Abstract
Glomerular diseases are an important cause of chronic renal failure in developing countries. The spectrum of diseases causing nephrotic syndrome is changing globally in the last few decades. The aim of this prospective study was to look at this spectrum at a tertiary care center in North India and to analyze the changing trends over the last five decades. Patients in the age group 18-60 years with nephrotic syndrome were consecutively included in the study. Renal biopsies were performed in all patients and were subjected to light microscopy, immunofluorescence (IF) and electron microscopy (EM). While the IF was performed in 78% of cases, EM was available in one-fourth of cases. During 2002-2007, 364 patients (60.2% males) were included in the study. The mean age was 31.5 years. Primary glomerular diseases accounted for 89% of cases while lupus nephritis was the most common secondary glomerular disease. Focal segmental glomerulosclerosis (FSGS) accounted for 30.6% of primary glomerular diseases making it the most common cause of nephrotic syndrome. It was followed by membranous glomerulonephritis (MGN) in 24.4%, mesangiocapillary glomerulonephritis in 17.9% and minimal change disease in 14.8%. In the age group >40 years, MGN was the most common lesion (32.5%) followed by FSGS (27.7%). Over the last five decades, there was a nearly five-fold increase in the incidence of FSGS, 3-fold increase in MGN and a 10-fold reduction in diffuse proliferative glomerulonephritis while there was no major change in incidence of other diseases. The biopsy diagnosis of FSGS has increased considerably in last few decades and it is now the most common cause of nephrotic syndrome in adults in North India. MGN is the most common lesion in patients over 40 years of age.
Keywords: Chronic kidney disease, focal segmental glomerulosclerosis, idiopathic nephrotic syndrome, membranous nephropathy, minimal change disease, renal biopsy
Introduction
Chronic glomerulonephritis, until recently has been the most common cause of chronic kidney disease (CKD) in developing countries like India.[1,2] However, with the increase in diabetes mellitus, the majority of CKD patients in India now have diabetic nephropathy as their underlying etiology. According to the recently published report of Indian National CKD registry, diabetic nephropathy accounts for 31.3% of CKD while glomerulonephritis is the second most common cause.[3] Glomerular diseases in tropical countries is vastly different in epidemiology, etiology and natural history from those seen in temperate countries; and their prevalence also varies according to socio-economic conditions, race, age and indications for renal biopsy.[1] Over the last few years, studies have shown a changing pattern of these diseases. Previous studies showed that membranous glomerulonephritis (MGN) was the most common cause of adult nephrotic syndrome in the United States and Europe.[4] However, more recent studies have shown that the focal segmental glomerulosclerosis (FSGS) is increasing significantly and it has become the most common glomerular disease in African-Americans and Hispanic populations.[4,5] Some studies from India have shown declining incidence of mesangiocapillary glomerulonephritis (MPGN) along with an increase in FSGS, though there are others which have not confirmed this trend.[6,7]
Post Graduate Institute of Medical Education and Research, Chandigarh is a tertiary care referral center in Northern India, catering to a large number of patients not only from Northern India, but also from other parts of the country as well as from Bangladesh, Nepal and Tibet. We first described the spectrum of renal diseases at our center in 1984, when we had analyzed 2998 cases seen from 1964 onwards.[8] Subsequently, in 1998, a further experience of 2947 cases was published.[9,10] The present study was conducted to ascertain the histologic spectrum of nephrotic syndrome in adults at our institute during a 5-year period from 2002 to 2007 and to note the change in the spectrum of these diseases over last five decades.
Materials and Methods
All adults between 18 years and 60 years of age, with nephrotic range proteinuria undergoing renal biopsy over the 5-year period from July 2002 to June 2007 were consecutively included in this prospective study. Patients with long standing type 2 diabetes mellitus in whom diabetic nephropathy was suspected were not biopsied. Nephrotic range proteinuria was defined as proteinuria >3.5 g/1.73 m2 body surface area/day or >50 mg/kg/day. Proteinuria less than this range, but associated with serum albumin <3.0 g/dL was also classified as nephrotic range.
Blood samples were checked for hemoglobin, platelet count, serum creatinine, blood urea, serum albumin, lipid profile, coagulation profile, antinuclear antibody (ANA), hepatitis B surface antigen and anti-hepatitis C virus for all patients. Additional investigations such as blood sugars, anti-double stranded deoxyribonucleic acid, complement levels and anti-neutrophil cytoplasmic antibody were done as and when indicated. All patients underwent an ultrasound evaluation of the kidneys followed by renal biopsy. The biopsy was performed under ultrasound guidance and local anesthesia using 14 G Bard Trucut biopsy gun. Patients were observed for 24 h for procedure related complications. The biopsy material was subjected to histopathology (HP), immunofluorescence (IF) and electron microscopic (EM) examination. All the slides for HP examination were studied after staining with H and E and periodic acid Schiff stains. Jones silver staining and Masson's trichrome staining were done in selected cases. IF examination was done by direct method using fluorescein isothiocyanate conjugated antibodies against immunoglobulin G, A and M as well as for complement C3 and C1q. EM examination was done by epon embedding, 65-70 nm sections, uranyl acetate-lead acetate staining and Ziess 906 visualizing. While the IF was performed in 78% of cases, EM was available in one-fourth of cases.
The data was entered on Microsoft excel sheet and descriptive statistic was used. The Statistical Package for Social Sciences version 20.0, (SPSS, IBM, USA) was used for the analysis. Based on the renal biopsy and the clinical findings, patients were classified into primary and secondary glomerular diseases.
Results
During this 5-year period, 364 patients fulfilling the inclusion criteria were included. Of these, 219 (60.2%) were males and the mean age was 31.5 ± 11 years.
Clinical features
All patients had edema, 25% were hypertensive while 10.4% had oliguria. Mean 24 h urine protein excretion was 4.91 ± 1.98 g, mean serum creatinine was 1.85 ± 2.1 mg/dL, mean serum albumin was 2.6 ± 0.53 g/dL and mean cholesterol was 325 ± 109.9 mg/dL. Microscopic hematuria was observed in 24 patients (5.6%); while none of the patients had macroscopic hematuria. ANA was positive in 28 patients (7.7%) while one patient each was positive for hepatitis B surface antigen and anti-hepatitis C antibody.
Spectrum of glomerular lesions
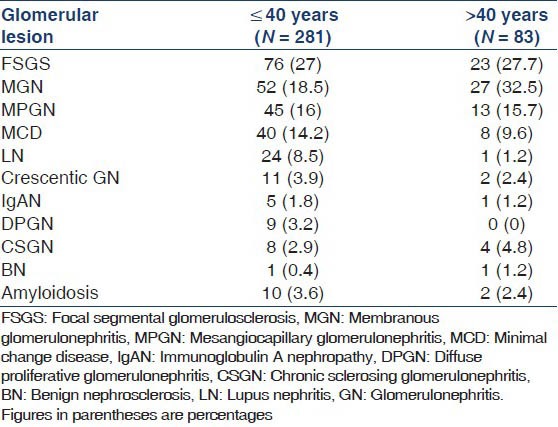
Primary glomerular diseases accounted for 89% of cases while the rest were secondary. The biopsy spectrum is summarized in Table 1. Among the primary glomerular diseases, FSGS was most common followed by MGN; lupus nephritis (LN) was the most common secondary glomerular disease. Class IV LN was the most common (60%) followed by class V (28%). All glomerular diseases, except for LN were more common in males. Hypertension was most common in benign nephrosclerosis (BNS) and chronic sclerosing glomerulonephritis (CSGN) while only 28.3% of FSGS, 25.3% MPGN, 16.5% MGN and 18.7% minimal change disease (MCD) cases had hypertension. FSGS was also the most common glomerular lesion in patients less than 40 years of age while MGN was most common in patients greater than 40 years [Table 2].
Table 1.
Spectrum of glomerular lesions in renal biopsy
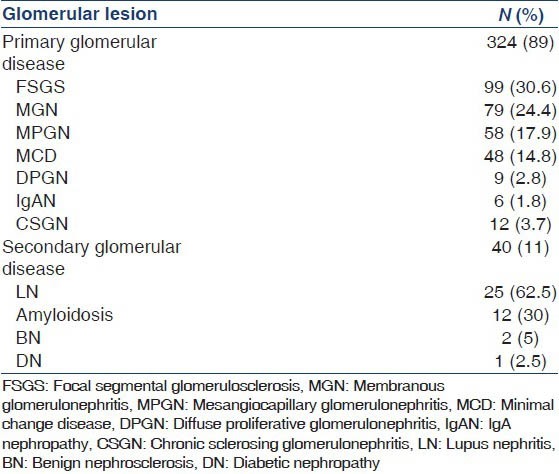
Table 2.
Distribution of glomerular lesions according to age
Renal functional status
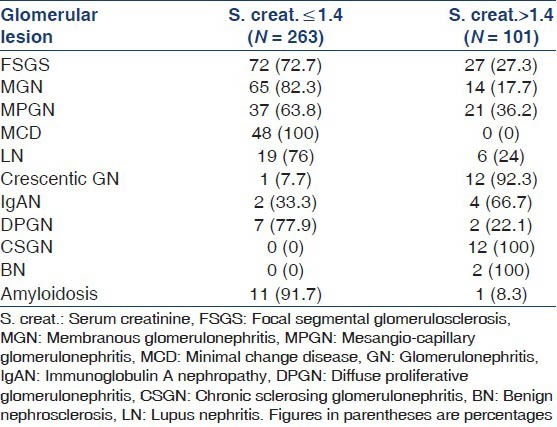
It was observed that serum creatinine was high in all patients with CSGN and BNS. Significant number of patients with crescentic glomerulonephritis (92.3%), Immunoglobulin A nephropathy (IgAN) (66.7%), MPGN (36.2%), FSGS (27.3%) and diffuse proliferative glomerulonephritis (DPGN) (22.1%) had deranged renal functions. All patients with MCD had normal serum creatinine [Table 3].
Table 3.
Distribution of glomerular lesions according to renal function
Changing spectrum over five decades
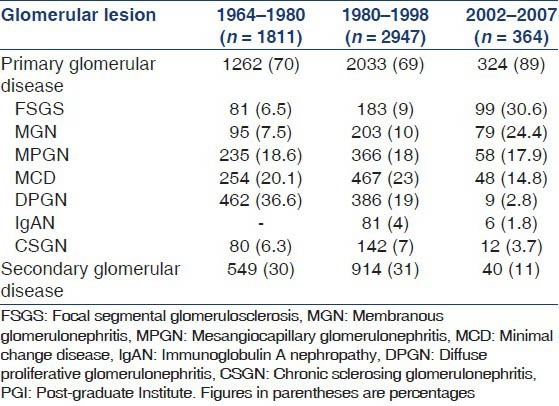
We had previously analyzed 2998 cases of renal disease at our center between 1964 and 1980. Of these, 1811 had glomerular disease.[8] Subsequently again in 1998, a further experience of 2947 cases was reported.[9,10] A comparison of the etiology of biopsy proven nephrotic syndrome at our center during the last five decades is summarized in Table 4. During this period, there has been a five-fold increase in the frequency of FSGS as a cause of nephrotic syndrome along with a three-fold increase in MGN. The frequency of MPGN remained more or less same while there was a slight decline in MCD. The DPGN decreased significantly from 36.6% in 1960-80 to about 3% in the present study while there was no significant change in the frequency of IgAN or CSGN.
Table 4.
Changing spectrum of glomerular diseases at PGI Chandigarh
Discussion
Glomerular diseases are an important cause of end-stage kidney disease. The histologic spectrum of these is different in adults as compared with children as well as in tropical as compared with temperate countries. In the present study, primary glomerular diseases accounted for 89% cases of nephrotic syndrome, while LN was the most common secondary cause. Overall, FSGS was the most common cause of nephrotic syndrome; particularly, if the age was less than 40 years. In greater than 40 years age group, MGN was the most common cause.
An analysis of the spectrum of primary glomerular diseases as a cause of nephrotic syndrome during the last five decades has revealed a 5-fold increase in the frequency of FSGS. Along with that, there was a 3-fold increase in the frequency of MGN making it the second most cause of nephrotic syndrome while DPGN decreased to one-tenth of its earlier prevalence. There was no significant change in MCD or MPGN.[8,9,10] This trend is similar to the emerging global trend, which indicates an increase in the incidence of FSGS making it the number one cause of nephrotic syndrome world-wide.[4,5] There can be a variety of reasons for this changing spectrum. This may be related to improvement in the overall quality-of-life, decreased rate of infections, better socio-economic status, increased incidence of obesity and changing pattern of indications for renal biopsy. A more widespread use of IF and electron microscopy in the analysis of renal biopsy can explain increased diagnosis of MGN and FSGS, which are otherwise likely to be misdiagnosed as MCD.
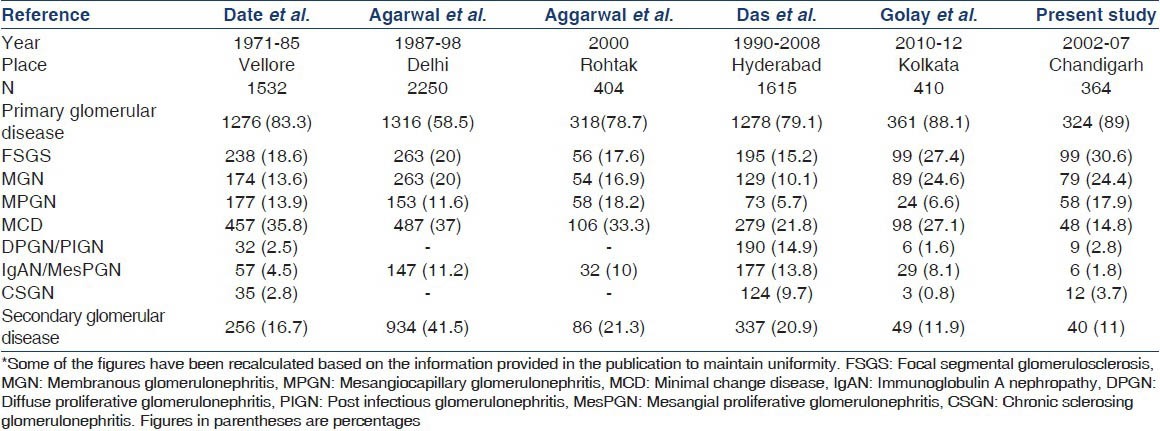
A summary of other studies on the same subject from India is presented in Table 5. While the earlier studies found MCD to be the most common cause, more recent ones show results similar to our study. The study done from Vellore in 1970's noted that MCD accounted for about 35% of all cases of nephrotic syndrome.[6] Similarly, studies from Delhi and Rohtak, also found MCD to be responsible for more than one-third of nephrotic syndrome.[11,12] The study done at Vellore in 1990's found that the incidence of FSGS had increased from 15% to 19% and it became the most common underlying etiology for primary nephrotic syndrome.[7] In a recent study published from Kolkata, Golay et al., found that FSGS was underlying disease in 27.4% of their patients making it the most common one while MGN was third most common accounting for about 25%.[13] This figure is very similar to our present data, where FSGS and MGN were responsible for 31% and 25% of cases respectively. However, they found MCD in 27.1% of cases, making it the second most common cause of nephrotic syndrome while MPGN was seen in only 7%. This is in contrast to our data where MPGN was seen in about 18% of cases while MCD was seen in around 15% of cases. The exact reason for this difference is not clear. A possible explanation may be that only 5.6% of patients in their study were subjected to EM examination in contrast to about one-fourth in our study. In a study published by Siegel et al., it was observed that EM is essential for correct diagnosis in 11% of cases and for confirmation of diagnosis in an additional 36%.[14] The incidence of IgAN was also less at 2% in our study as compared to 4-14% in other studies from India probably since the majority of those with IgAN do not have a nephrotic syndrome.
Table 5.
Comparison of glomerular lesions among nephrotic syndrome in adults in different Indian studies*
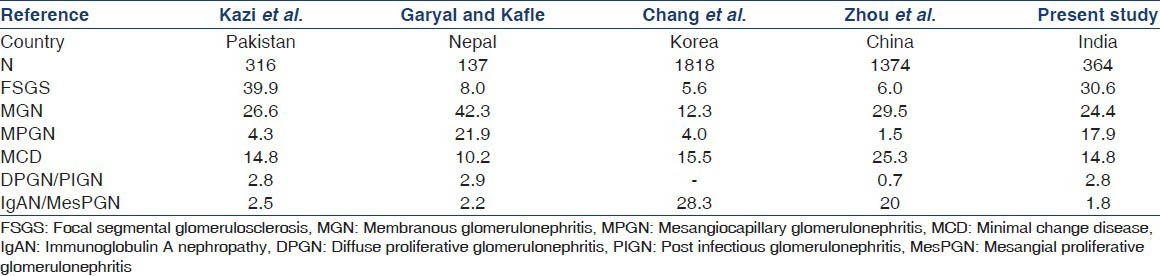
A comparison with other studies from the Asian region is summarized in Table 6 and it shows certain interesting and conflicting data. While studies from Pakistan and Nepal have shown that IgAN is an infrequent cause of nephrotic syndrome with figures of around 2%[15,16] the ones from China and Korea have found it to be very common. Chang et al., observed that IgAN was responsible for 28.3% of nephrotic syndrome making it the most common cause in Korea.[17] Zhou et al., found IgAN to be the second most common cause of nephrotic syndrome after MGN, accounting for 20% of cases in China.[18] However, Kazi et al., from Pakistan have found FSGS to be the most common cause accounting for almost 40% of their cases, followed by MGN (26.6%) and MCD (14.8%).[15]
Table 6.
Comparison of glomerular lesions among nephrotic syndrome in adults in different Asian studies
The data from the West are also conflicting. Studies done in USA have clearly demonstrated increasing incidence of FSGS particularly in African-Americans making it the most common cause of nephrotic syndrome in their adult population.[4,5,19] Not only this, the proportion of FSGS as cause of end-stage renal disease in USA has increased almost ten times in the last two decades.[20] Similarly, studies done from some other parts of the world have shown FSGS to be the most common cause of adult nephrotic syndrome.[21,22,23] There is emerging evidence that its incidence in children is also increasing and a study done in Indian pediatric patients has demonstrated FSGS to be the most common cause in adolescents as compared with MCD in younger patients.[24] However, the data from some European countries including registry data do not agree with this trend. Studies done from Italy and Spain have shown MGN to be the most common cause of adult nephrotic syndrome,[25,26] while those from Denmark, the Czech republic and Romania have shown MCD, IgAN and MPGN respectively to be the most common lesions.[27,28,29]
Thus, in conclusion, there has been considerable heterogeneity in the histologic spectrum of the nephrotic syndrome. However, recent data from USA as well as from the recent studies in India have clearly shown an increase in the incidence of FSGS making it the most common cause of nephrotic syndrome in our population. FSGS was also the most common biopsy diagnosis in our patients with nephrotic syndrome.
The main limitation of the present study is the small sample size. Furthermore, the fact that our institute caters mainly to the population of North India and to some extent East India, thus these results may not be applicable to other parts of the country. While the IF was performed in 78% of cases, EM was available in only one-fourth of cases and a greater use of these methods may further change the spectrum of nephrotic syndrome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sakhuja V, Jha V, Ghosh AK, Ahmed S, Saha TK. Chronic renal failure in India. Nephrol Dial Transplant. 1994;9:871–2. [PubMed] [Google Scholar]
- 2.Jha V. End-stage renal care in developing countries: The India experience. Ren Fail. 2004;26:201–8. doi: 10.1081/jdi-120039516. [DOI] [PubMed] [Google Scholar]
- 3.Rajapurkar MM, John GT, Kirpalani AL, Abraham G, Agarwal SK, Almeida AF, et al. What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10. doi: 10.1186/1471-2369-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis. 1997;30:621–31. doi: 10.1016/s0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 5.Kitiyakara C, Kopp JB, Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:172–82. doi: 10.1053/snep.2003.50025. [DOI] [PubMed] [Google Scholar]
- 6.Date A, Raghavan R, John TJ, Richard J, Kirubakaran MG, Shastry JC. Renal disease in adult Indians: A clinicopathological study of 2,827 patients. Q J Med. 1987;64:729–37. [PubMed] [Google Scholar]
- 7.Balakrishnan N, John GT, Korula A, Visalakshi J, Talaulikar GS, Thomas PP, et al. Spectrum of biopsy proven renal disease and changing trends at a tropical tertiary care centre 1990-2001. Indian J Nephrol. 2003;13:29–35. [Google Scholar]
- 8.Chugh KS, Sakhuja V. Renal diseases in Northern India. In: Kibukamusoke JW, editor. Tropical Nephrology. Canberra, Australia: City Forge Pty Ltd; 1984. pp. 428–40. [Google Scholar]
- 9.Chugh KS. Renal disease in India. Am J Kidney Dis. 1998;31:Ivii–Iix. [PubMed] [Google Scholar]
- 10.Chugh KS, Sakhuja V. Glomerular diseases in the tropics. Am J Nephrol. 1990;10:437–50. doi: 10.1159/000168167. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal SK, Dash SC. Spectrum of renal diseases in Indian adults. J Assoc Physicians India. 2000;48:594–600. [PubMed] [Google Scholar]
- 12.Aggarwal HK, Yashodara BM, Nand N, Sonia, Chakrabarti D, Bharti K. Spectrum of renal disorders in a tertiary care hospital in Haryana. J Assoc Physicians India. 2007;55:198–202. [PubMed] [Google Scholar]
- 13.Golay V, Trivedi M, Kurien AA, Sarkar D, Roychowdhary A, Pandey R. Spectrum of nephrotic syndrome in adults: Clinicopathological study from a single center in India. Ren Fail. 2013;35:487–91. doi: 10.3109/0886022X.2013.768939. [DOI] [PubMed] [Google Scholar]
- 14.Siegel NJ, Spargo BH, Kashgarian M, Hayslett JP. An evaluation of routine electron microscopy in the examination of renal biopsies. Nephron. 1973;10:209–15. doi: 10.1159/000180189. [DOI] [PubMed] [Google Scholar]
- 15.Kazi JI, Mubarak M, Ahmed E, Akhter F, Naqvi SA, Rizvi SA. Spectrum of glomerulonephritides in adults with nephrotic syndrome in Pakistan. Clin Exp Nephrol. 2009;13:38–43. doi: 10.1007/s10157-008-0075-0. [DOI] [PubMed] [Google Scholar]
- 16.Garyal, Kafle RK. Hisopathological spectrum of glomerular disease in Nepal: A seven-year retrospective study. Nepal Med Coll J. 2008;10:126–8. [PubMed] [Google Scholar]
- 17.Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, et al. Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant. 2009;24:2406–10. doi: 10.1093/ndt/gfp091. [DOI] [PubMed] [Google Scholar]
- 18.Zhou FD, Shen HY, Chen M, Liu G, Zou WZ, Zhao MH, et al. The renal histopathological spectrum of patients with nephrotic syndrome: An analysis of 1523 patients in a single Chinese centre. Nephrol Dial Transplant. 2011;26:3993–7. doi: 10.1093/ndt/gfr166. [DOI] [PubMed] [Google Scholar]
- 19.Korbet SM, Genchi RM, Borok RZ, Schwartz MM. The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis. 1996;27:647–51. doi: 10.1016/s0272-6386(96)90098-0. [DOI] [PubMed] [Google Scholar]
- 20.Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815–25. [PubMed] [Google Scholar]
- 21.Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, et al. Japan Renal biopsy registry: The first nationwide, web-based, and prospective registry system of renal biopsies in Japan. Clin Exp Nephrol. 2011;15:493–503. doi: 10.1007/s10157-011-0430-4. [DOI] [PubMed] [Google Scholar]
- 22.Jha V. Current status of chronic kidney disease care in Southeast Asia. Semin Nephrol. 2009;29:487–96. doi: 10.1016/j.semnephrol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Das U, Dakshinamurty KV, Prayaga A. Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol. 2011;21:250–7. doi: 10.4103/0971-4065.85482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulati S, Sural S, Sharma RK, Gupta A, Gupta RK. Spectrum of adolescent-onset nephrotic syndrome in Indian children. Pediatr Nephrol. 2001;16:1045–8. doi: 10.1007/s004670100023. [DOI] [PubMed] [Google Scholar]
- 25.Gesualdo L, Di Palma AM, Morrone LF, Strippoli GF, Schena FP Italian immunopathology group, Italian society of nephrology. The Italian experience of the national registry of renal biopsies. Kidney Int. 2004;66:890–4. doi: 10.1111/j.1523-1755.2004.00831.x. [DOI] [PubMed] [Google Scholar]
- 26.Rivera F, López-Gómez JM, Pérez-García R Spanish Registry of glomerulonephritis. Clinicopathologic correlations of renal pathology in Spain. Kidney Int. 2004;66:898–904. doi: 10.1111/j.1523-1755.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 27.Heaf J, Løkkegaard H, Larsen S. The epidemiology and prognosis of glomerulonephritis in Denmark 1985-1997. Nephrol Dial Transplant. 1999;14:1889–97. doi: 10.1093/ndt/14.8.1889. [DOI] [PubMed] [Google Scholar]
- 28.Rychlík I, Jancová E, Tesar V, Kolsky A, Lácha J, Stejskal J, et al. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19:3040–9. doi: 10.1093/ndt/gfh521. [DOI] [PubMed] [Google Scholar]
- 29.Covic A, Schiller A, Volovat C, Gluhovschi G, Gusbeth-Tatomir P, Petrica L, et al. Epidemiology of renal disease in Romania: A 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant. 2006;21:419–24. doi: 10.1093/ndt/gfi207. [DOI] [PubMed] [Google Scholar]


