Abstract
Respiratory complications and renal failure are the leading causes for morbidity and mortality due to influenza (H1N1) virus infection. There has been limited information on histopathology of H1N1 influenza-related acute kidney injury (AKI). We describe AKI with H1N1 infection in a 52-year-old female. Renal biopsy showed mesangial proliferative glomerulonephritis with acute tubule interstitial nephritis. Her condition improved rapidly with oseltamivir, fluid replacement, steroid and dialysis. Our case suggests that H1N1 infection may have a causative link to the development of mesangial proliferative glomerulonephritis with acute tubulointerstitial nephritis.
Keywords: Acute kidney injury, glomerulonephritis, histopathology, H1N1 influenza
Introduction
Influenza is an acute respiratory illness caused by infection with influenza viruses. Respiratory complications and renal failure are the leading causes for hospitalization. There have been a few studies on acute kidney injury (AKI) during the 2009 influenza (H1N1) pandemic. Respiratory failure requiring ventilator support was associated with 50% mortality.[1] AKI requiring renal replacement therapy (RRT) occurred in 9.3-21% of critically ill-patients with influenza A infection and has been independently associated with poor outcome world-wide.[1]
Although the pathogenesis of renal injuries due to influenza A virus is incompletely described, some hypotheses have been postulated, including acute tubular necrosis (ATN) due to renal hypoperfusion or rhabdomyolysis, glomerular microthrombosis due to DIC, direct viral injury to the kidney and an altered immune system, like systemic mononuclear cell activation following influenza A virus infection.[2]
Despite various reports of diffuse alveolar damage on histopathology of lung in these patients,[3] there has been limited information on histopathology of H1N1 influenza-related AKI. In this report we describe the clinical features and kidney biopsy findings in patient with AKI with H1N1 infection.
Case Report
A 52-year-old female patient was admitted in March 2012 with fever, cough, breathlessness and progressive dyspnea with respiratory distress and decrease urine output of 10 days duration. There was no history of abdominal pain, dysuria or trauma and no recent use of non-steroidal anti-inflammatory medication.
On examination, she was breathless with blood pressure of 150/90 mmHg, temperature 40°C, respiratory rate of 30 breaths/min, heart rate of 100 bpm. Laboratory investigations revealed hemoglobin, 10.3 g/l; total white cell count, 9.88 × 103/μl (differential count: 74% neutrophils, 20% lymphocytes, 4% monocytes and 2% eosinophils); platelet count, 211 × 103/μl; serum creatinine (SCr), 10.8 mg/dl sodium, 138 mEq/l; potassium, 5.3 mEq/l; blood urea, 76 mg/dl; and blood glucose, 72 mg/dl.
Renal ultrasound showed normal sized kidneys with hyper reflective cortex. Serological tests for malaria, leptospirosis, dengue and viral hepatitis were negative. The initial blood, urine and sputum cultures were sterile. Urinalysis showed proteinuria, hematuria and two to five fine granular casts. SCr phosphokinase levels and liver function test chest radiograph were unremarkable while tests for antinuclear antibody, anti-double-stranded deoxyribonucleic acid, anti-neutrophil cytoplasmic antibody, anti-glomerular basement membrane antibody, coombs’ test, cryoglobulins and C-reactive protein were all negative and complement components (C3 and C4) were low. Enzyme-linked immunosorbent assays test for human immunodeficiency virus (HIV), hepatitis B surface antigen and hepatitis C virus were negative. Patients had influenza H1N1 virus subtype specific ribonucleic acid detected in a nasopharyngeal swab using reverse transcriptase-polymerase chain reaction. She had a history of exposure to swine flu positive patient and was managed using oseltamivir, antibiotic, fluid replacement and dialysis. Patients received oseltamivir, 75 mg within 48 h of presumed diagnosis for 5 days and supportive measures as needed. Initially, she was treated with peritoneal dialysis and she remained oliguric and required intermittent hemodialysis for a total of 5 times through temporary jugular dialysis catheter for 4 h each.
Renal biopsy [Figure 1] revealed two fragment of renal tissue containing 11 glomeruli with associated tubules and vessels. All the glomeruli show uniform mesangial prominence. Capillary lumina were open, and membranes were of normal thickness. Tubules showed mild to moderate degeneration. Interstitium was mildly prominent for uniform edema and diffuse infiltration with leucocytes. Immunofluorescence microscopy did not reveal staining with anti-human sera tested (IgG, IgM, IgA and C1q). Influenza nucleoprotein (InfA-NP) antigen-positive cells were not detected in kidney tissue. A diagnosis of mesangial proliferative glomerulonephritis with acute tubule interstitial nephritis was made. The patient was treated with methyl prednisolone 500 mg for 3 days followed by oral prednisolone 1 mg/kg/day. Her condition also improved rapidly and she was discharged with SCr of 1.5 mg/dl. Her SCr was 1 mg/dl at 1 month follow-up.
Figure 1.
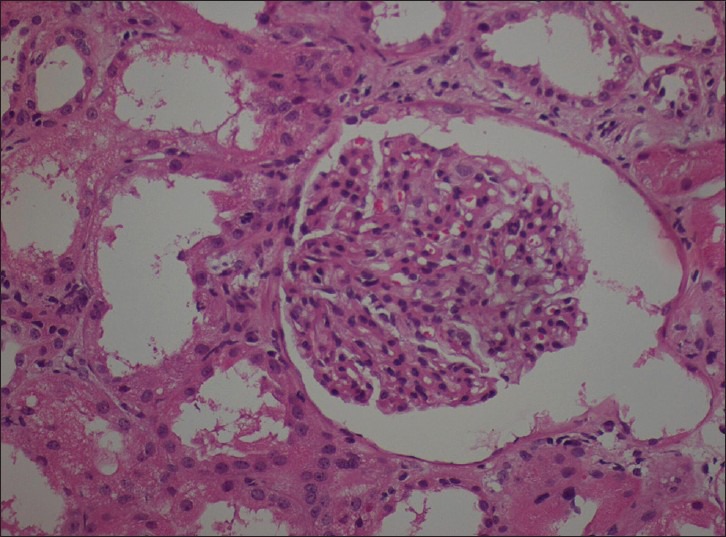
Renal biopsy (H and E, ×400) revealed mesangial proliferative glomerulonephritis with acute tubule interstitial nephritis
Discussion
Several mechanisms for renal injury have been implicated in subjects with H1N1 influenza infection, including pigment injury due to elevations in creatine kinase, hemodynamic effects, direct effect of the H1N1 virus on the kidney and/or ATN in the context of coexistent culture proven bacterial sepsis or secondary to hypovolemia. A direct renal effect of the virus has been proposed as a contributing mechanism to AKI.[3,4,5,6,7,8,9,10]
The detection of InfA-NP in tissue sections is evidence of influenza virus infection and specific findings. There are no histopathological findings specific to H1N1 influenza infection, such as a specific inclusion body. There were several reasons why the antigen was not detected in our case, such as effective antiviral treatment, clearance of viral antigens after a long duration of illness and the condition of formalin-fixed and paraffin-embedded samples.
The clinicopathologic features in 46 fatal cases with confirmed pandemic 2009 H1N1 virus infection during August 2009 to October 2010 were reported in an Indian study. Histopathological findings in kidney tissues were mild interstitial space mononuclear cells; infiltration (75%), ATN (50%). The virus antigen was not detected in kidney tissues. Main lung pathology is diffuse alveolar damage, which is basically the histological prototype of acute lung injury.[1]
AKI was noted in 18% and dialysis requiring renal failure in 3% of study subjects. Mortality was associated with multiple organ failure, sepsis, shock, prolonged ventilator requirement, intensive care unit stay and AKI.
In a recent autopsy report of 21 patients, renal findings included mild-to-moderate ATN, presence of myoglobin and thrombotic microangiopathy.[11] To et al.,[12] detected the H1N1 virus in one urine sample of 14 patients with influenza A infection, whereas Abdulkader et al.,[13] reported the presence of H1N1 in one urine sample of two. The close relationship between membranous glomerulonephritis and antigens is known, particularly in secondary forms, which occur after viral infections and vaccinations. A 56-year-old man developed membranous glomerulonephritis 23 days after the vaccination against influenza A (H1N1) virus.[14] Influenza vaccinations have been implicated as possible triggers for Henoch-Schönlein purpura.[15] Although a causal effect cannot be confirmed, few data suggests that influenza A: H1N1 should be considered in the differential diagnosis of AGN and AKI. AGN is most commonly post-infectious and caused by streptococcal throat infection (serotype 12) and less frequently, skin infection (serotype 49). Hepatitis A, B and HIV infections are well-known to cause AGN, but infection with the H1N1 subtype of influenza A (Swine flu) has been less commonly reported in the literature.[16,17,18]
Kidney pathological changes in influenza A (H1N1) virus infection were consistent with ATN. Bowman's capsule epithelial cells and distal tubular cells seem to actively replicate the virus.[19] The limited histopathological data available show that, ATN and rhabdomyolysis contributed to H1N1-induced tubular injury.
Conclusion
Our case suggests that H1N1 infection may have a causative link to the development of mesangial proliferative glomerulonephritis with acute tubulointerstitial nephritis and should be considered in the differential diagnosis of such condition in such patients. It responded well to steroid therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Demirjian SG, Raina R, Bhimraj A, Navaneethan SD, Gordon SM, Schreiber MJ, Jr, et al. 2009 influenza A infection and acute kidney injury: Incidence, risk factors, and complications. Am J Nephrol. 2011;34:1–8. doi: 10.1159/000328386. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T. Renal complications of seasonal and pandemic influenza A virus infections. Eur J Pediatr. 2013;172:15–22. doi: 10.1007/s00431-012-1854-x. [DOI] [PubMed] [Google Scholar]
- 3.Shelke VN, Kolhapure RM, Kadam D, Sangle S, Chadha MC, Basu A, et al. Pathologic study of pandemic influenza A (H1N1) 2009 cases from India. Pathol Int. 2012;62:36–42. doi: 10.1111/j.1440-1827.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 5.Sood MM, Rigatto C, Zarychanski R, Komenda P, Sood AR, Bueti J, et al. Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A (H1N1): Report from a Canadian province. Am J Kidney Dis. 2010;55:848–55. doi: 10.1053/j.ajkd.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayala E, Kagawa FT, Wehner JH, Tam J, Upadhyay D. Rhabdomyolysis associated with 2009 influenza A (H1N1) JAMA. 2009;302:1863–4. doi: 10.1001/jama.2009.1582. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo-Esper R, Ornelas-Arroyo S, Pérez-Bustos E, Sánchez-Zúñiga J, Uribe-Esquivel M. Rhabdomyolysis and acute renal failure in human influenza A H1N1 mediated infection. Gac Med Me×. 2009;145:519–21. [PubMed] [Google Scholar]
- 8.Parikh M, Dolson G, Ramanathan V, Sangsiraprapha W. Novel H1N1-associated rhabdomyolysis leading to acute renal failure. Clin Microbiol Infect. 2010;16:330–2. doi: 10.1111/j.1469-0691.2010.03185.x. [DOI] [PubMed] [Google Scholar]
- 9.Lai CC, Wang CY, Lin HI. Rhabdomyolysis and acute kidney injury associated with 2009 pandemic influenza A (H1N1) Am J Kidney Dis. 2010;55:615. doi: 10.1053/j.ajkd.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Kute VB, Godara SM, Goplani KR, Gumber MR, Shah PR, Vanikar AV, et al. High mortality in critically ill patients infected with 2009 pandemic influenza A (H1N1) with pneumonia and acute kidney injury. Saudi J Kidney Dis Transpl. 2011;22:83–9. [PubMed] [Google Scholar]
- 11.Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2010;181:72–9. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 12.To KK, Chan KH, Li IW, Tsang TY, Tse H, Chan JF, et al. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol. 2010;82:1–7. doi: 10.1002/jmv.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdulkader RC, Ho YL, de Sousa Santos S, Caires R, Arantes MF, Andrade L. Characteristics of acute kidney injury in patients infected with the 2009 influenza A (H1N1) virus. Clin J Am Soc Nephrol. 2010;5:1916–21. doi: 10.2215/CJN.00840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutlucan A, Gonen I, Yildizhan E, Aydin Y, Sav T, Yildirim U. Can influenza H1N1 vaccination lead to the membranous glomerulonephritis? Indian J Pathol Microbiol. 2012;55:239–41. doi: 10.4103/0377-4929.97893. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T. Henoch-Schönlein purpura following influenza vaccinations during the pandemic of influenza A (H1N1) Pediatr Nephrol. 2011;26:795–8. doi: 10.1007/s00467-010-1722-8. [DOI] [PubMed] [Google Scholar]
- 16.Kupferman JC, Trachtman H, Spitzer ED. Acute glomerulonephritis and acute kidney injury associated with 2009 influenza A: H1N1 in an infant. Pediatr Nephrol. 2011;26:153–4. doi: 10.1007/s00467-010-1633-8. [DOI] [PubMed] [Google Scholar]
- 17.Ghiggeri GM, Losurdo G, Ansaldi F, Canepa A, Magnasco A. Two cases of swine H1N1 influenza presenting with hematuria as prodrome. Pediatr Nephrol. 2010;25:779–80. doi: 10.1007/s00467-009-1380-x. [DOI] [PubMed] [Google Scholar]
- 18.Jain T, Hemington L, Etuwewe B. A case of post-infectious glomerulonephritis following infection with influenza A subtype H1N1. Pediatr Nephrol. 2011;26:151–2. doi: 10.1007/s00467-010-1559-1. [DOI] [PubMed] [Google Scholar]
- 19.Nin N, Lorente JA, Sánchez-Rodríguez C, Granados R, Ver LS, Soto L, et al. Kidney histopathological findings in fatal pandemic 2009 influenza A (H1N1) Intensive Care Med. 2011;37:880–1. doi: 10.1007/s00134-011-2183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


