Abstract
Background:
Geohelminth infections are predominant in Nigeria and communities at greatest risks are those with poor environmental/sanitary conditions and unhygienic habits. Chemokine ligands (CXCL) a class under chemokine group play important roles in the immune system by either mediating susceptible or protective immune responses to parasitic infections.
Aim:
This study was to assess the impact of Ascaris lumbricoides sole infection and co-infection on some serum chemokines (CXCL5, CXCL9, and CXCL11) in infected Nigerians.
Materials and Methods:
A total of 194 individuals attending Agbor general hospital were examined for A. lumbricoides and hookworm infections. Thereafter, sera were obtained from positive volunteers and control group using enzyme-linked immunosorbent assay to examine the impact of these helminth infections on the serum concentration of some chemokines (CXCL5, CXCL9, and CXCL11).
Results:
The mean sera levels of CXCL5 and CXCL9 in infected volunteers were higher than the control subjects. Also, positive correlation was recorded for CXCL9 (P > 0.05), while negative responses were seen for CXCL5 and CXCL11 (P > 0.05) in relation to increase in the intensities of infections. CXCL9 was more expressed in A. lumbricoides + hookworm co-infections than single. Furthermore, the mean concentration of CXCL5 was higher in infected females than males (P < 0.05).
Conclusion:
The proinflammatory responses of CXCL5 and CXCL9 to A. lumbricoides and hookworm infections could be an indication of the meditating roles of these chemokines in the immune system to either confer some form of host/parasite immunity or susceptibility.
Keywords: A. lumbricoides, Chemokines, Co-infection, Hookworm, Nigeria, Single infection
Introduction
Geohelminths such as Ascaris lumbricoides and hookworms causes parasitic diseases among a large percentage of people in sub-Saharan Africa. The most vulnerable groups are school-aged children and pregnant women.[1,2] The disease burden and morbidity of soil transmitted helminths are influenced by a complexity of immune responses partly due to increased number of eosinophil cell population.[3] Eosinophils modulate the immune system by releasing cytokines and chemokines.[4] Chemokines may enhance, suppress, and regulate the expression of immunity to intravascular and intestinal parasites. However, varied data on the general role of eosinophils to helminth infections have been documented,[5,6] and consequently a likely disparity in chemokines responses may be observed.
Cytokines and chemokines are key players in the regulation and polarization of cellular reactivity and antibody responses to parasitic infections. Generally, prepatency of helminth infections are associated with an initial Th1 immune response, while during patency a Th2-type response prevails,[7,8] and further predominates with chronic infections.[9] In areas where polyparasitism is highly prevalent, the impact of multiple parasites on host responses is underestimated.[10] For instance, in a study comparing immunological responses in individuals with mono- and co- helminth infections, it was noted that the results were in contrast with studies on animal models,[11,12] in a way that coinfected individuals had a further down-modulated Th1 cytokine [interferon- γ (IFN-γ)] and CXCL10 with an increase in humoral responses.[13] It has also been suggested that there is an important influence of host sex on the development of Th2 responses to gastrointestinal nematodes.[14] Thus, this work highlights the role of sex on chemokines host responses to A. lumbricoides infection.
We had earlier carried out few immunological studies to assess cytokines host responses to some parasitic infections in Nigeria.[15,16] However, information on the role of chemokines in helminth infection is currently lacking in endemic areas despite its importance in disease progression. This paper for the first time examines the impact of A. lumbricoides sole infection and co-infection with hookworm on some serum chemokines (CXCL5, CXCL9, and CXCL11) in infected Nigerians.
Materials and Methods
Study area
The study was carried out in Agbor (6°15'9.93“N 6°11'58.79”E) in Delta State, Nigeria. Stool and serum samples were collected from 194 individuals attending the Agbor general hospital either for routine check-up or otherwise. The age of these volunteers ranged between 8 and 50 years. There are some villages around the main Agbor town, where the Agbor general hospital is located and so people come from these surrounding communities to seek medical attention. On the average, the toilet facilities used by inhabitants are the water systems and pit toilets. However, some still defecate in bushes and farms and most children indiscriminately defecate in the environment. Again, waste management system to a large extent is poor in Agbor. For instance, in an effort to dispose of wastes, a good number of people construct pits and bury these wastes therein; and sometimes there is unchecked indiscriminate disposal of wastes in surrounding bushes. The inhabitants also depend on well water, borehole, rain water, streams, and rivers for their sources of drinking water. The major preoccupation of the people is farming, while a few are civil servants and traders.
Ethical consideration
Before the commencement of the screening exercise, we obtained ethical clearance from the Delta State Ministry of Health, Asaba. Also, informed consents of individuals were sought and obtained and then drawn to participate in the survey. For volunteers between the ages of 8 and 12 years, parental informed consents were equally obtained.
Parasite investigation
Each subject was given a clean, dry, and well labelled specimen bottle with which to collect stool samples. Stool samples were collected from 194 individuals and immediately processed. Fresh stool samples mixed with saline were dispersed on slides and examined under the microscope for parasite detection and identification. A. lumbricoides and hookworm eggs were quantified using Kato-Katz technique. The degrees of infections were measured as number of eggs per gram (epg). The intensities of infection for A. lumbricoides were estimated as light (1-4999), moderate (5000-49999), and heavy (≥50000); while for hookworm, they were similarly estimated as light (1-1999); moderate (2000-3999), and heavy (≥4000).[17]
Exclusion criteria and chemokines analysis
We excluded volunteers with overt diseases like viral hepatitis B, human immunodeficiency virus, or sickle cell anaemia using standard procedures. Also, volunteers with other parasitic infections like malaria were not included for the chemokines analysis. Sera from the positive volunteers and 20 control subjects drawn from the 194 surveyed volunteers were analysed using enzyme-linked immunosorbent assay for CXCL5, CXCL9, and CXCL11 following the manufacturer's instruction (Abcam, UK). Results were expressed in pg/mL on the basis of standard curves.
Statistical analysis
Data obtained were subjected to linear regression, student's t-test, and analysis of variance analysis using InStat statistical package.
Results
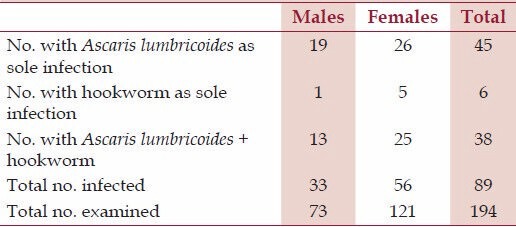
A total number of 194 patients were examined and 89 volunteers were infected with A. lumbricoides or/and hookworm infections [Table 1]. Others were either completely free of these parasites or were positive with other infections.
Table 1.
Number of positive volunteers with helminth infection(s)
The concentrations of chemokines to A. lumbricoides infection are shown in Table 2. The levels of CXCL5 and CXCL9 in infected volunteers were higher than their control subjects at P < 0.05 and P < 0.001, respectively. However, the levels of CXCL11 for the two groups were unchanged statistically, an indication of little or no impact of infection on this particular chemokine.
Table 2.
Comparison of chemokines responses to Ascaris. lumbricoides-infected and noninfected volunteers
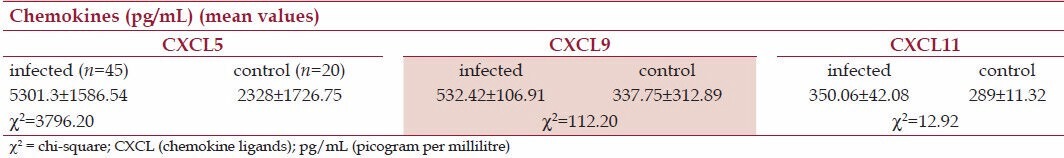
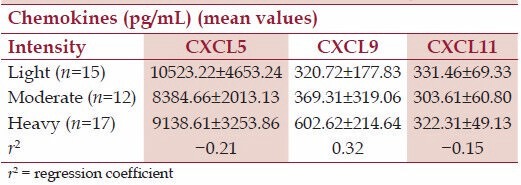
Table 3 presents the correlation between chemokines and the intensities of A. lumbricoides infection. The concentration of CXCL9 in relation to the intensities of infection was positively correlated though not statistically significantly (P > 0.05). Conversely, for CXCL5 and CXCL11, a negative correlation was observed (P > 0.05).
Table 3.
Relationship between intensities of Ascaris lumbricoides infection and chemokines responses
The comparison in the profile of chemokines levels between single and co-infections is shown in Table 4. Single infection with A. lumbricoides was higher than A. lumbricoides + hookworm co-infection for CXCL9 (P < 0.05), but no significant change was seen for CXCL5 and CXCL11 (P > 0.05).
Table 4.
Chemokines profiles of Ascaris lumbricoides single infection and co-infection with hookworm
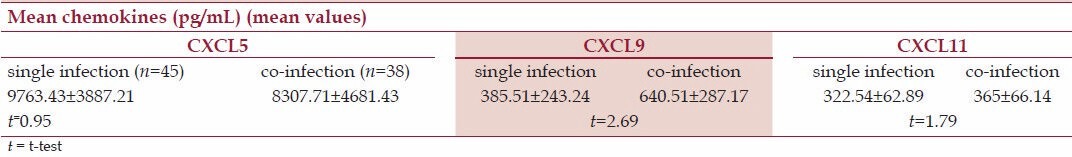
Table 5 summarizes the impact of A. lumbricoides infection on chemokines concentration in relation to sex. Although there were no significant differences between sexes for CXCL9 and CXCL11, the mean concentration of CXCL 5 was higher in females than males (P < 0.05).
Table 5.
Chemokines responses to Ascaris lumbricoides-infected volunteers in relation to sex

Discussion
In an effort to assess the impact of A. lumbricoides infection on some chemokines, we examined the changes seen in infected patients against noninfected individuals. It was demonstrated that serum levels of CXCL5 and CXCL9 among the infected was upregulated. Our observation is in line with a study that asserts that helminth infection stimulates increase in chemokine proinflammatory responses and downplayed Th2 [interleukin (IL)-13] responses.[18] A lack of IL-13 promotes chronic intestinal nematode infection in mice, whereas reconstitution of IL-13 expels Trichuris muris from their gut.[19] Such an amplified IL-13 production will enhance secretion of immunoglobin E (IgE)-mediated cellular cytotoxicity that protects against helminth infections.[20]
Human intestinal infections are associated with characteristic features of Th2 responses and there is evidence that strong type 2 responses are correlated negatively with infection intensity.[21,22] Type 2 immune responses are composed of three major features: inflammation, wound repair, and most importantly resistance to helminths. The analysis of chemokines data in relation to intensities of infection showed that CXCL11 and CXCL5 were negatively correlated. These results could possibly produce similar outcomes like in the case where Th2 responses involved CD4 + Th2 cells, cytokines interleukins (IL-4, IL-5, IL-9, IL-10, and IL-13) and IgE in driving the process of recruiting eosinophils, basophils, and mast cells in order to enhance host defence against parasitic infections.[23] Conversely, CXCL9 was positively correlated with intensity of A. lumbricoides infection and heavily infected volunteers were remarkably higher than other degrees of infection. Since a proinflammatory response was observed for CXCL9 with the evidence demonstrated by these authors,[24] we suggest, therefore, that this chemokine could have partly mediated the process of macrophage inhibition with a direct chemotactic effect on the human monocytes in a way that ensured some form of protective immunity to heavily infected patients.
This study reports that CXCL11 and CXCL5 profiles of individuals coinfected with A. lumbricoides + hookworm showed similar responses with single infection. However, CXCL9 profile for co-infection was higher than single infection. This probably emphasises the important role of CXCL9 in A. lumbricoides + hookworm co-infection. Obviously, the upregulation of CXCL9 could be attributed to the presence of hookworm in this association supporting the findings of,[18] CXCL9 is a member of the alpha subfamily of inflammatory chemokine. It is inducible in macrophages, hepatocytes, and endothelial cells by IFN-γ which is a Th1 immune response. Existing reports show that helminth infection can ensure susceptibility to certain pathogens against which Th1 responses are protective and more resistant to other pathogens against which Th2 responses are defensive.[25] Whichever case, the central question of whether multiple helminth infections drive host immune responses toward phenotypes different from those of a single infection remains to be answered.[26]
Multiple factors including sex could determine the ability of a host to generate protective type 2 cytokines in response to helminth infection. For example, some authors demonstrated that resolution of Trichuris infection is typically characterized by the production of type 2 cytokines such as IL-4 and IL-13.[27] Previous studies with BALB/c mice deficient in IL-4 demonstrated the ability to mount an effective immune response against Trichuris due to a compensatory increase in IL-13, whereas mice lacking IL-13 were rendered susceptible to chronic infection.[19] Interestingly, however, this compensatory mechanism was only present in female knockout (KO)3 mice of same strain, and not their male counterparts, suggesting an important influence of host sex on the development of Th2 responses to gastrointestinal nematodes.[14] In our study therefore, out of the three chemokines analysed in A. lumbricoides-infected volunteers, only serum CXCL5 was boosted in females. This result suggests that CXCL5 could be mediating a protective response,[27,28] bias toward the female sex. The possible explanation for this is quite unclear and thus further investigation should be carried out along this line.
In conclusion, we have shown that A. lumbricoides could impact on the serum levels of CXCL5 and CXCL9. The implication of this is that these chemokines may play important roles in the helminth-disease outcomes. On one hand, CXCL5 and CXCL9 could mediate debilitating effects of A. lumbricoides infection on its hosts, while on the other in relation to co-infections, CXCL9 might be a key player in the process of immune regulation in expressing either protective or susceptible responses on parasites or host.
Acknowledgments
We thank the management of Agbor general hospital for granting us access to their facilities and also to the hospital volunteers.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Omorodion AO, Nmorsi OP, Isaac C, Umukoro DO, Akhile AO. Distribution of intestinal parasites among school-age children in Delta and Edo States of Nigeria. Parasitol Union J. 2012;5:1–6. [Google Scholar]
- 2.Hall A, Hewitt G, Tuffrey V, de Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4:118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: Cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 4.Kita H. The eosinophils: A cytokine-producing cell. J? Allergy Clin Immunol. 1996;97:889–92. doi: 10.1016/s0091-6749(96)80061-3. [DOI] [PubMed] [Google Scholar]
- 5.de Andres B, Rakasz E, Hagen M, McCormik ML, Mueller AL, Elliot D, et al. Lack of Fc-epsilon receptors on murine eosinopjils: Implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–36. [PubMed] [Google Scholar]
- 6.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–9. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 7.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, et al. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;10:1322–7. [PubMed] [Google Scholar]
- 8.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Down regulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;10:159–66. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Díaz A, Allen JE. Mapping immune response profiles: The emerging scenario from helminth immunology. Eur J Immunol. 2007;10:3319–26. doi: 10.1002/eji.200737765. [DOI] [PubMed] [Google Scholar]
- 10.Metenou S, Kovacs M, Dembele B, Coulibaly YI, Klion AD, Nutman TB. Interferon regulatory factor modulation underlines the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filarial-malaria co-infection. Eur J Immunol. 2012;42:641–50. doi: 10.1002/eji.201141991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behnke JM. Structure in parasite component communities in wild rodents: Predictability, stability, associations and interactions or pure randomness? Parasitology. 2008;135:751–66. doi: 10.1017/S0031182008000334. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida A, Maruyama H, Yabu Y, Amano T, Kobayakawa T, Ohta N. Immune responses against protozoal and nematodal infection in mice with underlying Schistosoma mansoni infection. Parasitol Int. 1999;48:73–9. doi: 10.1016/s1383-5769(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 13.Geiger SM, Alexander ND, Fujiwara RT, Brooker S, Cundill B, Diemert JD, et al. Necator americanus and helminth co-infections: Further down-modulation of hookworm-specific type 1 immune responses. PLoS Negl Trop Dis. 2011;5:e1280. doi: 10.1371/journal.pntd.0001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bancroft AJ, Artis D, Donaldson DD, Sypek JP, Grencis RK. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. Eur J Immunol. 2000;30:2083–91. doi: 10.1002/1521-4141(200007)30:7<2083::AID-IMMU2083>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Isaac C, Nmorsi OP, Igbinosa IB, Umukoro DO, Imarenezor EP, Ihimire IG, et al. Interleukin (IL)-10 and tumor necrosis factor-alpha (TNF-á) profiles of Nigerians with Trypanosoma brucei gambiense infection. Ann Biol Res. 2010;1:62–7. [Google Scholar]
- 16.Nmorsi OP, Osagie DI, Maliki A. Serum cytokines profiles in Nigerian children with Ascaris lumbricoides infection. Asian Pac J Trop Med. 2010;3:288–91. [Google Scholar]
- 17.WHO. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization; 1998. [Google Scholar]
- 18.Hamm DM, Agossou A, Gantin RG, Kocherscheidt L, Banla M, Dietz K, et al. Co-infections with Schistosoma haematobium, Necator americanus, and Entamoeba histolytica/Entamoeba dispar in children: Chemokine and cytokine responses and changes after antiparasite treatment. J Infect Dis. 2009;199:1583–91. doi: 10.1086/598950. [DOI] [PubMed] [Google Scholar]
- 19.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–61. [PubMed] [Google Scholar]
- 20.Mitre E, Nutman TB. IgE memory: Persistence of antigen-specific IgE responses years after treatment of human filarial infections. J Allergy Clin Immunol. 2006;117:939–45. doi: 10.1016/j.jaci.2005.12.1341. [DOI] [PubMed] [Google Scholar]
- 21.Loukas A, Prociv P. Immune responses in hookworm infections. Clin Microbiol Rev. 2001;14:689–703. doi: 10.1128/CMR.14.4.689-703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deschoolmeester ML, Else KJ. Cytokine and chemokine responses underlying acute and chronic Trichuris muris infection. Int Rev Immunol. 2002;21:439–67. doi: 10.1080/08830180213278. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood is a signature of Th2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SK, Cho MK, Park HK, Lee KH, Lee SJ, Choi SH, et al. Macrophage migration inhibitory factor homologs of Anisakis Simplex. Suppress Th2 response in allergic airway inflammation model via CD4+CD25+ Foxp3Tcell recruitment. J Immunol. 2009;182:6907–14. doi: 10.4049/jimmunol.0803533. [DOI] [PubMed] [Google Scholar]
- 25.Marshall AJ, Brunet LR, van Gessel Y, Alcaraz A, Bliss SK, Pearce EJ, et al. Toxoplasma gondii and Schistosomamansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-alpha and early death in C57BL/6 mice. J Immunol. 1999;163:2089–97. [PubMed] [Google Scholar]
- 26.Bradley JE, Jackson JA. Measuring immune system variation to help understand host-pathogen community dynamics. Parasitology. 2008;135:807–23. doi: 10.1017/S0031182008000322. [DOI] [PubMed] [Google Scholar]
- 27.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: A new mechanism of parasite expulsion. Science. 2005;308:1463–5. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 28.Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc Natl Acad Sci U S A. 2000;97:767–72. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]


