Abstract
The regulation of chlorophyll synthesis in Chlorella was examined at the level of the formation and metabolism of δ-aminolevulinic acid. δ-Aminolevulinic acid synthetase activity could not be detected in broken cell preparations, and exogenously supplied δ-aminolevulinic acid was taken up only in the presence of dimethylsulfoxide, with a corresponding production of porphobilinogen.
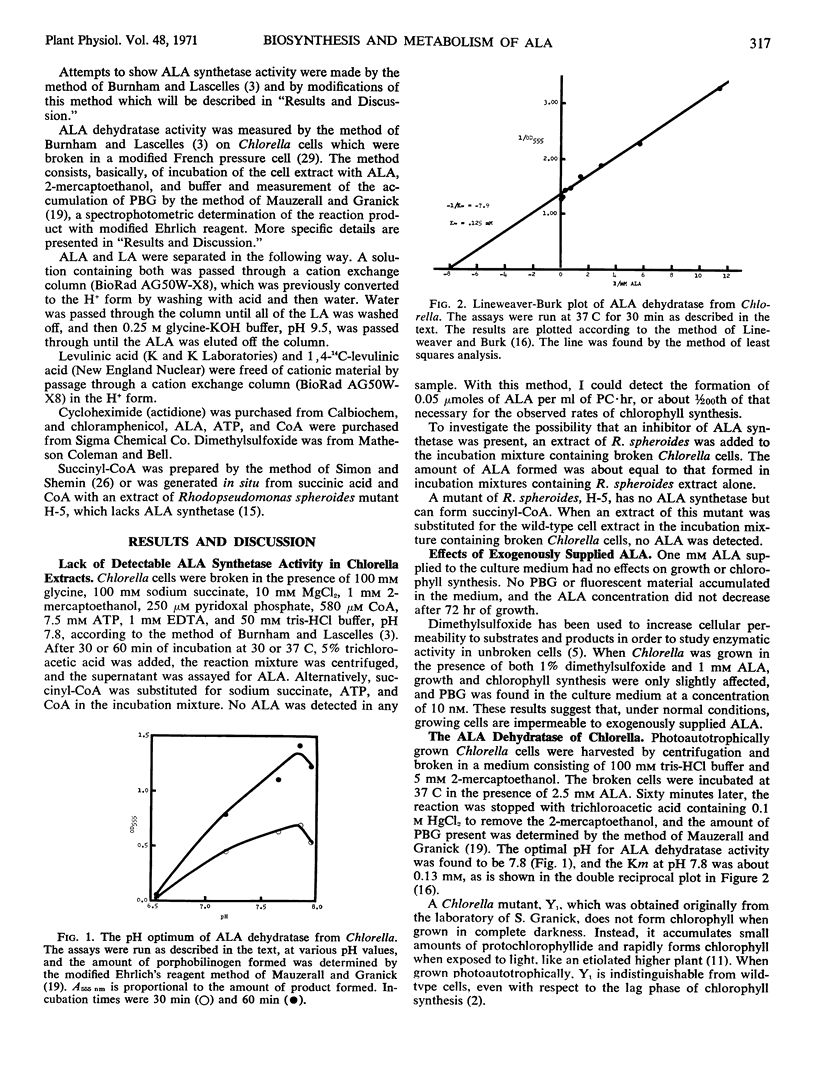
The δ-aminolevulinic acid dehydratase of Chlorella has a pH optimum of 7.8 and at this pH the Michaelis constant for δ-aminolevulinic acid is 0.13 millimolar.
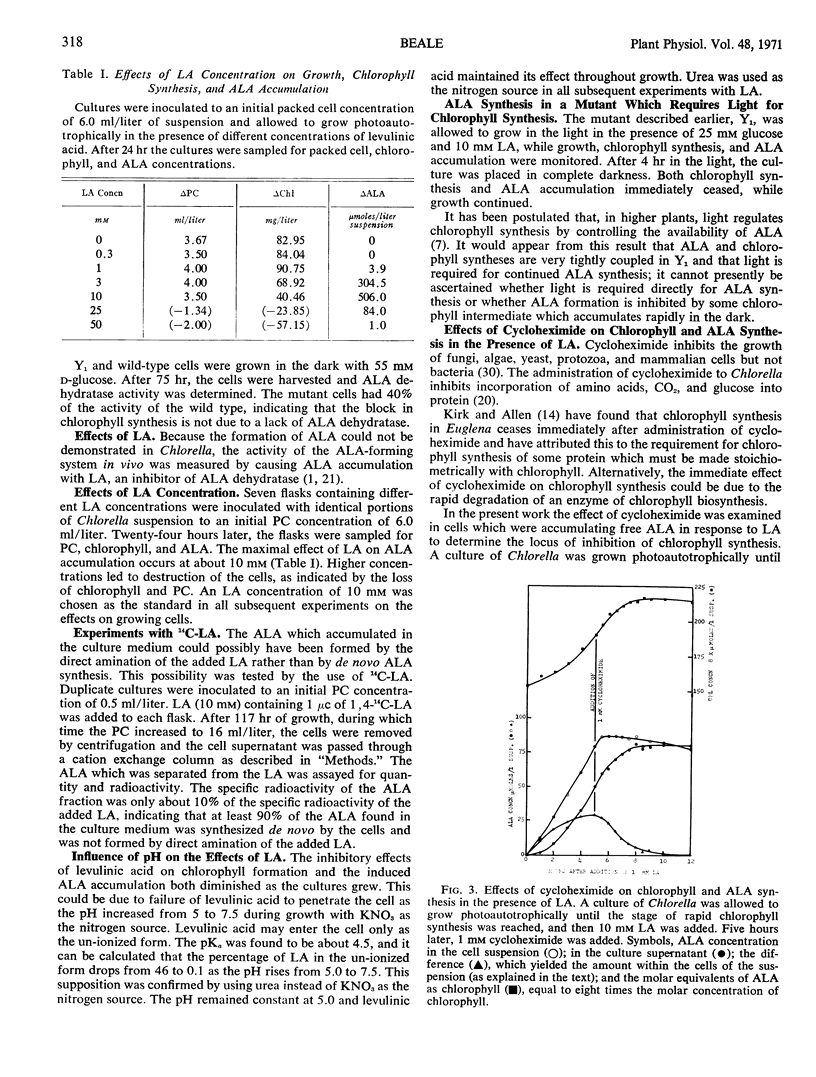
δ-Aminolevulinic acid excretion in the presence of levulinic acid, a competitive inhibitor of δ-aminolevulinic acid dehydratase, allowed measurement of the relative rates of δ-aminolevulinic acid synthesis under various growth conditions. A mutant which requires light for chlorophyll synthesis also requires light for δ-aminolevulinic acid accumulation in the presence of levulinic acid. This same mutant has 40% of the δ-aminolevulinic acid dehydratase activity of the wild-type Chlorella during growth in the dark on glucose.
The necessity for protein synthesis during chlorophyll synthesis is due primarily to the requirement for protein synthesis during δ-aminolevulinic acid formation.
It is concluded that the rate of chlorophyll formation and the cellular chlorophyll content are regulated by the relative rates of synthesis and breakdown of an enzyme responsible for δ-aminolevulinic acid biosynthesis and that this enzyme has an in vivo lifetime of about 30 minutes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Appleman D. Chlorophyll synthesis in chlorella: regulation by degree of light limitation of growth. Plant Physiol. 1971 Feb;47(2):230–235. doi: 10.1104/pp.47.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I. The biosynthesis of delta-aminolevulinic acid in Chlorella. Plant Physiol. 1970 Apr;45(4):504–506. doi: 10.1104/pp.45.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLA ROSA R. J., ALTMAN K. I., SALOMON K. The biosynthesis of chlorophyll as studied with labeled glycine and acetic acid. J Biol Chem. 1953 Jun;202(2):771–779. [PubMed] [Google Scholar]
- Delmer D. P., Mills S. E. A technique for the assay of enzymes in intact plant cells in the presence of dimethylsulfoxide. Plant Physiol. 1969 Jan;44(1):153–155. doi: 10.1104/pp.44.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., SCOTT J. J. The purification and properties of delta-aminolaevulic acid dehydrase. Biochem J. 1955 Dec;61(4):618–629. doi: 10.1042/bj0610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Enzymatic conversion of delta-amino levulinic acid to porphobilinogen. Science. 1954 Dec 31;120(3131):1105–1106. doi: 10.1126/science.120.3131.1105. [DOI] [PubMed] [Google Scholar]
- GRANICK S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1948 1949;Series 44:220–245. [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Control of chlorophyll production in rapidly greening bean leaves. Plant Physiol. 1967 Jun;42(6):774–780. doi: 10.1104/pp.42.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Pluscec J., Bogorad L. delta-Aminolevulinic Acid Transaminase in Chlorella vulgaris. Plant Physiol. 1968 Sep;43(9):1411–1414. doi: 10.1104/pp.43.9.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIKUCHI G., KUMAR A., TALMAGE P., SHEMIN D. The enzymatic synthesis of delta-aminolevulinic acid. J Biol Chem. 1958 Nov;233(5):1214–1219. [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Lascelles J., Altschuler T. Mutant strains of Rhodopseudomonas spheroides lacking delta-aminolevulinate synthase: growth, heme, and bacteriochlorophyll synthesis. J Bacteriol. 1969 May;98(2):721–727. doi: 10.1128/jb.98.2.721-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Nandi D. L., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. 3. Mechanism of porphobilinogen synthesis. J Biol Chem. 1968 Mar 25;243(6):1236–1242. [PubMed] [Google Scholar]
- ROBERTS D. W., PERKINS H. J. Chlorophyll biosynthesis and turnover in wheat leaves. Biochim Biophys Acta. 1962 Apr 23;58:499–506. doi: 10.1016/0006-3002(62)90060-4. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Perkins H. J. The incorporation of the two carbons of acetate and glycine into the phorbide and phytol moieties of chlorophylls a and b. Biochim Biophys Acta. 1966 Sep 26;127(1):42–46. doi: 10.1016/0304-4165(66)90473-9. [DOI] [PubMed] [Google Scholar]
- Shetty A. S., Miller G. W. Purification and general properties of delta-aminolaevulate dehydratase from Nicotiana tabacum L. Biochem J. 1969 Sep;114(2):331–337. doi: 10.1042/bj1140331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer B. T., Gibbs M. Delta-aminolevulnic Acid dehydrase in greening bean leaves. Plant Physiol. 1969 May;44(5):781–783. doi: 10.1104/pp.44.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigier H. A., Batlle A. M., Locascio G. A. Porphyrin biosynthesis in the soybean callus tissue system. II. Improved purification and some properties of delta amino-laevulic acid dehydratase. Enzymologia. 1970 Jan 30;38(1):43–56. [PubMed] [Google Scholar]
- Walters J. R., Stahly D. P. Modification of the valve of the French pressure cell. Appl Microbiol. 1968 Oct;16(10):1605–1605. doi: 10.1128/am.16.10.1605-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


