Abstract
Arteriogenesis as a way to restore blood flow after arterial occlusion has been under investigation for the treatment of coronary artery disease (CAD) for decades. Therapeutic approaches so far have included delivery of cytokines and growth factors as well as mechanical stimulation such as external counterpulsation. As knowledge on the mechanisms of arteriogenesis expanded, new therapeutic approaches have emerged.
This review summarizes recent attempts to stimulate the growth of the coronary vasculature and discusses their potential in clinical application. This article also delivers an overview of current studies and trials on coronary arteriogenesis.
Keywords: Coronary artery disease, arteriogenesis.
INTRODUCTION
For decades ischemic heart disease (IHD) has been the leading cause of death in industrialized western societies [1]. Macrovascular atherosclerosis leading to coronary artery obstruction and impairment of blood flow towards myocardial tissue is the underlying pathophysiology leading to IHD. Interventional and surgical revascularization strategies provide the hallmark of treatment options in this setting. Having unequivocally demonstrated improved survival in acute coronary syndromes (ACS) [2, 3], revascularization by percutaneous coronary intervention (PCI) has hitherto failed to yield prognostic benefit in stable coronary artery disease (CAD) [4]. Next to emergency revascularization, the presence of natural pre-existing bypasses improves survival in ACS [5]. Sufficiently developed collateral arteries, which are present in about one third of patients with CAD and also in about one fifth of patients without CAD [6], are associated with reduced long-term cardiac and overall mortality [7]. Therefore, stimulation of collateral artery growth (arteriogenesis) has been proposed in the management of IHD. Notably, there are several different mechanisms of vessel growth serving different purposes. While vasculogenesis describes the formation of blood vessels in embryogenesis, the term angiogenesis is used for ischemia-induced sprouting of new capillaries from existing blood vessels [8]. Finally, the term arteriogenesis has been coined to describe an increase in diameter of preexisting collateral anastomoses and their maturation into leading arterial vessels [9]. Potentially allowing bulk blood flow, only arteriogenesis is capable of supplying sufficient blood flow after occlusion of the native vessel by increasing blood flow up to factor 20 following a two-fold enlargement of a collateral artery [10, 11].
The following review describes different approaches including inflammatory as well as mechanical stimuli but also highlighting new experimental approaches and possible downsides of an increased coronary collateral vasculature.
INFLAMMATORY CYTOKINE THERAPY – ALREADY OUTDATED?
Approaches to promote collateral artery growth have advanced from animal studies to clinical investigations studies. Arteriogenesis being an inflammatory process [12] it seemed logical to promote inflammation by systemic application of proinflammatory cytokines. First small studies based on positive data from experimental studies showed promising results. However, most randomized studies were unable to demonstrate therapeutic benefit of putative pro-arteriogenic substances when compared to placebo or showed severe side effects such as promotion of atherosclerosis and development of ACS, which is to be demonstrated below.
Most of the studies first concentrated on angiogenesis as the main mechanism of blood flow restoration. The VIVA trial [13] (Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis), in which vascular endothelial growth factor (VEGF) was tested versus placebo in IHD had neutral results. Similarly, the AGENT [14] (Angiogenic GENe Therapy) and FIRST [15] (FGF Initiating RevaScularization Trial) studies enquiring the effects of fibroblast growth factor (FGF) on capillary growth showed no benefit over placebo treatment. In the AGENT trial, an adenoviral vector containing a human fibroblast growth factor gene was applied via an intracoronary route in patients with stable IHD. Treadmill exercise testing was used to evaluate efficacy of treatment. Overall, there was a non-significant increase in exercise time between treatment and placebo group. A significant difference in the increase of exercise time was only reported in patients with baseline exercise time of less than 10 minutes. Similarly, the FIRST trial assessed single-shot intracoronary application of recombinant FGF2 (rhFGF2) for improvement of exercise tolerance in patients with coronary artery disease. Again, there was no significant difference reported in comparison to placebo treatment. The VIVA trial focused on recombinant human vascular endothelial growth factor (rhVEGF) in angina patients. Study endpoint was angina frequency and exercise time evaluated by treadmill test. The intracoronary application of rhVEGF resulted in no significant difference of angina frequency and exercise time when compared to placebo.
In an attempt to stimulate specifically arteriogenesis which in the meantime had proven superior to angiogenesis in restoring blood flow after arterial occlusion [10, 11], granulocyte-monocyte-colony stimulating factor (GM-CSF) was administered in a small study by Seiler et al. [16]. GM-CSF is a colony stimulating factor mobilizing progenitor cells of the granulocyte and macrophage cell line from the bone marrow and protecting monocytes – which play an important role in arteriogenesis [17] – from apoptosis. The application of this substance had been validated in several different animal models and also showed an increase of the coronary flow index in the 11 CAD patients included in the study when compared to placebo treatment [18].
However, in a further study investigating the effects of a subcutaneous-only therapy with GM-CSF concerns about the safety of the drug arouse, with two of seven patients in the treatment group suffering an acute coronary syndrome [19]. To reduce side effects, the authors of the GM-CSF studies started investigating the effect of granulocyte-colony stimulating factor (G-CSF) only. A short-term treatment period with subcutaneous G-CSF significantly enhanced coronary collateralization as invasively measured by collateral flow index [20]. A study using pegylated G-CSF with reduced injection frequency and reduced side-effects is currently being carried out (clinicaltrials.gov identifier NCT00886509).
Atherogenesis itself being an inflammatory process [21], attempted stimulation of arteriogenesis through induction of systemic inflammation via application of proinflammatory cytokines poses several possible risks. This notion was exemplified by experimental investigations on monocyte chemoattractant protein 1 (MCP1). While administration of MCP1 was demonstrated to increase collateral artery formation in mice, it was also shown to lead to plaque progression in dyslipidemia mouse models [22, 23]. Likewise, VEGF has been reported to enhance not only angiogenesis but also plaque progression [24].
Of the aforementioned clinical studies, one involving the subcutaneous application of GM-CSF had to be terminated prematurely when two patients developed an acute coronary syndrome [25]. Also, most of the cytokines thought to promote collateral artery growth are found at relatively high local concentrations in patients with insufficient coronary collateralization and might thus not be the ideal therapeutic target [26].
In an attempt to stimulate arteriogenesis through administration of a mediator without proinflammatory properties and the concurrent atherogenic side effects, Neuropeptide Y (NPY) was tested in swine suffering from metabolic syndrome. Treatment with NPY resulted in up-regulation of pro-arteriogenic proteins while down-regulating anti-angiogenic factors [27] and could therefore be another possible target for the stimulation of collateral artery growth in the future. However, the effects described might not specifically stimulate arteriogenesis, but also angiogenesis.
Studies involving a visual angiographic grading of collateralization and analyzing serum concentrations of inflammatory cytokines proved modulation of inflammatory cytokines in patients with good collateralization. Angiostatic ligands such as C-X-C motif chemokine 10 (CXCL10) and C-X-C motif chemokine 11 (CXCL11) were decreased, while higher concentrations of angiogenic ligands were associated with a well-developed collateral circulation [28].
MECHANICAL STIMULATION – THE IDEAL APPROACH?
In accordance with the pathophysiology of arteriogenesis corroborating animal experiments, physical exercise was shown to have positive effects on collateral artery growth in the clinical setting [29, 30]. The additional mechanical stress on the vessel walls induced by physical exercise is believed to be one of the main mechanisms in this model. An additional inflammatory-modulating effect of exercise as the underlying mechanism, independent of shear stress, is currently under investigation (ClinicalTrials.gov Identifier: NCT01432639). Arteriogenesis unlike angiogenesis is among other factors dependent on mechanical stress, in the coronary circulation foremost fluid shear stress during diastole [31, 32]. Fluid shear stress in pre-existent collateral anastomoses is substantially increased after vascular occlusion, as in coronary artery disease. Since coronary arteries are perfused passively during diastole, there is no discernable pulse wave. Hence, circumferential wall stress, which is another mechanical factor inducing arteriogenesis [33], appears to be negligible in coronary circulation. Mechanic factors such as fluid shear stress and circumferential wall stress activate the mechanosensitive channels of the transient receptor potential cation channel family (TrpV4) [34] in the vessel walls which in turn induce inflammatory mediators [35] and concurrent attraction of mononuclear cells (MNCs) [36]. Also, enzymes such as endothelial nitric oxide synthase (eNOS) and MNC-based inducible nitric oxide synthase (iNOS) are activated, leading to a proarteriogenic milieu [37]. Induced factors include MCP1, transforming growth factor-beta (TGFß) and adhesion molecules such as intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM). These lead to further infiltration with circulating monocytes, which initiate extracellular matrix degradation [38]. Simultaneously, liberated nitric oxide (NO) triggers expression of Actin-binding rho activating protein (ABRA) [39] and thus induces a proliferative phenotype in vascular smooth muscle cells [9]. Consequently, collateral artery growth occurs, leading to typical corkscrew-shaped conducting arterioles [36]. Generally, this growth is associated with a process called pruning, leading to obliteration of smaller collaterals and selection of fewer, but larger arteriolar vessels [40]. However, sufficient physical exercise inducing collateral artery growth seems difficult for patients with recurring and severe angina or patients suffering from heart failure.
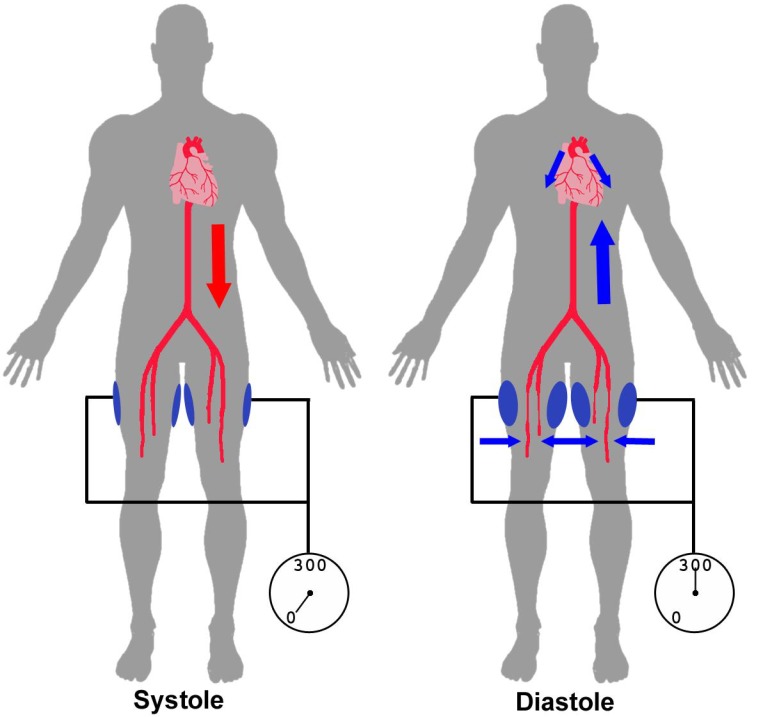
Given the fact that mechanical factors influence arteriogenesis [33], there has been increasing focus on a treatment that has been primarily used in the management of refractory angina [41]. Because of the difficulties of active exercise training, a passive method of blood flow augmentation has been investigated. During enhanced external counterpulsation (ECP), external hydraulic pressure of up to 300 mmHg is applied to the lower limbs at three different places triggered by electrocardiogram (ECG), causing a diastolic augmentation of coronary blood flow and increasing cardiac output, by raising preload (venous return) and lowering afterload (diastolic augmentation of aortic perfusion pressure) (Fig. 1). In the MUST-EECP-study (MUlticenter STudy of Enhanced External CounterPulsation) an increase of coronary flow reserve and alleviation of angina were reported after treatment with ECP [42]. This is possibly due to the increased diastolic blood flow in the main coronary artery through increased cardiac preload and thus enhanced passive myocardial perfusion but also due to enhanced collateral artery growth based on increased fluid shear stress in pre-existing collateral anastomoses.
Fig. (1).
Principle of ECP. During systole pressure cuffs, which are applied to the complete lower limb, are deflated allowing normal arterial blood flow in the lower limbs. During diastole cuffs are inflated to 300mmHg causing retrograde aortic blood flow and increased myocardial perfusion, consequently leading to an increase in coronary vascular shear stress.
Two studies have directly tested the hypothesis that ECP enhances coronary collateral artery growth.
In the non-randomized Art.Net.-2 (Arteriogenesis Network) trial patients with stable coronary artery disease underwent a 35 1-h sessions of ECP in 7 weeks [43]. They showed an increased coronary flow index (CFI) and fractional flow reserve (FFR) as well as an improvement in clinical endpoints such as angina frequency and dyspnea after ECP.
Another investigation by Gloekler et al. on patients with chronic stable CAD contained a sham group (80 mmHg; mean diastolic pressure was 73 mmHg at baseline) next to an ECP-group (300 mmHg) [44]. Patients were exposed to 30 h of ECP spread over 4 weeks (twenty 90 min sessions). Since 80 mmHg cuff pressure applied in the sham group was only negligibly higher than diastolic pressure, and therefore has only little effect, it seems that venous return has the least impact, compared to diastolic augmentation. This pressure would still increase venous return to the right atrium, but could not interfere with the arterial system, being well below diastolic blood pressure. Primary endpoint was the collateral flow index (CFI) which increased in ECP-treated patients compared to sham: CFI changed from 0.125 at baseline to 0.174 at follow-up in the ECP group, while it did not improve significantly in the sham group.
In an attempt to reduce angina in symptomatic CAD-patients not suitable for revascularization, the Coronary Sinus Reducer was implanted in fifteen patients. The Coronary Sinus Reducer is an expandable stainless steel stent inserted percutaneously through the internal jugular vein narrowing the coronary sinus (CS) and thus increasing coronary venous pressure. After 6 months, angina was reduced as well as myocardial ischemia as evaluated by dobutamine echocardiography and thallium single-photon emission computed tomography (SPECT) [45]. Considering the data from this study, implantation of a Coronary Sinus Reducer could be a possible treatment not only for the alleviation of symptoms in angina patients, but also in the improvement of myocardial perfusion.
An active means of increasing collateral flow and shear stress, particularly in peripheral artery disease (PAD), is supervised exercise (SE). The CLEVER study (CLaudication: Exercise Vs. Endoluminal Revascularization) has proven SE to be even superior to stent revascularization (ST) and sole optimal medical care (OMC) in patients with aortoiliac peripheral artery disease by a significant increase in peak walking distance [46]. Both SE and OMC presented a greater enhancement in high-density lipoprotein (HDL) cholesterol compared with the ST group, due to physical exercise and lipid lowering drugs, respectively. However, looking at the ankle-brachial index (ABI) as a secondary endpoint, only patients with ST showed significantly better results. SE is a non-invasive way of improving PAD. Of note, however, published data on the beneficial effects of supervised exercise lack proof of increased collateral formation as the main underlying mechanism as there is currently no validated method available to quantify collateral formation in the peripheral circulation.
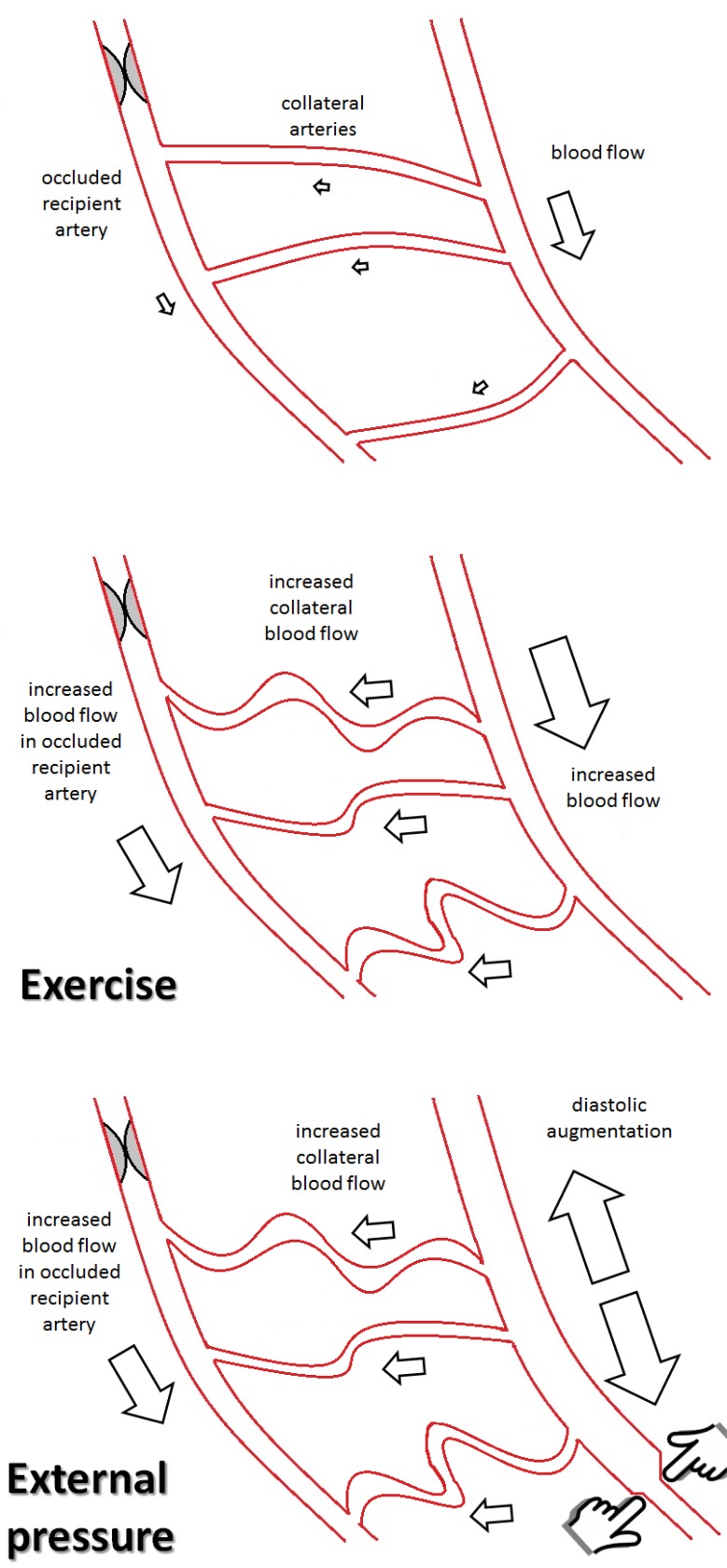
Because of similar mechanisms a beneficial effect of exercise can be expected on the coronary circulation as well. In an individual athlete’s report, different levels of physical activity resulted in an increase on both CFI and coronary flow reserve (CFR). At baseline fitness level the healthy sportsman experienced anginal chest pain during a one minute left anterior descending coronary artery (LAD) occlusion, which he did not suffer from at a higher fitness level, suggesting a shear-stress induced arteriogenesis of pre-existing coronary collaterals [47]. A clinical study examining different training modalities and their effects on coronary arteriogenesis is currently enrolling a patient collective [48]. The EXCITE-trial (Impact of Intensive Exercise Training on Coronary Collateral Circulation in Patients With Stable CAD) will be evaluating high-intensity training and moderate-intensity training with optimal medical therapy (OMT) versus OMT only in patients with significant coronary stenosis. Primary endpoint will be the change in collateral blood flow as assessed by coronary catheterization during balloon occlusion. See also (Fig. 2).
Fig. (2).
Poor blood flow over small preexisting collateral arteries. The area distal of the occluded artery is not supplied sufficiently. Exercise leads to an increased blood flow in the native artery, causing increased flow in the collateral arteries and arteriogenesis. Blood flow in the occluded recipient artery is improved. External pressure as in ECP leads to increased collateral flow and arteriogenesis through diastolic augmentation, leading to an increase in blood flow in the recipient artery.
ALTERNATIVE IDEAS FROM EXPERIMENTAL MODELS
Pipp et al. for the first time increased fluid shear stress (FSS) by creation of arteriovenous (AV) side-to-side-anastomoses in the porcine peripheral circulation following femoral artery occlusion [49]. Here, AV-shunt results in profoundly decreased arterial pressure (equaling venous pressure) distal to the arterial occlusion, thereby increasing pressure gradient over the pre-existent collateral circulation and consecutively enhanced shear stress. Expression of proarteriogenic genes was markedly increased after the procedure as well as collateral flow. This model which was later repeated in smaller animals [39] for the first time provided evidence, that perfusion restoration following experimental arterial occlusion can exceed 100% of the healthy situation if the stimulus is strong enough. Gene expression analyses identified genes which potentially transduce the mechanical force into collateral artery growth, and application of the corresponding proteins in-vivo has been sought to find a means to translate mechanical arteriogenesis into an exogenously applicable pro-arteriogenic drug. Nevertheless, the conception of an arteriovenous shunt is potentially conceivable in the clinical situation as well, where percutaneous or surgical creation of a coronary AV-shunt in a patient with end-stage coronary artery disease and lack of therapeutic options is theoretically feasible.
As mentioned above, eNOS is induced during arteriogenesis. Past studies have shown that the presence of NO is critical in arterial growth. Application of NO donating moieties has been demonstrated to stimulate arteriogenesis [37]. Substances used in vascular prevention such as aspirin or statins have already been linked to an NO-donating moiety [50] making this approach feasible also in the clinical situation.
Animal studies showed increased flow restoration in a femoral artery ligation model following heart rate reduction by If-channel blockade through administration of Ivabradine. In this study, the augmented pulse amplitude is most likely the key to increased circumferential wall stress and increased arteriogenesis [51]. However, coronary circulation and peripheral circulation differ strongly. For instance, increased pulse wave amplitude has a much higher impact on peripheral vessels than on coronary vessels. In the clinical setting, however, prolongation of diastole as a major hemodynamic effect of bradycardia, results in increased coronary flow and shear stress and can thus likely have a positive effect on arteriogenesis. In fact, low heart rate has earlier been positively related to collateral flow index [52]. A clinical study assessing the effect of Ivabradine on coronary collateral circulation is currently carried out; results are being expected in the course of 2013 (clinicaltrials.gov identifier NCT01039389).
Ivabradine is the first substance assessed for the stimulation of arteriogenesis which has demonstrated proarteriogenic capabilities while attenuating systemic inflammation [51]. Strong inflammatory cytokine prodution has been associated with decreased arteriogenesis, suggesting pro- and antiarteriogenic properties of different inflammatory pathways [53, 54]. Therefore, modulation of these pathways inducing a proarteriogenic milieu rather than induction of full-scale inflammation could be a possible approach for future investigation.
Cell therapy has gained vast interest in regenerative medicine in the past years. A prospective study on cell therapy for the stimulation of collateral artery growth using quantitative outcome measures (CFI) is currently lacking. One promising approach could lie in the application of vascular progenitor cells. A recent paper has shown the possibility of applying reprogrammed vascular progenitor cells to increase collateral artery growth [55, 56]. This method rather than the use of pluripotent stem cells seems promising and shows a significantly reduced risk of unwanted side effects when compared to stem cell therapy. Also, in earlier investigations, a correlation between circulating progenitor cells and coronary collateralization was discovered [57]. Nonetheless, the multitude of effects of stem cells and the use thereof are still not completely clear and require further research in these fields.
DOWNSIDES – A WELL-DEVELOPED COLLATERAL CIRCULATION AT ALL COST?
There are possible downsides to a well-developed coronary collateral circulation that should be considered carefully. A meta-analysis comprising seven studies on restenosis after percutaneous coronary intervention (PCI) and drug-eluting stent (DES) implantation shows that good collateralization before intervention is associated with a 40% increase in restenosis when compared to patients with poor collateralization [58]. In another study, a higher recruitable CFI was associated with a better developed collateral circulation and an increase in angiographic restenosis >50% after 9 months [59]. The effects reported could possibly result from reduced antegrade blood flow through the native vessel. A similar effect has been described in collateral arteries after bypass grafting [60]. In a single study, the negative effects on restenosis of a well-developed collateral circulation in patients having undergone bare metal stent (BMS) implantation did not occur [61]. Of note, a good collateralization is associated with increased survival regardless of the increase in restenoses after PCI [62].
Table 1.
Overview of Studies on Stimulation of Coronary Arteriogenesis. First Column Names the Study, Second and Third Columns Describe the Stimulus Used and Fourth Column Presents the Outcome.
| Trial | Stimulus | Substance/route of Administration | Outcome |
|---|---|---|---|
| Seiler et al. | chemical | GM-CSF (i.c., s.c.) | Increase in coronary collateralization, increase of CFI |
| Belardinalli et al. | chemical | Dipyridamole (p.o.) | Increase in coronary collateralization, combined with exercise |
| VIVA | chemical | rhVEGF (i.c.) | No difference in angina frequency or exercise tolerance |
| AGENT | chemical | Adenoviral FGF4 (i.c.) | Non-significant increase in exercise-time after 4 weeks |
| FIRST | chemical | FGF2 (i.c.) | No difference in exercise tolerance when compared to placebo |
| Zbinden et al. | chemical | GM-CSF (s.c.) | Increase in CFI, terminated when 2 patients developed ACS |
| Meier et al. | chemical | G-CSF (s.c.) | Increase in CFI, more often lack of ST-segment elevation |
| Gloekler et al. | mechanical | ECP | Increased CFI compared to Sham treatment (augmentation of venous return alone) |
| Art.Net.-2 | mechanical | ECP | Increased CFI and FFR, improvement in angina frequency and dyspnea |
ACKNOWLEGEMENTS
The authors are supported by the Deutsche Forschungsgemeinschaft and the Deutsche Herzstiftung.
SUMMARY AND CONCLUSION
Because of the impact of ischemic heart disease, a non-invasive approach to stimulate natural bypasses has been subject of extensive research. At the current stage, the focus of coronary arteriogenesis has shifted from cytokine-based therapy towards a mechanical approach where physical exercise and external counterpulsation therapy appear to be most promising means for the stimulation of collateral artery growth. Interestingly, these therapies decrease rather than increase systemic inflammation, questioning the earlier accepted belief that a pro-arteriogenic therapy is always pro-inflammatory. Currently, the results of different studies examining several of the aforementioned approaches are still pending or awaiting clinical trial stage.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.World Health Organization. Global status report on noncommunicable diseases. 2010 [Google Scholar]
- 2.Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006;48(7):1319–25. doi: 10.1016/j.jacc.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 3.O'Donoghue M, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction a meta-analysis. JAMA. 2008;300(1):71–80. doi: 10.1001/jama.300.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without pci for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 5.Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–31. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- 6.Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries. Circulation. 2003;107(17):2213–20. doi: 10.1161/01.CIR.0000066321.03474.DA. [DOI] [PubMed] [Google Scholar]
- 7.Meier P, Gloekler S, Zbinden R, et al. Beneficial effect of recruitable collaterals.A 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–83. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 9.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23(7):1143–51. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 10.Van Royen N, Piek JJ, Schaper W, Bode C, Buschmann I. Arteriogenesis: mechanisms and modulation of collateral artery development. J Nucl Cardiol. 2001;8(6):687–93. doi: 10.1067/mnc.2001.118924. [DOI] [PubMed] [Google Scholar]
- 11.Schirmer SH, van Royen N. Stimulation of collateral artery growth a potential treatment for peripheral artery disease. Expert Rev Cardiovasc Ther. 2004;2(4):581–8. doi: 10.1586/14779072.2.4.581. [DOI] [PubMed] [Google Scholar]
- 12.Buschmann I, Heil M, Jost M, Schaper W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation. 2003;10(3-4):371–9. doi: 10.1038/sj.mn.7800199. [DOI] [PubMed] [Google Scholar]
- 13.Henry TD, Annex BH, McKendall GR, et al. The viva trial Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 14.Grines CL, Watkins MW, Helmer G, et al. Angiogenic gene therapy (agent) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–7. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 15.Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–93. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 16.Seiler C, Pohl T, Wustmann K, et al. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease a randomized double-blind placebo-controlled study. Circulation. 2001;104(17):2012–7. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- 17.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3:353. doi: 10.3389/fphys.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiler C, Pohl T, Wustmann K, et al. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease.A randomized double-blind placebo-controlled study. Circulation. 2001;104:2012–7. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- 19.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636–42. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 20.Meier P, Gloekler S, de Marchi SF, et al. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease.A controlled randomized trial. Circulation. 2009;120(14):1355–63. doi: 10.1161/CIRCULATIONAHA.109.866269. [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 22.Aiello RJ, Bourassa PA, Lindsey S, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–25. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 23.van Royen N, Hoefer I, Böttinger M, et al. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression neointimal formation and plaque progression. Circ Res. 2003;92(2):218–25. doi: 10.1161/01.res.0000052313.23087.3f. [DOI] [PubMed] [Google Scholar]
- 24.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7(4):425–9. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 25.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46(9):1636–42. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 26.Schirmer SH, van Royen N, Moerland PD, et al. Local cytokine concentrations and oxygen pressure are related to maturation of the collateral circulation in humans. J Am Coll Cardiol. 2009;53:2141–7. doi: 10.1016/j.jacc.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 27.Matyal R, Chu L, Mahmood F, et al. Neuropeptide Y improves myocardial perfusion and function in a swine model of hypercholesterolemia and chronic myocardial ischemia. J Mol Cell Cardiol. 2012;53(6):891–8. doi: 10.1016/j.yjmcc.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Keeley EC, Moorman JR, Liu L, et al. Plasma chemokine levels are associated with the presence and extent of angiographic coronary collaterals in chronic ischemic heart disease. PLoS One. 2011;6(6):e21174. doi: 10.1371/journal.pone.0021174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naylor LH, O'Driscoll G, Fitzsimons M, Arnolda LF, Green DJ. Effects of training resumption on conduit arterial diameter in elite rowers. Med Sci Sports Exerc. 2006;38(1):86–92. doi: 10.1249/01.mss.0000181220.03855.1c. [DOI] [PubMed] [Google Scholar]
- 30.Yang HT, Prior BM, Lloyd PG, et al. Training-induced vascular adaptations to ischemic muscle. J Physiol Pharmacol. 2008;59(Suppl 7 ):57–70. [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen LP, Zhang RL, Zheng W, et al. Postmyocardial infarction remodeling and coronary reserve effects of ivabradine and beta blockade therapy. Am J Physiol Heart Circ Physiol. 2009;297(1):H322–30. doi: 10.1152/ajpheart.01337.2008. [DOI] [PubMed] [Google Scholar]
- 32.Lamping KG, Zheng W, Xing D, Christensen LP, Martins J, Tomanek RJ. Bradycardia stimulates vascular growth during gradual coronary occlusion. Arterioscler Thromb Vasc Biol. 2005;25(10):2122–7. doi: 10.1161/01.ATV.0000179598.57819.77. [DOI] [PubMed] [Google Scholar]
- 33.Heil M, Schaper W. Influence of mechanical cellular and molecular factors on collateral artery growth (arteriogenesis). Circ Res. 2004;95(5):449–58. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 34.Troidl C, Troidl K, Schierling W, et al. Trpv4 induces collateral vessel growth during regeneration of the arterial circulation. J Cell Mol Med. 2009;13(8B ):2613–21. doi: 10.1111/j.1582-4934.2008.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye L, Kleiner S, Wu J, et al. TRPV4 is a regulator of adipose oxidative metabolism inflammation and energy ho-meostasis. Cell. 2012;151(1):96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buschmann I, Schaper W. Arteriogenesis Versus Angiogenesis Two Mechanisms of Vessel Growth. News Physiol Sci. 1999;14:121–5. doi: 10.1152/physiologyonline.1999.14.3.121. [DOI] [PubMed] [Google Scholar]
- 37.Troidl K, Tribulova S, Cai WJ, et al. Effects of endogenous nitric oxide and of DETA NONOate in arteriogenesis. J Cardiovasc Pharmacol. 2010;55(2):153–60. doi: 10.1097/FJC.0b013e3181c9556f. [DOI] [PubMed] [Google Scholar]
- 38.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai). 2008;40(8):681–92. [PubMed] [Google Scholar]
- 39.Troidl K, Rüding I, Cai WJ, et al. Actin-binding rho activating protein (Abra) is essential for fluid shear stress-induced arteriogenesis. Arterioscler Thromb Vasc Biol. 2009;29(12):2093–101. doi: 10.1161/ATVBAHA.109.195305. [DOI] [PubMed] [Google Scholar]
- 40.Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49(3):609–17. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 41.Braverman DL. Enhanced external counterpulsation an innovative physical therapy for refractory angina. PMR. 2009;1(3):268–76. doi: 10.1016/j.pmrj.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP) effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33(7):1833–40. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 43.Buschmann EE, Utz W, Pagonas N, et al. Improvement of fractional flow reserve and collateral flow by treatment with external counterpulsation (Art.et.-2 Trial). Eur J Clin Invest. 2009;39(10):866–75. doi: 10.1111/j.1365-2362.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 44.Gloekler S, Meier P, de Marchi SF, et al. Coronary collateral growth by external counterpulsation a randomised controlled trial. Heart. 2010;96(3):202–7. doi: 10.1136/hrt.2009.184507. [DOI] [PubMed] [Google Scholar]
- 45.Banai S, Ben Muvhar S, Parikh KH, et al. Coronary sinus reducer stent for the treatment of chronic refractory angina pectoris a prospective open-label multicenter safety feasibility first-in-man study. J Am Coll Cardiol. 2007;49(17):1783–9. doi: 10.1016/j.jacc.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 46.Murphy TP, Cutlip DE, Regensteiner JG, et al. CLEVER Study Investigators.Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease six-month outcomes from the claudication exercise versus endoluminal revascularization (CLEVER) study. . Circulation. 2012;125(1):130–9. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zbinden R, Zbinden S, Windecker S, Meier B, Seiler C. Direct demonstration of coronary collateral growth by physical endurance exercise in a healthy marathon runner. Heart. 2004;90(11):1350–1. doi: 10.1136/hrt.2003.023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhlemann M, Adams V, Lenk K, et al. Impact of different exercise training modalities on the coronary collateral circulation and plaque composition in patients with significant coronary artery disease (EXCITE trial) study protocol for a randomized controlled trial. Trials. 2012;13:167. doi: 10.1186/1745-6215-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pipp F, Boehm S, Cai WJ, et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24(9):1664–8. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 50.Momi S, Monopoli A, Alberti PF, et al. Nitric oxide enhances the anti-inflammatory and anti-atherogenic activity of atorvastatin in a mouse model of accelerated atherosclerosis. Cardiovasc Res. 2012;94(3):428–38. doi: 10.1093/cvr/cvs100. [DOI] [PubMed] [Google Scholar]
- 51.Schirmer SH, Degen A, Baumhäkel M, et al. Heart-rate reduction by if-channel inhibition with ivabradine restores collateral artery growth in hypercholesterolemic atherosclerosis. Eur Heart J. 2012;33:1223–1. doi: 10.1093/eurheartj/ehr255. [DOI] [PubMed] [Google Scholar]
- 52.de Marchi SF, Gloekler S, Meier P, et al. Determinants of preformed collateral vessels in the human heart without coronary artery disease. Cardiology. 2011;118(3):198–206. doi: 10.1159/000328648. [DOI] [PubMed] [Google Scholar]
- 53.Schirmer SH, Bot PT, Fledderus JO, et al. Blocking interferon {beta} stimulates vascular smooth muscle cell proliferation and arteriogenesis. J Biol Chem. 2010;285(45):34677–85. doi: 10.1074/jbc.M110.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schirmer SH, Fledderus JO, Bot PT, et al. Interferon-beta signaling is enhanced in patients with insufficient coronary collateral artery development and inhibits arteriogenesis in mice. Circ Res. 2008;102(10):1286–94. doi: 10.1161/CIRCRESAHA.108.171827. [DOI] [PubMed] [Google Scholar]
- 55.Yin L, Ohanyan V, Pung YF, et al. Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth. Circ Res. 2012;110(2):241–52. doi: 10.1161/CIRCRESAHA.111.250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faber JE. Reprogrammed endothelial cells cell therapy for coronary collateral growth. Circ Res. 2012;110(2):192–4. doi: 10.1161/CIRCRESAHA.111.261495. [DOI] [PubMed] [Google Scholar]
- 57.Tokgözoglu L, Yorgun H, Gürses KM, et al. The association between circulating endothelial progenitor cells and coronary collateral formation. Atherosclerosis. 2011;219(2):851–4. doi: 10.1016/j.atherosclerosis.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 58.Meier P, Indermuehle A, Pitt B, et al. Coronary collaterals and risk for restenosis after percutaneous coronary inter-ventions.A meta-analysis. BMC Medicine. 2012;10:62. doi: 10.1186/1741-7015-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen LO, Thayssen P, Lassen JF, et al. Recruitable collateral blood flow index predicts coronary instent restenosis after percutaneous coronary intervention. Eur Heart J. 2007;28:1820–26. doi: 10.1093/eurheartj/ehm067. [DOI] [PubMed] [Google Scholar]
- 60.Aldridge HE, Trimble AS. Progression of proximal coronary artery lesions to total occlusion after aorta-coronary saphenous vein bypass grafting. J Thorac Cardiovasc Surg. 1971;62(1):7–11. [PubMed] [Google Scholar]
- 61.Perera D, Postema P, Rashid R, et al. Does a well developed collateral circulation predispose to restenosis after percutaneous coronary intervention.An intravascular ultrasound study. Heart. 2006;92:763–67. doi: 10.1136/hrt.2005.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality a meta-analysis. Eur Heart J. 2012;33(5):614–21. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]