637 | 455 | 73,24,3 69,28,3 |
ga vs. gg aa vs. gg |
None | 0.8 (0.7–1.1) 1.0 (0.5–2.1) |
[30] |
| ATM D1853V | Caucasians | Population | 637 | 455 | 99,1,0 99,1,0 |
ta vs. aa | None | 0.9 (0.2–3.3) | [30] |
| ATM ivs38-8t>c | Caucasians | Population | 637 | 455 | 93,7,0 93,7,0 |
ct/cc vs. tt | None | 1.0 (0.6–1.6) | [30] |
| ATM ivs38-15g>c | Caucasians | Population | 637 | 455 | 98,2,0 99,1,0 |
gc vs. gg | None | 1.8 (0.6–5.7) | [30] |
| ATMP1054R | Caucasians | Population | 637 | 455 | 92,7,0 96,4,0 |
PR/RR vs. PP | None | 2.1 (1.2–3.9) | [30] |
| hOGG1 a10660t | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 65,32, 4 61,32, 7 |
tt vs. aa | Age | NS | [16]. |
| hOGG1 a11657g | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 70,25, 5 74,25, 1 |
gg vs. aa | Age | 9.8 (1.2–76.9) | [16]. |
| Mixed (84% Caucasians) |
Family | 159 | 222 | 67,29, 5 74,25, 1 |
gg vs. aa | Age | 13.9 (1.6–125) | ||
| hOGG1 a11657g | Mixed (84% Caucasians) |
Hospital | 996 | 1092 | 70,27, 3 71,25, 4 |
ag vs. aa gg vs. aa |
Age | NS | [26] |
| hOGG1 a11826t | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 66,31, 3 60,33, 7 |
tt vs. aa | Age | NS | [16]. |
| hOGG1 a7143g | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 68,26, 5 71,28, 1 |
gg vs. aa | Age | 5.1 (1.1–23.3) | [16]. |
| Mixed (84% Caucasians) |
Family | 159 | 222 | 64,32, 5 71,28, 1 |
gg vs. aa | Age | 8.2 (1.5–45.5) | ||
| hOGG1 a9110g | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 66,31, 3 60,34, 7 |
gg vs. aa | Age | NS | [16]. |
| hOGG1 c10629g | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 28,44,27 30,41,30 |
gg vs. cc | Age | NS | [16]. |
| hOGG1 g3402a | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 40,44,16 43,45,12 |
aa vs. gg | Age | NS | [16]. |
| hOGG1 g3574a | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 67,28, 5 60,34, 6 |
aa vs. gg | Age | NS | [16]. |
| hOGG1 g6170c | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 63,33, 4 58,34, 7 |
cc vs. gg | Age | NS | [16]. |
| hOGG1 S326C | Caucasians | Hospital | 84 | 252 | 58,35, 7 74,25, 1 |
SC vs. SS CC vs. SS |
Age, S | 1.8 (1.0–3.3) 7.8 (1.7–36) |
[14] |
| hOGG1 S326C | Mixed (93% Caucasians) |
Hospital | 245 | 222 | 61,36, 3 55,36, 9 |
CC vs. SS | Age | 0.3 (0.1–0.8) | [16]. |
| Mixed (84% Caucasians) |
Family | 159 | 222 | 61,35, 4 55,36, 9 |
CC vs. SS | Age | 0.5 (0.2–1.7) | ||
| hOGG1 S326C | Mixed (84% Caucasians) |
Hospital | 996 | 1092 | 60,35, 5 57,35, 8 |
SC vs. SS CC vs. SS |
Age, race, FH, DRE, PSA |
0.9 (0.8–1.1) 0.7 (0.5–1.0) |
[26] |
| hOGG1 S326C | Mixed (90% Caucasians) |
Family | 439 | 479 | 64,31, 5 54,30, 6 |
SC vs. SS CC vs. SS |
Age | 1.1 (0.7–1.6) 0.7 (0.3–1.7) |
[18] |
| MGMT I143V | Chinese | Population | 162 | 251 | 96, 3, 1 98, 2, 0 |
VV/IV vs. II | Age | 1.9 (0.6–6.2) | [12] |
| MGMT L84F | Chinese | Population | 162 | 251 | 76,22,1 86,13,1 |
LF vs. LL FF va. LL |
Age | 2.0 (1.2–3.3) 3.4 (0.3–38.1) |
[12] |
| hHR23B A249V | Mixed (90% Caucasians) |
Hospital | 494 | 470 | 293 | AA vs. AV/VV | Age,BPH, FH, S |
1.1 (0.8–1.4) | [17] |
| NBS1E185Q | unknown | Family | 121 | 200 | 33,52,15 44,40,16 |
EQ vs. EE QQ vs. EE |
none | 1.4 (0.7–2.8) 0.8 (0.4–1.6) |
[29] |
| Population | 200 | 200 | 41,47,12 44,40,16 |
EQ vs. EE QQ vs. EE |
1.6 (0.9–2.9) 1.2 (0.7–2.3) |
||||
| XPC A499V | Mixed (90% Caucasians) |
Hospital | 494 | 470 | 243 | AA/AV vs. VV | Age,BPH, FH, S |
0.9(0.5–1.5) | [17] |
| XPC K939Q | Mixed (90% Caucasians) |
Hospital | 494 | 470 | 383 | KK/KQ vs. QQ | Age,BPH, FH, S |
1.0 (0.7–1.4) | [17] |
| XPC K939Q | Japanese | Hospital | 165 | 165 | 47,47,6 44.42.14 |
KK/KQ vs. QQ | Age | 2.5 (1.1–5.5) | [10] |
| XPD c-114g | Taiwanese | Hospital | 123 | 479 | 28,53,19 31,46,23 |
DN/NN vs. DD | 1.0 (0.7–1.5) | [20] | |
| XPD D312N | Mixed | Siblings | 637 | 480 | 44,45,12 44,48, 8 |
NN vs. DN/DD | None | 1.6 (1.0–2.5) | [13] |
| Caucasian | Siblings | 572 | 437 | 40,47,13 41,50, 9 |
NN vs. DN/DD | None | 1.6 (1.0–2.5) | ||
| XPD D312N | Mixed (90% Caucasians) |
Hospital | 494 | 470 | 413 | NN vs. DN/DD | Age,BPH, FH, S |
0.8 (0.5–1.2) | [17] |
| XPD D312N | Taiwanese | Hospital | 123 | 479 | 50,32,18 65,22,13 |
DN/NN vs. DD | 1.8 (1.2–2.7) | [20] | |
| XPD K751Q | Mixed (90% Caucasians) |
Hospital | 494 | 470 | 353 | KQ/QQ vs. KK | Age,BPH, FH, S |
0.9 (0.6–1.4) | [17] |
| XPD K751Q | Chinese | Population | 162 | 251 | 88,12,0 86,13,1 |
KQ/QQ vs. KK | Age | 0.8 (0.5–1.5) | [12] |
| XPD K751Q | Mixed | Siblings | 637 | 480 | 40,47,13 41,47,12 |
QQ vs. KQ/KK | None | 1.1 ( 0.7–1.8) | [13] |
| Caucasian | Siblings | 572 | 437 | 39,48,13 41,47,12 |
QQ vs. KQ/KK | None | 1.2 (0.7–2.0) | ||
| XPD K751Q | Taiwanese | Hospital | 123 | 479 | 91,7,2 92,7,1 |
DN/NN vs. DD | 1.3 (0.6–2.5) | [20] | |
| XPF/ERCC4 R415Q | Mixed (90% Caucasians) |
Hospital | 494 | 470 | 93 | RQ vs. RR | Age, BPH, S. FH |
1.4 (1.0–2.0) | [17] |
| XPG/ERCC5 D1104H | Mixed (90% Caucasians) |
Hospital | 494 | 470 | 463 | DD/DH vs. HH | Age, BPH, S, FH | 0.8 (0.5–1.5) | [17] |
| XRCC1 R194W | Mixed (90% Caucasians) |
Population | 76 | 182 | 88,12,0 84,15,1 |
RW/WW vs. RR |
Age, race | 0.7 (0.3–1.6) | [15] |
| XRCC1 R194W | Japanese | Hospital | 165 | 165 | 42,48,13 52,38,10 |
RW/WW vs. RR |
Age | 1.5 (0.9–2.2) | [10] |
| XRCC1 R194W | Chinese | Hospital | 207 | 235 | 50,41,9 39,50,11 |
RW/WW vs. RR |
Age,S,AL FH |
0.6 (1.1–2.5) | [31] |
| XRCC1 R280H | Mixed (90% Caucasians) |
Population | 76 | 182 | 87,13,0 90,10,0 |
RH vs. RR | Age, race | 1.5 (0.7–3.6) | [15] |
| XRCC1 R280H | Chinese | Hospital | 207 | 235 | 80,19,1 82,17,1 |
RR/RH vs. HH |
Age,S,AL FH |
1.1 (0.7–1.9) | [31] |
| XRCC1 R399Q | Caucasian | Hospital | 228 | 219 | 42,46,12 50,40,10 |
RQ vs. RR QQ vs. RR |
Age, S, FH | 1.6 (1.1–2.5) 1.6 (0.8–3.1) |
[11] |
| African Americans |
Population | 124 | 116 | 73,24,3 73,24,3 |
RQ vs. RR QQ vs. RR |
Age, S | 1.2 (0.6–2.2) 1.5 (0.3–8.2) |
||
| XRCC1 R399Q | Chinese | Hospital | 207 | 235 | 52,41,7 65,31,4 |
RR/RQ vs. |
Age,S,AL FH |
1.7 (1.1–2.5) | [31] |
| XRCC1 R399Q | Chinese | Population | 162 | 251 | 55,34,11 54,41,5 |
RQ vs. RR QQ vs. RR |
Age | 0.8 (0.5–1.3) 2.2 (1.0–4.8) |
[12] |
| XRCC1 R399Q | Japanese | Hospital | 165 | 165 | 53,38,9 52,42,6 |
RQ/QQ vs. RR | Age | 1.0 (0.6–1.5) | [10] |
| XRCC1 R399Q | Mixed | Siblings | 637 | 480 | 46,43,11 45,43,12 |
QQ vs. RQ/RR | None | 0.9(0.6–1.4) | [13] |
| Caucasians | Siblings | 572 | 437 | 43,45,12 41,46,13 |
QQ vs. RQ/RR | None | 0.9(0.5–1.4) | ||
| XRCC1 R399Q | Mixed (90% Caucasians) |
Population | 76 | 182 | 49,39,12 42,43,15 |
RQ vs. RR QQ vs. RR |
Age, race | 0.8 (0.5–1.4) 0.7 (0.3–1.6) |
[15] |
| XRCC1 R399Q | Mixed (92% Caucasians) |
Population | 77 | 174 | 49, (51)4 43, (57) |
RQ vs. RR QQ vs. RR |
none | 0.8 (0.4–1.5) 0.7 (0.3–1.7) |
[27] |
| XRCC3 T241M | Chinese | Population | 162 | 251 | 87,11,2 87,13,1 |
TM vs. TT MM vs. TT |
Age | 0.8 (0.5–1.6) 2.2 (0.4–13.7) |
[12] |
| XRCC7 g6721t | Japanese | Hospital | 165 | 165 | 7,48,45 7,41,52 |
gt/tt vs. gg | Age | 1.0 (0.5–1.2) | [10] |
numbers are percentages of each genotypes, and single number indicates percentage of minor allele frequency
BPH benign prostate hyperplasia, FH: family history of prostate cancer, S, Smoking, AL, alcohol
minor allelic frequency
heteozygous and homozygous polymorphic genotypes were combined
Table 2 displays the SNPs in DNA repair genes included in this chapter with allele frequencies, SNP identification number and their potential functional effects.
Table 2.
SNPs in DNA repair genes and their functional relation.
| Pathway | Gene | SNP ID# | Minor Allele Frequency |
Function | Reference | ||
|---|---|---|---|---|---|---|---|
| White | Black | Asian | |||||
| BER | ADPRT V762A | rs1136410 | 0.11 | 0.06 | 0.33 | Decrease enzyme activities in response to H2O2 | [28] |
| BER | APE1 D148E | rs3136820 | 0.49 | 0.32 | 0.28 | Hypersensitivity to ionizing radiation | [66] |
| BER | APE1 Q51H | rs1048945 | 0.03 | 0 | 0 | Regulate the DNA binding activity | [73] |
| DRCC | ATM P1054R | rs1800057 | 0.02 | 0 | 0 | Affect the cellular response after exposure to ionizing radiation | [30] |
| BER | hOGG1 S326C | rs1052133. | 0.23 | 0.38 | 0.10 | No difference in DNA repair capacity between genotypes. | [41–46, 103] |
| 326Cys allele was associated with a decrease in p53 mutations. | [47] | ||||||
| Suppression of mutagenesis is lower in hOGG1 326Cys. | [38] | ||||||
| No difference in adducts between genotypes. | [40], | ||||||
| Adduct level was higher in CC genotypes | [48, 104] [105] | ||||||
| DR | MGMT I143V | rs2308321 | 0.13 | 0 | 0 | More resistant to inactivation by MGMT pseudosubstrate, O6-(4-bromothenyl) guanine. | [98] |
| DR | MGMT L84F | rs12917 | 0.11 | 0.17 | 0.11 | No affect on cell survival after exposure to N-methyl-N '-nitro-N-nitrosoguanidine. | [97] |
| NER | hHR23B A249V | rs1805329 | 0.15 | 0.26 | 0 | No affect NER activity by plasmid-based NER assay | [17] |
| NER | XPC A499V | rs2228000 | 0.10 | 0.05 | 0 | Affect NER activity by plasmid-based NER assay | [17] |
| NER | XPC K939Q | rs2228001 | 0.13 | 0.13 | 0 | Linkage disequilibrium with intron 9, 5bp deletion which cause alternative splicing. | [106] |
| NER | XPD/ERCC2 –c114g | rs3810366 | 0.42 | 0.91 | 0.51 | SNP in the promoter region | [20] |
| NER | XPD/ERCC2 D312N | rs1799793 | 0.31 | 0 | 0.06 | Affect NER activity by plasmid-based NER assay | [17] |
| NER | XPD/ERCC2 K751Q | rs1052559 | 0.27 | 0.04 | 0.17 | Higher number of chromatid aberrations. | [84] |
| No association in SCE frequency or DNA adduct level. | [85] | ||||||
| Higher adduct level among never smokers. | [65] | ||||||
| NER | XPF/ERCC4 R415Q | rs1800067 | 0.05 | 0 | 0 | Reduced repair of aromatic DNA adducts | [83] |
| NER | XPG/ERCC5 D1104H | rs17655 | 0.27 | 0.44 | 0.46 | Affect NER activity by plasmid-based NER assay | [17] |
| BER | XRCC1 R194W | rs1799782 | 0.05 | 0.24 | 0.08 | No association with DNA-adduct levels, mutation rates, or sensitivity to ionizing radiation | [66–68, 70] |
| Lower bleomycin and benzo(a)pyrene diol epoxide sensitivity in vitro. | [64] | ||||||
| BER | XRCC1 R280H | rs25489 | 0.03 | 0.08 | 0.06 | Higher bleomycin sensitivity | [71, 72] |
| BER | XRCC1 R399Q | rs25487 | 0.47 | 0.45 | 0.10 | Higher levels of aflatoxin B1-DNA adducts and higher bleomycin sensitivity | [68, 70, 107] |
| DSBR | XRCC3 T241M | rs861539 | 0.42 | 0.15 | 0.24 | Hypersensitive to DNA damaging agents | [89] |
1. Base excision repair (BER) pathway
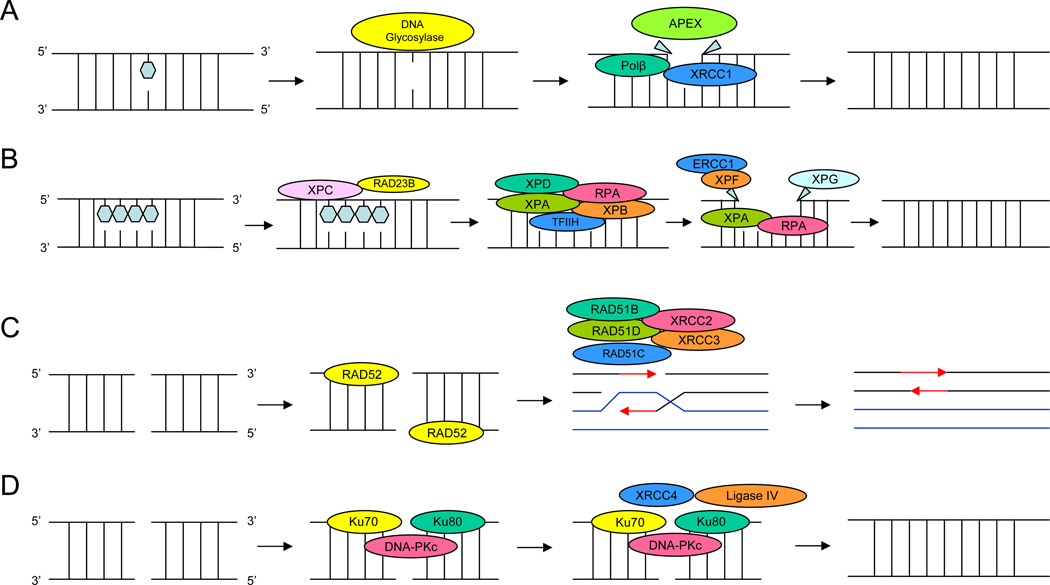
Base excision repair pathway targets DNA damaged during replication or by environmental agents. Repair of DNA mutations is necessary so that sequence errors are not transmitted to daughter cells. The single damaged base in DNA caused by endogenous metabolism or environmental oxidizing agents result in DNA adducts. This damage has been proposed to play a critical role in carcinogenesis in prostate tissue. Base excision repair involves removing the mutated base out of the DNA and repairing the base alone (Figure 1A).
Figure 1.
1A: Base excision repair (BER) pathway targets DNA damaged during replication or by environmental agents. The single damaged base in DNA caused by endogenous metabolism or environmental oxidizing agents result in DNA adducts. Base excision repair involves removing the mutated base out of the DNA and repairing the base alone. 1B: Nucleotide excision repair (NER) is associated with the repair of bulky adducts induced by several suspected environmental prostate cancer carcinogens. The NER pathway is a complex biochemical process that requires at least four steps: (a) damage recognition by a complex of bound proteins including xeroderma pigmentosum complementation group C (XPC), XPA, and replication protein A (RPA); (b) unwinding of the DNA by the transcription factor IIH (TFIIH) complex that includes XPD(ERCC2); (c) removal of the damaged single-stranded fragment (usually about 27–30 bp) by molecules including an ERCC1 and XPF complex and XPG; and (d) synthesis by DNA polymerases. 1C: Double-strand breaks are produced by replication failure or by DNA damaging agents. Two repair pathways exist to repair double strand breaks. The homologous recombination repair relies on DNA sequence complementarity between the intact chromatid and the damaged chromatid as the bases of stand exchange and repair. 1D: The non-homologous end-joining repair pathway requires direct DNA joining of the two double-strand-break ends.
Repair of a mutated base is primarily conducted by enzymes involved in BER with apurinic/apyrimidic (A/P) endonuclease (APE1), human 8-oxoguanine DNA glycosylase (hOGG1), DNA ligase, DNA polymerase delta (POLD1), X-ray repair cross-complementing group 1 (XRCC1), and poly (ADP-ribose) polymerase (ADPRT) [32–34].
1.a. human 8-oxoguanine DNA glycosylase (hOGG1)
The enzyme hOGG1 catalyzes the excision and removal of single base adducts [35, 36]. Base excision repair by hOGG1 enzyme leaves a single nucleotide space. This space is filled by DNA polymerase b, and the nick is sealed by the DNA ligase III/XRCC1 complex, which acts as a scaffold for interaction with other BER enzymes [37]. It is expressed as twelve alternatively-spliced isoforms with only the 1α-form containing a nuclear translocation signal [38]. Relatively high levels of expression of hOGG1 have been shown in several human tissues, including prostate [14, 21]. Public database (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=4968) lists 10 SNPs at the hOGG1 locus [39]. hOGG1 codon 326 polymorphism (rs1052123) in the 1α-specific exon 7 of the hOGG1 results in an amino acid substitution from serine to cysteine (Table 1). Results of studies for functional impact of the hOGG1 S326C polymorphism are inconsistent (Table 2). These studies used different measuring methods, HPLC, flow cytometry, and different specimens, such as cell lines, leukocytes, and tissues. No difference in catalytic activities was observed between the hOGG1 326C and hOGG1 326S alleles in several studies [40–46]. However, the hOGG1 encoded by the wild-type 326S allele exhibited higher DNA repair activity than the hOGG1 326C variant in other studies [38, 47–50].
The role of hOGG1 326 polymorphism in susceptibility to prostate cancer was assessed in four studies conducted in the US and Canada [14, 16, 18, 26]. The first was a population and family-based study that identified a significantly decreased risk associated with the hOGG1 326CC genotype [16]. This association was significant in non-familial prostate cancer patients, but not for familial prostate cancer. In contrast, the second, hospital-based study observed a positive relationship with prostate cancer risk [14]. The other two larger studies (996 and 439 cases) found no association between hOGG1 S326C polymorphism and prostate cancer risk [18, 26]. These inconsistent results could be explained by small sample sizes of first two studies (n=84 and 245 cases). Epidemiological studies of the hOGG1 S326C polymorphism with risk of other cancers show consistent evidence for an increased risk for esophageal [51], lung [52–59], nasopharyngeal [60], upper aero-digestive tract [60–62] and colon [63] cancers.
Xu et al. (2002) investigated other hOGG1 polymorphisms in addition to codon 326 polymorphism. Among 9 polymorphisms investigated in the hOGG1 promoter region, significantly increased risks were observed for homozygous polymorphic genotypes of two variants as compared with wild types (hOGG1 a7143g and a11657g) [16]. A larger study (n=996 cases) did not replicate the hOGG1 a11657g finding [26]. The difference of these two studies is sample sizes. The first study observed a significant result based upon 1 control and 11 patients with homozygous hOGG1 11657gg genotype.
1.b. X-ray repair cross-complementing group 1 (XRCC1)
After base excision by hOGG1 enzyme, the XRCC1/DNA ligase III complex seals the space [37]. Although 32 SNPs in XRCC1 have been reported [39], only three SNPs have been investigated as potential prostate cancer risk factors [R194W (rs17997820), R280H (rs25489) and R399Q (rs25487)]. The functional significance of the XRCC1 194W allele is somewhat controversial. One study reported lower bleomycin and benzo(a)pyrene diol epoxide sensitivity in vitro [64], but these results were not confirmed by other investigators [65–68] (Table 2). However, the XRCC1 R194W polymorphism may have detectable effect on DNA-adduct levels, mutation rates, or sensitivity to ionizing radiation [66, 67, 69, 70]. The functional significance of codon R280H polymorphism is not yet well-established; however, the codon 280 amino acid is located in the proliferating cell nuclear antigen binding region which has been associated with higher bleomycin sensitivity [71, 72]. The XRCC1 399Q allele has been associated with higher levels of aflatoxin B1-DNA adducts and higher bleomycin sensitivity [64, 69, 70].
Consistent with the functional data, the XRCC1 R194W does not appear to influence risk in two small studies (n=76 and 165 cases) [10, 15]. However, a recent study suggested 194W allele provides a protective effect [31]. The XRCC1 R280H polymorphism which has been evaluated in two small studies, can not reach any conclusion on effect with prostate cancer [15, 31].
The XRCC1 R399Q polymorphism has been the most frequently investigated of the BER genes. Recently, Chen et al. (2006) reported a significant association between XRCC1 R399Q polymorphism and prostate cancer risk among Caucasians, but not in African Americans [11]. Two studies in China observed a significantly increased risk with the XRCC1 R399Q polymorphism [12, 31]. The largest study to date [13] and three smaller studies [10, 15, 27] did not replicate the positive association. However, two small studies reported a significantly higher risk among men with the XRCC1 399 QQ genotype and low vitamin E intake/antioxidants [15, 27].
In summary, the XRCC1 R399Q polymorphism has been associated with risk in 5 of 7 studies, but only among men with low antioxidant, vitamin intake in two of five studies. Additional studies are needed to clarify these potential associations.
1.c. Apurinic/apyrimidic (A/P) endonuclease (APE1)
When BER enzymes initiate repair, the phosphodiester bond at the 5’ side of the intact apurinic/apyrimidinic site is incised by APE1, which is the rate-limiting enzyme. Six polymorphisms in APE1 have been reported, including relatively common alleles at codons Q51H (rs1048945) and D148E (rs3136820) [39]. Although the functional significance of APE1 51H allele has not been reported, it is conserved in most mammals and located in the Ref1 domain, which is essential for redox regulation of DNA binding proteins, such as p53 [73]. Therefore, APE1 Q51H polymorphism may affect the ability of APE1 to regulate DNA binding activity. The APE1 D148E polymorphism is associated with mitotic delay of lymphocytes from healthy subjects, implying higher sensitivity to ionizing radiation [66]. However, this variant was predicted as no impact on endonuclease and DNA binding activity in in vitro functional analysis [73],[74].
Both APE1 Q51H and D148E polymorphisms have been examined in a hospital based study of Caucasians and African Americans. No associations between these polymorphisms and prostate cancer risk were observed [11].
1.d. Poly (ADP-ribose) polymerase (ADPRT)
ADPRT is involved in DNA-damage signaling, genomic stability of damaged cells, BER, recombination and the transcriptional regulation of tumor suppressor genes [75, 76]. ADPRT recognizes and binds DNA damage, recruits other DNA-repair enzymes to the site of damage, and provides support for ligation [77]. Twenty-five polymorphisms in ADPRT have been reported, including the relatively common ADPRT V762A (rs1136410) [39].
The change from valine to alanine moves the codon 762 residue further away from the codon 888G residue, which is a part of the active site [77]. Locket et al. (2004) observed that ADPRT V762A is significantly associated with prostate cancer risk and decreased enzyme function in response to oxidative damage [28].
2. Nucleotide excision repair (NER) pathway
NER is associated with the repair of bulky adducts [78, 79] induced by several suspected environmental prostate cancer carcinogens, such as PAHs, heterocyclic aromatic amines from well-done meats, and pesticides. The NER pathway is a complex biochemical process that requires 20–25 enzymes and at least four steps: (a) damage recognition by a complex of bound proteins including xeroderma pigmentosum complementation group C (XPC), XPA, and replication protein A (RPA); (b) unwinding of the DNA by the transcription factor IIH (TFIIH) complex that includes XPD(ERCC2); (c) removal of the damaged single-stranded fragment (usually about 27–30 bp) by molecules including an ERCC1 and XPF complex and XPG; and (d) synthesis by DNA polymerases [6](Figure 1B).
2.a. Xeroderma pigmentosum complementation group D (XPD)
The XPD (ERCC2) gene product is a subunit of TFIIH (DNA helicase), promotes bubble formation, and is necessary for NER and transcription. Fourteen polymorphisms in XPD have been reported [39], including common alleles at codons D312N (rs1799793) and K751Q (rs1052559). Several studies reported that subjects with wild-type genotypes for XPD K751Q and D312N polymorphisms exhibit the highest NER activity, while homozygous variant genotypes of either polymorphism show low NER activity [80, 81]. Hou et al. (2002) reported that the XPD 312N allele have reduced capacity to repair aromatic DNA adducts [82, 83]. Lunn et al. (2000) reported that XPD K751Q was associated with higher levels of chromatid aberrations in white blood cells [84]. Conversely, Duell et al. (2000) [85] evaluated phenotypic effects of codons 312 and 751 polymorphisms by measuring two markers of DNA damage, sister chromatid exchange (SCE) frequencies and polyphenol DNA adducts. Both polymorphisms were unrelated to SCE frequency or DNA adduct level [85].
A potential role of XPD codons D312N and K751Q with prostate cancer risk has been investigated in four studies [12, 13, 17, 20]. All four studies observed no association between XPD K751Q polymorphism and prostate cancer risk in US, Taiwanese and Chinese populations. Rybicki et al. (2004) [13] and Bau et al. (2007) [20] observed a significant risk increase with the D312N polymorphism. However, this was not replicated by Lockett et al. [17] after adjustment for age, BPH, family history and smoking. Bau et al. (2007) reported that no a significant difference in the frequency of the XPD promoter -114 polymorphism between cases and controls
2.b. Xeroderma pigmentosum complementation group F (XPF)/(ERCC4)
XPF is a key enzyme responsible for excising bulky adducts from damaged DNA. XPF interacts with the ERCC1 together to form a complex which is required to repair DNA interstrand cross-linking damage [86]. Ten polymorphisms in XPF have been reported, [39] but only R415Q (rs1800067) has been studied in an epidemiological investigation. Lockett et al. (2005) reported that XPF R415Q polymorphism was associated with a moderate, near significant increase in prostate cancer risk (OR=1.4) [17].
2.c. Xeroderma pigmentosum complementation group G (XPG)/ (ERCC5)
XPG is responsible for a structure-specific endonuclease activity that is essential for the two incision steps in NER [86]. The XPG enzyme has been suggested to act on the single-stranded region created as a result of the combined action of the XPB helicase and the XPD helicase at the DNA damage site. XPG incises the 3’ side of damaged DNA before the 5’ incision made by XPF-ERCC1 complexes. XPG has a structural function in the complex of the DNA-hR23B. Twelve SNPs were reported including XPG D1104H (rs17655) [39]. The functional effects of D1104H SNP are still unknown, but the lack of association with prostate cancer risk [17] decreases the incentives to pursue small studies.
2.d. Xeroderma pigmentosum complementation group C (XPC)
In the early steps of the NER process, the XPC-hR23B protein complex has a structure-specific affinity for certain defined lesions. Thus, this complex can bind damaged DNA and change the DNA conformation around the lesion. DNA damage recognition is carried out by the XPC-hR23B protein complex [87], followed by recruitment of the transcription factor IIH (TFIIH) complex. Among twenty known SNPs [39], two common polymorphisms at codons A499V (rs2228000) and K939Q (rs2228001) have been investigated. There are no published data on their potential functional significance. Lockett et al. (2005) observed no significant association between these polymorphisms and prostate cancer risk [17]. However, a small study (n=165 cases) observed a significant 2.5 fold risk increase among Japanese men with the 939K allele [10].
2.e. human homolog RAD23B (hR23B)
The hR23B enzyme, which is the human homolog of the yeast NER protein RAD23, forms a complex with XPC. The XPC-hR23B-TFIIH complex unwinds the DNA duplex around the damaged site. Five SNPs have been reported [39], but only the A249V has been investigated. Lockett et al. (2005) found no association between this SNP and prostate cancer risk [17], and the absence of functional data tempers interest.
3. Double-strand break repair pathway
Double-strand breaks are produced by replication failure or by DNA damaging agents such as ionizing radiation. Two repair pathways exist to repair double strand breaks: (a) the homologous recombination repair relies on DNA sequence complementarity between the intact chromatid and the damaged chromatid as the bases of stand exchange and repair (Figure 1C); (b) the non-homologous end-joining repair pathway requires direct DNA joining of the two double-strand-break ends [88] (Figure 1D).
3.a. Xeroderma pigmentosum complementation group 3 (XRCC3)
XRCC3 is involved in homologous recombination repair process and at least 6 SNPs have been identified [39]. Araujo et al. (2002) reported that the variant XRCC3 enzyme (T241M) was functionally active for homology-directed repair (HDR) determined by a quantitative fluorescence assay. They also found that cells expressing this variant have been found to be no more sensitive to DNA damaging agents than cells expressing the wild-type enzyme [89].
XRCC3 T241M polymorphism has been analyzed in relation to prostate cancer risk in a population-based study in China [12]. This relatively small study detected no statistically significant association between XRCC3 T241M polymorphism and prostate cancer risk, but homozygous carriers deserve further study. Relative to men with the TT genotype and a low intake of preserved foods, those with the MT+MM genotype and having a higher intake of nitrosamines and nitrosamine precursors, had a significantly higher risk of prostate cancer (OR=2.6; 95% CI=1.1–6.1). In contrast, men with the MT+MM genotype and a low intake of preserved foods had a significant reduction in risk (OR=0.3; 95% CI=0.1–0.96). These data suggest that diet factors, such as preserved foods, may influence prostate cancer risk in combination with genetic susceptibility in DNA repair pathways.
3.b. Xeroderma pigmentosum complementation group 7 (XRCC7)
XRCC7/PRKDC (protein kinase, DNA-activated, catalytic polypeptide) is a key enzyme that becomes activated upon incubation with DNA. Genetic defects in this enzyme result in immunodeficiency, radiosensitivity, and premature aging [90, 91]. These phenotypes are due to the defect of DNA double strand breaks repair processes. Recent studies reveal that XRCC7 also participates in signal transduction cascades related to apoptotic cell death, telomere maintenance and other pathways of genome surveillance [92]. Only one epidemiological study has been reported, one of the 9 known SNPs [39], only g6721t polymorphism located intron 8 was investigated. No significant association between XRCC7 g6721t polymorphism and prostate cancer risk was observed in this small hospital-based study of Japanese man [10]. The functional significance of the XRCC7 g6721t polymorphism is not firmly established, but it may regulate splicing and cause mRNA instability [93].
3.c. Nijmegen breakage syndrome1 (NBS1)
The Nijmegen breakage syndrome 1 (NBS1) is part of a protein complex that forms in response to DNA damage to maintain chromosomal integrity. The exact role of NBS1 in DNA repair is not fully understood because NBS1 does not have a DNA binding site or kinase activity, which is usually required in DNA repair. However, the N-terminal domain binds to γH2AX, and this may be an important step to recruit the NBS1 protein complex to the proximity of DNA repair [94]. Thirty-eight polymorphisms in NBS1 have been reported, including codon E185Q (rs1805794) [39]. Although there is no information regarding changes in the activity of the NBS1-185Q variant, the region between amino acid 108–196 of the NBS1 enzyme constitutes a BRCA1 COOH-terminus domain that is presumably involved in cell-cycle checkpoints or in DNA repair [95]. In this same report, all individuals with the NBS1 185QQ genotype had lung tumors with p53 mutations in contrast with only 46% of p53 mutations in tumors from individuals with 185EE genotype [95]. In the only study of this variant in relation to prostate cancer, Hebbring et al. (2005) observed that NBS1 E185Q polymorphism was not strongly associated with familial or sporadic prostate cancer risk [29].
4. Direct Reversal (DR) pathway
The biologically significant DNA lesions produced by both carcinogenic and chemotherapeutic alkylating agents are O6-alkylguanine adducts, which can pair with thymine instead of cytosine during DNA replication. Therefore, O6-alkylguanine adducts may be responsible for the increase in the frequency of mutations following exposure to alkylating agents, and carcinogenesis [96].
4.a. Methylguanine-DNA methyltransferase (MGMT)
The only known enzyme in the DR pathway is methylguanine-DNA methyltransferase (MGMT). MGMT transfers the alkyl group at the O6 position of guanine to a cysteine residue within its active site, leading to the direct restoration of the natural chemical composition of DNA without the need for genomic reconstruction. Defective MGMT activity often increases mutation because O6-MeG mispairs with thymine during DNA replication [88].
Among 16 SNPs in MGMT [39], the functional effects of two common SNPs (L84F and I143V) have been examined [12]. Although L84F polymorphism did not affect cell survival after exposure to N-methyl-N-nitro-N-nitrosoguanidine [97], MGMT 143V allele was significantly more resistant to inactivation by MGMT pseudosubstrate, O6-(4-bromothenyl)guanine [98]. However, Liu et al (2003) reported that the relative gene expression level, evaluated by the real-time reverse transcription-PCR assay of MGMT in peripheral lymphocytes, was not significantly different between in prostate cancer patients and age- and ethnicity-matched controls [21]. Further, this I143V change may affect the isoleucine residue close to the alkyl acceptor cysteine residue at codon 145 [96].
Ritchey et al. (2005) examined MGMT L84F and I143V polymorphisms in a population-based case-control study of Chinese (162 cases, 251 controls). The MGMT L84F polymorphism was significantly associated with a 2 fold increased risk, but the I143V polymorphism was not [12].
5. Damage recognition cell cycle delay responses
Minimizing transmission of DNA mutation to daughter cells is biologically important. Therefore, some enzymes can recognize DNA damage and signal the status to initiate DNA replication [88]. DNA damage activates a cell cycle delay response pathway to earn time for damage repair [99]. Defects in this pathway may result in genomic instability, ultimately leading to cancer susceptibility. The key enzyme of this damage recognition cell delay response pathway is the ataxia telangiectasia-mutated (ATM) and the tumor suppressor protein p53.
5.a. Ataxia telangiectasia mutated protein (ATM)
ATM, which is the product of the gene mutated in patients with the autosomal recessive disorder ataxia telangiectasia, is one of key enzymes responsible for downstream signaling. ATM is activated by DNA damage and induces the trans-activation of various proteins involved in cell cycle arrest, apoptosis, DNA repair and centrosome duplication. In particular, ATM regulates phosphorylation of p53 protein, thereby allowing p53 to accumulate. ATM also regulates a wide variety of downstream proteins, including the tumor suppressor BRCA1, checkpoint kinase CHK2, checkpoint protein RAD50 and DNA repair protein NBS1 [100]. Nine polymorphisms in ATM have been reported [39]. Angele et al. (2004) investigated the association of 5 SNPs in ATM (D1853N, D1853V, ivs38-8t>c, ivs38-15g>c and P1054R) with prostate cancer risk [30]. The ATM P1054R variant is located in the beta-adaptin domain of the ATM protein and has been suggested to be linked to an increased cancer risk, particularly breast cancer [101, 102]. Only ATM 1054R allele was significantly associated with an increased risk of prostate cancer [30]. Further, in the same study, a lymphoblastoid cell line carrying P1054R polymorphism shows a significantly different cell progression to that seen in cell lines carry a wild type ATM after exposure to ionizing radiation. These results suggest that codon 1054 polymorphism confers an altered cellular phenotype and might be associated with prostate cancer risk.
6. Oligogenic Model
Results of epidemiological studies have been inconsistent. Although the exact basis for the inconsistency is unknown, a number of factors may be relevant, including various study design limitations (e.g., using mixed ethnic groups, polymorphisms with unknown functional effects, enzymes not expressed in target tissues, and use of prevalent cases), competing or overlapping DNA repair pathways, and grouping of genotypes, small sample sizes, or variations in allelic frequencies across populations. Many of the studies used convenience samples of cases and controls. However, one of main potential reasons is investigating only one SNP and one gene from a complex metabolic pathway.
Due to recent advance in high-throughput genotyping techniques, multiple polymorphisms within genes, multiple genes in the same pathway, and haplotype approaches are now available to greatly increase the depth of exploration. Although several studies analyze multiple SNPs within a gene, only two studies used a haplotype analysis [10, 11]. A few studies also analyzed multiple genes in the DNA repair pathway. This approach may provide more biologically plausible insight into the studied associations, including interaction effects of different alleles on prostate cancer risk.
When prostate cancer risk for combined effects of multiple polymorphisms in different DNA repair genes were estimated, we often find significant associations. Rybicki et al. (2004) reported that the OR for the combined effects of the XPD 312 DD and XRCC1 399 QQ genotype was 4.8 compared with XPD 312 DN/NN and XRCC1 RR/RQ genotypes [13]. In a separate study, similar combined effects were observed in individuals with APE1 D148E/XRCC1 R399Q polymorphisms. The OR for the combined effects of the APE1 51QQ and XRCC1 399RQ/QQ genotypes was 4.0 compared with APE1 QH/HH and XRCC1 399RR [11]. Recently, Hirata et al. (2007) reported that significant combined effects of SNPs in XPC and XRCC1 when two genes from different DNA repair pathway, were observed [10].
These combined effect with multiple SNPs and different genes suggest that severely defected DNA repair capacity may play a role in prostate cancer risk, particularly when the function of multiple DNA repair genes are compromised.
DISCUSSION
Fifteen published epidemiological studies have presented the association of 31 SNPs in 14 DNA repair genes with prostate cancer risk. Although more studies are warranted, the only pathway that shows significant associations is BER. The XRCC1 399Q allele is associated with increased risk for carriers alone or when the variant allele is combined with other DNA repair polymorphisms or low antioxidant diet [10–13, 15, 27]. Lockett et al. (2004) reported that ADPRT V762A variant contributed to prostate cancer risk and altered enzyme activity [28]. The hOGG1 S326C polymorphism needs additional studies. Particularly, results from epidemiological studies of other cancer sites show a consistent relation with increased risk [51–63].
SNPs in two NER genes, XPC and XPD, show significant associations with prostate cancer risk in some [10, 13], but not all studies [17]. Finally, a study from ataxia telangiectasia mutated protein (ATM) show a promising result [30].
Epidemiological studies of SNPs in DNA repair genes may inform individual susceptibility and provide insight on potential mechanisms of carcinogenesis. The current challenge is to validate the functional impact of important SNPs identified by epidemiological studies. Another challenge is to identify “causal SNPs” through epidemiological studies, especially in studies investigating the role of SNPs in complex prostate cancer. Results of many epidemiological studies are non-significant or border-line significant risk estimates. Most studies do not have enough power to investigate gene-gene and gene-environmental interactions. Studies investigating a single SNP in a DNA repair gene are not likely detecting difference of overall DNA repair activity. As we presented in the oligogenic model section, a large studies investigating multi-SNPs and multi-genes will generate significant data through combined genotype and haplotype analysis.
In the future, with a combination of relatively inexpensive high-throughput genotyping methods and more functional data will be available based on an individual’s genetic profile that affects the progression, metastasis, and response to therapy. The interpretation of epidemiological data and translation to patient care will be accelerated through pooled analysis and consortia.
References
- 1.American CS. In: Cancer Facts & Figures 2007. A.C. Society, editor. Atlanta, GA: 2007. [Google Scholar]
- 2.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85(1):60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6) Suppl 1:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Noble RL. The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer Res. 1977;37(6):1929–1933. [PubMed] [Google Scholar]
- 5.Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42(8):3232–3239. [PubMed] [Google Scholar]
- 6.Friedberg EC. How nucleotide excision repair protects against cancer. Nat Rev Cancer. 2001;1(1):22–33. doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- 7.Mullaart E, Lohman PH, Berends F, Vijg J. DNA damage metabolism and aging. Mutat Res. 1990;237(5–6):189–210. doi: 10.1016/0921-8734(90)90001-8. [DOI] [PubMed] [Google Scholar]
- 8.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291(5507):1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 9.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1513–1530. [PubMed] [Google Scholar]
- 10.Hirata H, et al. Polymorphisms of DNA repair genes are risk factors for prostate cancer. Eur J Cancer. 2007;43(2):231–237. doi: 10.1016/j.ejca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Ambrosone CB, Lee J, Sellers TA, Pow-Sang J, Park JY. Association between polymorphisms in the DNA repair genes XRCC1 and APE1, and the risk of prostate cancer in white and black Americans. J Urol. 2006;175(1):108–112. doi: 10.1016/S0022-5347(05)00042-X. discussion 112. [DOI] [PubMed] [Google Scholar]
- 12.Ritchey JD, et al. Genetic variants of DNA repair genes and prostate cancer: a population-based study. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1703–1709. doi: 10.1158/1055-9965.EPI-04-0809. [DOI] [PubMed] [Google Scholar]
- 13.Rybicki BA, Conti DV, Moreira A, Cicek M, Casey G, Witte JS. DNA repair gene XRCC1 and XPD polymorphisms and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(1):23–29. doi: 10.1158/1055-9965.epi-03-0053. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Elahi A, Pow-Sang J, Lazarus P, Park J. Association between polymorphism of human oxoguanine glycosylase 1 and risk of prostate cancer. J Urol. 2003;170(6 Pt 1):2471–2474. doi: 10.1097/01.ju.0000087498.23008.bb. [DOI] [PubMed] [Google Scholar]
- 15.van Gils CH, Bostick RM, Stern MC, Taylor JA. Differences in base excision repair capacity may modulate the effect of dietary antioxidant intake on prostate cancer risk: an example of polymorphisms in the XRCC1 gene. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1279–1284. [PubMed] [Google Scholar]
- 16.Xu J, et al. Associations between hOGG1 sequence variants and prostate cancer susceptibility. Cancer Res. 2002;62(8):2253–2257. [PubMed] [Google Scholar]
- 17.Lockett KL, Snowhite IV, Hu JJ. Nucleotide-excision repair and prostate cancer risk. Cancer Lett. 2005;220(2):125–135. doi: 10.1016/j.canlet.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Nock NL, et al. Polymorphisms in estrogen bioactivation, detoxification and oxidative DNA base excision repair genes and prostate cancer risk. Carcinogenesis. 2006;27(9):1842–1848. doi: 10.1093/carcin/bgl022. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, et al. Relationship between DNA repair gene XRCC1 Arg399Gln polymorphism and susceptibility to prostate cancer in the Han population in Jiangsu and Anhui. Zhonghua Nan Ke Xue. 2007;13(4):327–331. [PubMed] [Google Scholar]
- 20.Bau DT, et al. Association of XPD polymorphisms with prostate cancer in Taiwanese patients. Anticancer Res. 2007;27(4C):2893–2896. [PubMed] [Google Scholar]
- 21.Liu Z, et al. Overexpression of hMTH in peripheral lymphocytes and risk of prostate cancer: a case-control analysis. Mol Carcinog. 2003;36(3):123–129. doi: 10.1002/mc.10108. [DOI] [PubMed] [Google Scholar]
- 22.Strom SS, Spitz MR, Yamamura Y, Babaian RJ, Scardino PT, Wei Q. Reduced expression of hMSH2 and hMLH1 and risk of prostate cancer: a case-control study. Prostate. 2001;47(4):269–275. doi: 10.1002/pros.1071. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, et al. Alterations in PMS2, MSH2 and MLH1 expression in human prostate cancer. Int J Oncol. 2003;22(5):1033–1043. [PubMed] [Google Scholar]
- 24.Chen Y, et al. Defects of DNA mismatch repair in human prostate cancer. Cancer Res. 2001;61(10):4112–4121. [PubMed] [Google Scholar]
- 25.Hu JJ, et al. Deficient nucleotide excision repair capacity enhances human prostate cancer risk. Cancer Res. 2004;64(3):1197–1201. doi: 10.1158/0008-5472.can-03-2670. [DOI] [PubMed] [Google Scholar]
- 26.Nam RK, et al. The use of genetic markers to determine risk for prostate cancer at prostate biopsy. Clin Cancer Res. 2005;11(23):8391–8397. doi: 10.1158/1078-0432.CCR-05-1226. [DOI] [PubMed] [Google Scholar]
- 27.Goodman M, et al. Lycopene intake and prostate cancer risk: effect modification by plasma antioxidants and the XRCC1 genotype. Nutr Cancer. 2006;55(1):13–20. doi: 10.1207/s15327914nc5501_2. [DOI] [PubMed] [Google Scholar]
- 28.Lockett KL, et al. The ADPRT V762A genetic variant contributes to prostate cancer susceptibility and deficient enzyme function. Cancer Res. 2004;64(17):6344–6348. doi: 10.1158/0008-5472.CAN-04-0338. [DOI] [PubMed] [Google Scholar]
- 29.Hebbring SJ, et al. Role of the nijmegen breakage syndrome 1 gene in familial and sporadic prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(5):935–938. doi: 10.1158/1055-9965.EPI-05-0910. [DOI] [PubMed] [Google Scholar]
- 30.Angele S, et al. ATM polymorphisms as risk factors for prostate cancer development. Br J Cancer. 2004;91(4):783–787. doi: 10.1038/sj.bjc.6602007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, et al. Relationship between XRCC1 polymorphisms and susceptibility to prostate cancer in men from Han, Southern China. Asian J Androl. 2007;9(3):331–338. doi: 10.1111/j.1745-7262.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 32.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 33.Robson CN, Hickson ID. Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991;19(20):5519–5523. doi: 10.1093/nar/19.20.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson DM, 3rd, Barsky D. The major human abasic endonuclease: formation, consequences and repair of abasic lesions in DNA. Mutat Res. 2001;485(4):283–307. doi: 10.1016/s0921-8777(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 35.Boiteux S, Radicella JP. The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. Arch Biochem Biophys. 2000;377(1):1–8. doi: 10.1006/abbi.2000.1773. [DOI] [PubMed] [Google Scholar]
- 36.Sunaga N, et al. OGG1 protein suppresses G:C-->T:A mutation in a shuttle vector containing 8-hydroxyguanine in human cells. Carcinogenesis. 2001;22(9):1355–1362. doi: 10.1093/carcin/22.9.1355. [DOI] [PubMed] [Google Scholar]
- 37.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286(5446):1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 38.Kohno T, et al. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16(25):3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 39.NCBI. SNP500 Cancer. Cancer Genome Anatomy Project. 2006 p. http://snp500cancer.nci.nih.gov.
- 40.Shinmura K, Kohno T, Kasai H, Koda K, Sugimura H, Yokota J. Infrequent mutations of the hOGG1 gene, that is involved in the excision of 8-hydroxyguanine in damaged DNA, in human gastric cancer. Jpn J Cancer Res. 1998;89(8):825–828. doi: 10.1111/j.1349-7006.1998.tb00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen K, Schlink K, Gotte W, Hippler B, Kaina B, Oesch F. DNA repair activity of 8-oxoguanine DNA glycosylase 1 (OGG1) in human lymphocytes is not dependent on genetic polymorphism Ser326/Cys326. Mutat Res. 2001;486(3):207–216. doi: 10.1016/s0921-8777(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 42.Dherin C, Radicella JP, Dizdaroglu M, Boiteux S. Excision of oxidatively damaged DNA bases by the human alpha-hOgg1 protein and the polymorphic alpha-hOgg1(Ser326Cys) protein which is frequently found in human populations. Nucleic Acids Res. 1999;27(20):4001–4007. doi: 10.1093/nar/27.20.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardie LJ, et al. The effect of hOGG1 and glutathione peroxidase I genotypes and 3p chromosomal loss on 8-hydroxydeoxyguanosine levels in lung cancer. Carcinogenesis. 2000;21(2):167–172. doi: 10.1093/carcin/21.2.167. [DOI] [PubMed] [Google Scholar]
- 44.Park YJ, Choi EY, Choi JY, Park JG, You HJ, Chung MH. Genetic changes of hOGG1 and the activity of oh8Gua glycosylase in colon cancer. Eur J Cancer. 2001;37(3):340–346. doi: 10.1016/s0959-8049(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 45.Kondo S, et al. Overexpression of the hOGG1 gene and high 8-hydroxy-2'-deoxyguanosine (8-OHdG) lyase activity in human colorectal carcinoma: regulation mechanism of the 8-OHdG level in DNA. Clin Cancer Res. 2000;6(4):1394–1400. [PubMed] [Google Scholar]
- 46.Blons H, et al. Frequent allelic loss at chromosome 3p distinct from genetic alterations of the 8-oxoguanine DNA glycosylase 1 gene in head and neck cancer. Mol Carcinog. 1999;26(4):254–260. [PubMed] [Google Scholar]
- 47.Hu YC, Ahrendt SA. hOGG1 Ser326Cys polymorphism and G:C-to-T:A mutations: no evidence for a role in tobacco-related non small cell lung cancer. Int J Cancer. 2005;114(3):387–393. doi: 10.1002/ijc.20730. [DOI] [PubMed] [Google Scholar]
- 48.Tarng DC, Tsai TJ, Chen WT, Liu TY, Wei YH. Effect of human OGG1 1245C-->G gene polymorphism on 8-hydroxy-2'-deoxyguanosine levels of leukocyte DNA among patients undergoing chronic hemodialysis. J Am Soc Nephrol. 2001;12(11):2338–2347. doi: 10.1681/ASN.V12112338. [DOI] [PubMed] [Google Scholar]
- 49.Chen SK, et al. Age-associated decrease of oxidative repair enzymes, human 8-oxoguanine DNA glycosylases (hOgg1), in human aging. J Radiat Res (Tokyo) 2003;44(1):31–35. doi: 10.1269/jrr.44.31. [DOI] [PubMed] [Google Scholar]
- 50.Yamane A, et al. Differential ability of polymorphic OGG1 proteins to suppress mutagenesis induced by 8-hydroxyguanine in human cell in vivo. Carcinogenesis. 2004;25(9):1689–1694. doi: 10.1093/carcin/bgh166. [DOI] [PubMed] [Google Scholar]
- 51.Xing DY, Tan W, Song N, Lin DX. Ser326Cys polymorphism in hOGG1 gene and risk of esophageal cancer in a Chinese population. Int J Cancer. 2001;95(3):140–143. doi: 10.1002/1097-0215(20010520)95:3<140::aid-ijc1024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Sugimura H, et al. hOGG1 Ser326Cys polymorphism and lung cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1999;8(8):669–674. [PubMed] [Google Scholar]
- 53.Wikman H, et al. hOGG1 polymorphism and loss of heterozygosity (LOH): significance for lung cancer susceptibility in a caucasian population. Int J Cancer. 2000;88(6):932–927. doi: 10.1002/1097-0215(20001215)88:6<932::aid-ijc15>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 54.Ito H, et al. A limited association of OGG1 Ser326Cys polymorphism for adenocarcinoma of the lung. J Epidemiol. 2002;12(3):258–265. doi: 10.2188/jea.12.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Marchand L, Donlon T, Lum-Jones A, Seifried A, Wilkens LR. Association of the hOGG1 Ser326Cys polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(4):409–412. [PubMed] [Google Scholar]
- 56.Sunaga N, et al. Contribution of the NQO1 and GSTT1 polymorphisms to lung adenocarcinoma susceptibility. Cancer Epidemiol Biomarkers Prev. 2002;11(8):730–738. [PubMed] [Google Scholar]
- 57.Lan Q, et al. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25(11):2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- 58.Park J, Chen L, Tockman MS, Elahi A, Lazarus P. The human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) DNA repair enzyme and its association with lung cancer risk. Pharmacogenetics. 2004;14(2):103–109. doi: 10.1097/00008571-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Hung RJ, et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J Natl Cancer Inst. 2005;97(8):567–576. doi: 10.1093/jnci/dji101. [DOI] [PubMed] [Google Scholar]
- 60.Cho EY, et al. Nasopharyngeal carcinoma and genetic polymorphisms of DNA repair enzymes XRCC1 and hOGG1. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1100–1104. [PubMed] [Google Scholar]
- 61.Hao B, et al. Identification of genetic variants in base excision repair pathway and their associations with risk of esophageal squamous cell carcinoma. Cancer Res. 2004;64(12):4378–4384. doi: 10.1158/0008-5472.CAN-04-0372. [DOI] [PubMed] [Google Scholar]
- 62.Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis. 2002;23(7):1229–1234. doi: 10.1093/carcin/23.7.1229. [DOI] [PubMed] [Google Scholar]
- 63.Kim JI, et al. hOGG1 Ser326Cys polymorphism modifies the significance of the environmental risk factor for colon cancer. World J Gastroenterol. 2003;9(5):956–960. doi: 10.3748/wjg.v9.i5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Spitz MR, Zhu Y, Dong Q, Shete S, Wu X. From genotype to phenotype: correlating XRCC1 polymorphisms with mutagen sensitivity. DNA Repair (Amst) 2003;2(8):901–908. doi: 10.1016/s1568-7864(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 65.Matullo G, et al. DNA repair gene polymorphisms, bulky DNA adducts in white blood cells and bladder cancer in a case-control study. Int J Cancer. 2001;92(4):562–567. doi: 10.1002/ijc.1228. [DOI] [PubMed] [Google Scholar]
- 66.Hu JJ, Smith TR, Miller MS, Mohrenweiser HW, Golden A, Case LD. Amino acid substitution variants of APE1 and XRCC1 genes associated with ionizing radiation sensitivity. Carcinogenesis. 2001;22(6):917–922. doi: 10.1093/carcin/22.6.917. [DOI] [PubMed] [Google Scholar]
- 67.Hu JJ, Smith TR, Miller MS, Lohman K, Case LD. Genetic regulation of ionizing radiation sensitivity and breast cancer risk. Environ Mol Mutagen. 2002;39(2–3):208–215. doi: 10.1002/em.10058. [DOI] [PubMed] [Google Scholar]
- 68.Lunn RM, Bell DA, Mohler JL, Taylor JA. Prostate cancer risk and polymorphism in 17 hydroxylase (CYP17) and steroid reductase (SRD5A2) Carcinogenesis. 1999;20(9):1727–1731. doi: 10.1093/carcin/20.9.1727. [DOI] [PubMed] [Google Scholar]
- 69.Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA. XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res. 1999;59(11):2557–2561. [PubMed] [Google Scholar]
- 70.Matullo G, et al. XRCC1, XRCC3, XPD gene polymorphisms, smoking and (32)P-DNA adducts in a sample of healthy subjects. Carcinogenesis. 2001;22(9):1437–1445. doi: 10.1093/carcin/22.9.1437. [DOI] [PubMed] [Google Scholar]
- 71.Fan J, Otterlei M, Wong HK, Tomkinson AE, Wilson DM., 3rd XRCC1 co-localizes and physically interacts with PCNA. Nucleic Acids Res. 2004;32(7):2193–2201. doi: 10.1093/nar/gkh556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuimala J, Szekely G, Gundy S, Hirvonen A, Norppa H. Genetic polymorphisms of DNA repair and xenobiotic-metabolizing enzymes: role in mutagen sensitivity. Carcinogenesis. 2002;23(6):1003–1008. doi: 10.1093/carcin/23.6.1003. [DOI] [PubMed] [Google Scholar]
- 73.Xi T, Jones IM, Mohrenweiser HW. Many amino acid substitution variants identified in DNA repair genes during human population screenings are predicted to impact protein function. Genomics. 2004;83(6):970–979. doi: 10.1016/j.ygeno.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Hadi MZ, Coleman MA, Fidelis K, Mohrenweiser HW, Wilson DM., 3rd Functional characterization of Ape1 variants identified in the human population. Nucleic Acids Res. 2000;28(20):3871–3879. doi: 10.1093/nar/28.20.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dantzer F, et al. Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie. 1999;81(1–2):69–75. doi: 10.1016/s0300-9084(99)80040-6. [DOI] [PubMed] [Google Scholar]
- 76.Wieler S, Gagne JP, Vaziri H, Poirier GG, Benchimol S. Poly(ADP-ribose) polymerase-1 is a positive regulator of the p53-mediated G1 arrest response following ionizing radiation. J Biol Chem. 2003;278(21):18914–18921. doi: 10.1074/jbc.M211641200. [DOI] [PubMed] [Google Scholar]
- 77.Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994;14(1):68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sancar GB, Siede W, van Zeeland AA. Repair and processing of DNA damage: a summary of recent progress. Mutat Res. 1996;362(1):127–146. doi: 10.1016/0921-8777(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 79.Yu MW, et al. Polymorphisms in XRCC1 and glutathione S-transferase genes and hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2003;95(19):1485–1488. doi: 10.1093/jnci/djg051. [DOI] [PubMed] [Google Scholar]
- 80.Spitz MR, et al. Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer Res. 2001;61(4):1354–1357. [PubMed] [Google Scholar]
- 81.Baccarelli A, et al. XPD gene polymorphism and host characteristics in the association with cutaneous malignant melanoma risk. Br J Cancer. 2004;90(2):497–502. doi: 10.1038/sj.bjc.6601385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benhamou S, Sarasin A. ERCC2 /XPD gene polymorphisms and lung cancer: a HuGE review. Am J Epidemiol. 2005;161(1):1–14. doi: 10.1093/aje/kwi018. [DOI] [PubMed] [Google Scholar]
- 83.Hou SM, et al. The XPD variant alleles are associated with increased aromatic DNA adduct level and lung cancer risk. Carcinogenesis. 2002;23(4):599–603. doi: 10.1093/carcin/23.4.599. [DOI] [PubMed] [Google Scholar]
- 84.Lunn RM, et al. XPD polymorphisms: effects on DNA repair proficiency. Carcinogenesis. 2000;21(4):551–555. doi: 10.1093/carcin/21.4.551. [DOI] [PubMed] [Google Scholar]
- 85.Duell EJ, et al. Polymorphisms in the DNA repair genes XRCC1 and ERCC2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis. 2000;21(5):965–971. doi: 10.1093/carcin/21.5.965. [DOI] [PubMed] [Google Scholar]
- 86.Kiyohara C, Yoshimasu K. Genetic polymorphisms in the nucleotide excision repair pathway and lung cancer risk: A meta-analysis. Int J Med Sci. 2007;4(2):59–71. doi: 10.7150/ijms.4.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang WY, et al. Nucleotide excision repair gene polymorphisms and risk of advanced colorectal adenoma: XPC polymorphisms modify smoking-related risk. Cancer Epidemiol Biomarkers Prev. 2006;15(2):306–311. doi: 10.1158/1055-9965.EPI-05-0751. [DOI] [PubMed] [Google Scholar]
- 88.Mohrenweiser HW, Wilson DM, 3rd, Jones IM. Challenges and complexities in estimating both the functional impact and the disease risk associated with the extensive genetic variation in human DNA repair genes. Mutat Res. 2003;526(1–2):93–125. doi: 10.1016/s0027-5107(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 89.Araujo FD, Pierce AJ, Stark JM, Jasin M. Variant XRCC3 implicated in cancer is functional in homology-directed repair of double-strand breaks. Oncogene. 2002;21(26):4176–4180. doi: 10.1038/sj.onc.1205539. [DOI] [PubMed] [Google Scholar]
- 90.Nonoyama S, Ochs HD. Immune deficiency in SCID mice. Int Rev Immunol. 1996;13(4):289–300. doi: 10.3109/08830189609061753. [DOI] [PubMed] [Google Scholar]
- 91.Nicolas N, et al. A human severe combined immunodeficiency (SCID) condition with increased sensitivity to ionizing radiations and impaired V(D)J rearrangements defines a new DNA recombination/repair deficiency. J Exp Med. 1998;188(4):627–634. doi: 10.1084/jem.188.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dip R, Naegeli H. More than just strand breaks: the recognition of structural DNA discontinuities by DNA-dependent protein kinase catalytic subunit. Faseb J. 2005;19(7):704–715. doi: 10.1096/fj.04-3041rev. [DOI] [PubMed] [Google Scholar]
- 93.Sipley JD, Menninger JC, Hartley KO, Ward DC, Jackson SP, Anderson CW. Gene for the catalytic subunit of the human DNA-activated protein kinase maps to the site of the XRCC7 gene on chromosome 8. Proc Natl Acad Sci U S A. 1995;92(16):7515–7519. doi: 10.1073/pnas.92.16.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Zhou J, Lim CU. The role of NBS1 in DNA double strand break repair, telomere stability, and cell cycle checkpoint control. Cell Res. 2006;16(1):45–54. doi: 10.1038/sj.cr.7310007. [DOI] [PubMed] [Google Scholar]
- 95.Medina PP, Ahrendt SA, Pollan M, Fernandez P, Sidransky D, Sanchez-Cespedes M. Screening of homologous recombination gene polymorphisms in lung cancer patients reveals an association of the NBS1-185Gln variant and p53 gene mutations. Cancer Epidemiol Biomarkers Prev. 2003;12(8):699–704. [PubMed] [Google Scholar]
- 96.Margison GP, Povey AC, Kaina B, Santibanez Koref MF. Variability and regulation of O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 2003;24(4):625–635. doi: 10.1093/carcin/bgg005. [DOI] [PubMed] [Google Scholar]
- 97.Inoue R, Abe M, Nakabeppu Y, Sekiguchi M, Mori T, Suzuki T. Characterization of human polymorphic DNA repair methyltransferase. Pharmacogenetics. 2000;10(1):59–66. doi: 10.1097/00008571-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Margison GP, et al. Quantitative trait locus analysis reveals two intragenic sites that influence O6-alkylguanine-DNA alkyltransferase activity in peripheral blood mononuclear cells. Carcinogenesis. 2005;26(8):1473–1480. doi: 10.1093/carcin/bgi087. [DOI] [PubMed] [Google Scholar]
- 99.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97(19):10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JH, et al. Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet. 2006;15(7):1181–1186. doi: 10.1093/hmg/ddl033. [DOI] [PubMed] [Google Scholar]
- 101.Koren M, et al. ATM haplotypes and breast cancer risk in Jewish high-risk women. Br J Cancer. 2006;94(10):1537–1543. doi: 10.1038/sj.bjc.6603062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee KM, et al. Genetic polymorphisms of ataxia telangiectasia mutated and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14(4):821–825. doi: 10.1158/1055-9965.EPI-04-0330. [DOI] [PubMed] [Google Scholar]
- 103.Audebert M, Radicella JP, Dizdaroglu M. Effect of single mutations in the OGG1 gene found in human tumors on the substrate specificity of the Ogg1 protein. Nucleic Acids Res. 2000;28(14):2672–2678. doi: 10.1093/nar/28.14.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen C, et al. Endogenous sex hormones and prostate cancer risk: a case-control study nested within the Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1410–1416. [PubMed] [Google Scholar]
- 105.Peng T, et al. Oxidative DNA damage in peripheral leukocytes and its association with expression and polymorphisms of hOGG1: a study of adolescents in a high risk region for hepatocellular carcinoma in China. World J Gastroenterol. 2003;9(10):2186–2193. doi: 10.3748/wjg.v9.i10.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan SG, et al. A new xeroderma pigmentosum group C poly(AT) insertion/deletion polymorphism. Carcinogenesis. 2000;21(10):1821–1825. doi: 10.1093/carcin/21.10.1821. [DOI] [PubMed] [Google Scholar]
- 107.Wang CY, Jones RF, Debiec-Rychter M, Soos G, Haas GP. Correlation of the genotypes for N-acetyltransferases 1 and 2 with double bladder and prostate cancers in a case-comparison study. Anticancer Res. 2002;22(6B):3529–3535. [PubMed] [Google Scholar]