Abstract
Smoking is associated with an excessive morbidity and mortality from a variety of diseases. The aim of this study was to find out the effects of smoking on renal function study in non-diabetic, normotensive subjects. A community-based, prospective, cross-sectional cohort study was conducted on 120 subjects; 80 (66.66%) were smokers and 40 (33.33%) age matched non-smokers; with age range of 30 to 70 years. Measurement of fasting sugar, urea, creatinine, lipids and one time screening of urinary albumin and urinary creatinine was done. Smokers had significantly higher urinary albumin and albumin creatinine ratio (ACR) (52.84 ± 46.42 mg/L, 93.98 ± 78.68 μg/mg) than non-smokers (19.25 ± 7.77 mg/L, 18.99 ± 6.65 μg/mg), respectively (P =< 0.001, P =< 0.001). Microalbuminuria and urinary ACR level were directly related to the amount of smoking (pack-years). Among smokers, 73 (91.25%) had microalbuminuria (>20 mg/L) and 64 (80%) had increased urinary ACR (>30 μg/mg). Smoker had significantly lower high-density lipoprotein level (36.66 ± 10.28 mg/dl) compared to non-smokers (41.22 ± 11.72 mg/dl) (P = 0.031). Urea, creatinine, creatinine clearance, total cholesterol, low density lipoprotein, triglyceride levels were comparable (p = NS). In conclusion, smokers have a 4-fold higher prevalence of microalbuminuria than non-smokers.
Keywords: Creatinine clearance, microalbuminuria, non-diabetic normotensive, smoker, urinary albumin creatinine ratio
Introduction
Smoking affects vascular and hormonal systems and is also involved in the development of atherosclerosis, thrombogenesis and vascular occlusion.[1] Chronic smoking adversely influences the prognosis of nephropathies.[2]
In primary hypertension, urinary albumin excretion as an index of renal damage is highly correlated with smoking.[3,4] In established diabetic nephropathy, the rate of progression to renal failure amongst smokers is twice in both Type I and Type II diabetes.[5]
This study was intended for non-diabetic normotensive subjects to find out the effect of smoking on renal functions such as microalbuminuria, creatinine clearance and urinary albumin creatinine ratio (ACR).
Materials and Methods
This was a community-based prospective cross-sectional cohort study conducted on 120 non-diabetic normotensive subjects as per the inclusion criteria. It was approved by the institutional ethics committee and conducted in accordance with the guidelines of the Indian Council of Medical Research, international conference on harmonization-good clinical practices instructions of drug controller general of India New Delhi and institutional SOP with a proper informed consent.
Inclusion criteria were age 30-70 years, normotensive (≤139/≤89 mmHg), non-diabetic (fasting plasma glucose ≤125 mg/dl), non-obese (body mass index [BMI] <30 kg/m2), no family history of premature vascular disease, normal total cholesterol level (<200 mg/dl), normal renal function (urea ≤40 mg/dl and creatinine ≤1 mg/dl), clinically well and not on any regular cardiovascular medication and given informed consent. Exclusion criteria were age less than 30 and above 70 years, diabetics or using insulin or oral hypoglycaemic agents, hypertensive or using antihypertensive medication, hyperlipidemic or using lipid lowering drugs, obese (BMI ≥30 kg/m2), abnormal renal function (urea and creatinine), urinary tract infection, significant renal disease or using diuretics, angiotensin converting enzyme inhibitors, alcohol consumption or other significant drugs, fever, vigorous physical activity, menstruating/pregnant women and not willing to give consent.
Smoker was defined as anyone who had smoked at least 20 bidi/day for 5 years (5 pack-years) or equivalent. Smokers were divided into four sub-groups: Very light smoker (5-9 pack-years), light smoker (10-14 pack-years), moderate smoker (15-19 pack-years) and heavy smoker (>20 pack-years). Non-smoker neither smoked nor chewed tobacco in any form.
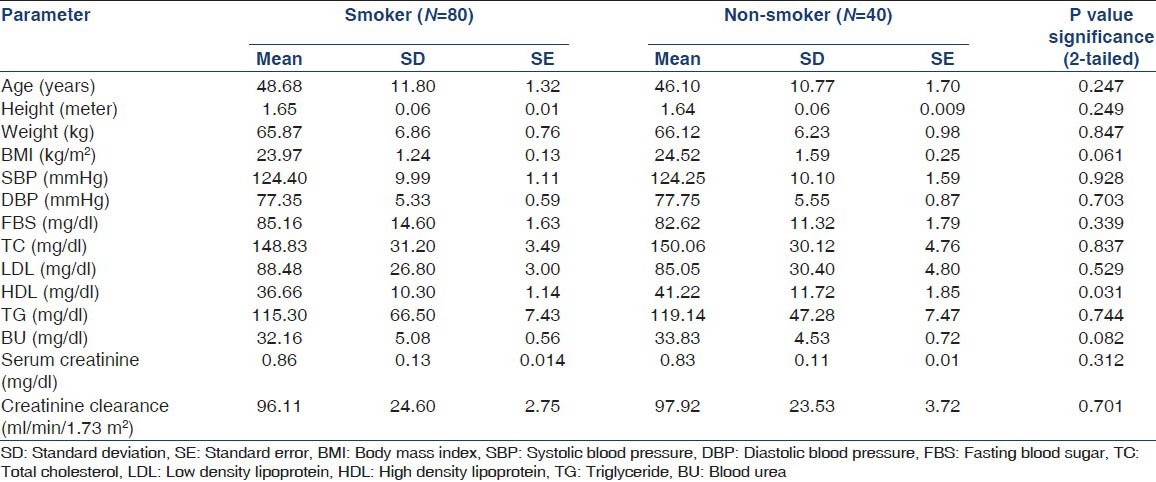
Out of 120 non-diabetic normotensive subjects, 80 (66.66%) were smokers and 40 (33.33%) were age matched non-smokers as control. The baseline physical characteristic and investigation were compared in these two groups using the statistical tests of significance (p = NS) [Table 1].
Table 1.
Baseline subjects characteristics and their comparison in both groups
Overnight fasting blood sugar, blood urea, serum creatinine and lipid profile were measured and first morning void (timed) quantitative mid-stream urine was sample taken for screening of urinary albumin and urinary creatinine concentrations [Table 1].
Laboratory methods used included; CLINITEK Micro albumin Reagent Strips[6] (urinary albumin and ACR), Jaffe colorimetric method (serum creatinine concentration), glucose enzymatic colorimetric (glucose oxidase/peroxidase) method (plasma glucose), enzymatic colorimetric cholesterol (cholesterol oxidase/peroxidase aminophenazone) method (serum lipid profile), urine analyzer URISCAN Optima II + Combistix SG, Korea (urinary leukocyte and erythrocyte).
All calculations were performed with the Statistical Package for the Social Sciences (SPSS) version 9.0 (SPSS, Chicago, USA) software by Chi-square analysis or analysis of variance.
Results
Out of 120 non-diabetic normotensive subjects, 80 (66.66%) were smoker and 40 (33.33%) were non-smoker. 83 subjects (69.16%) were male and 37 subjects (30.83%) were female. The mean age in smoker group was 48.68 ± 11.82 years and in non-smoker group was 46.10 ± 10.77 years, ranged from 30 to 70 years (P = 0.247) [Table 1]. The two groups were comparable in terms of all parameters except high-density lipoprotein (HDL) level (P = 0.031) [Table 1].
Smokers had higher mean urinary albumin level (52.84 mg/L) than non-smokers (19.25 mg/L) (P < 0.0001) [Figure 1]. Among smokers (n = 80), microalbuminuria was directly related to the amount of smoking (pack-years) [Figure 2]. Seventy three smokers (91.25%) and nine non-smokers (22.5%) had urinary albumin level > 20 mg/L (microalbuminuria). Seven smokers (8.75%) and 31 non-smokers (77.5%) had urinary albumin level < 20 mg/L [Figure 3].
Figure 1.
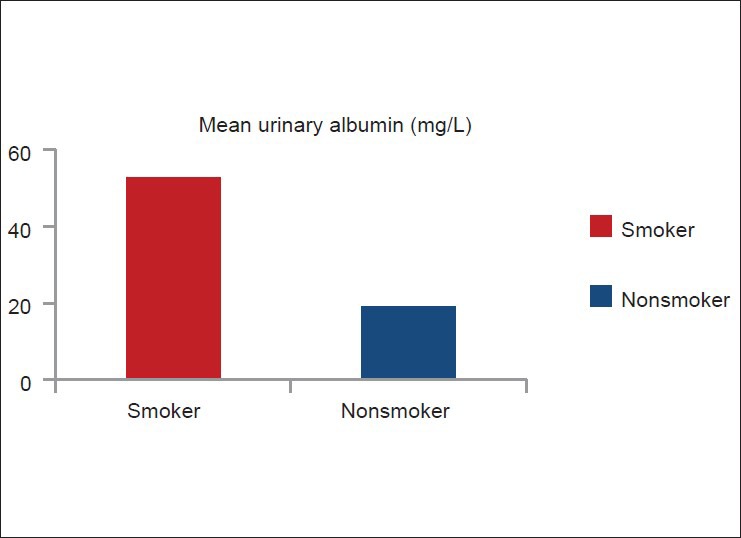
Comparison of mean urinary albumin level between smoker (n =80) and non-smoker (n =40)
Figure 2.
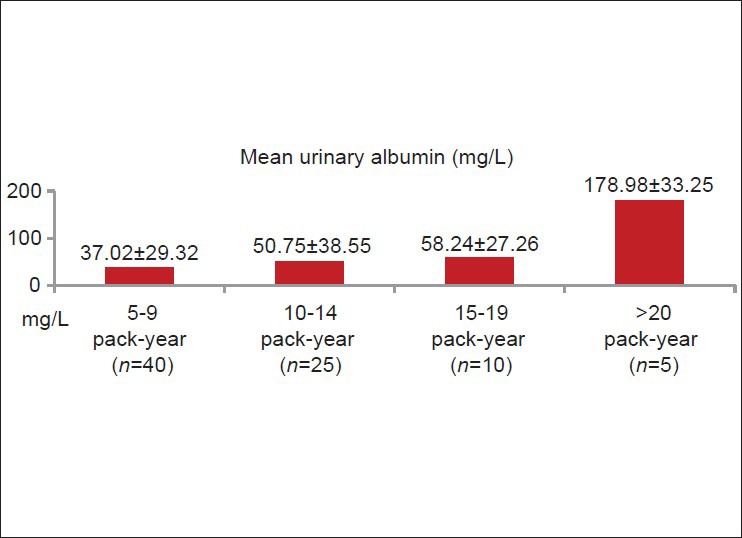
Relationship between quantity of smoking and mean urinary albumin in smoker
Figure 3.
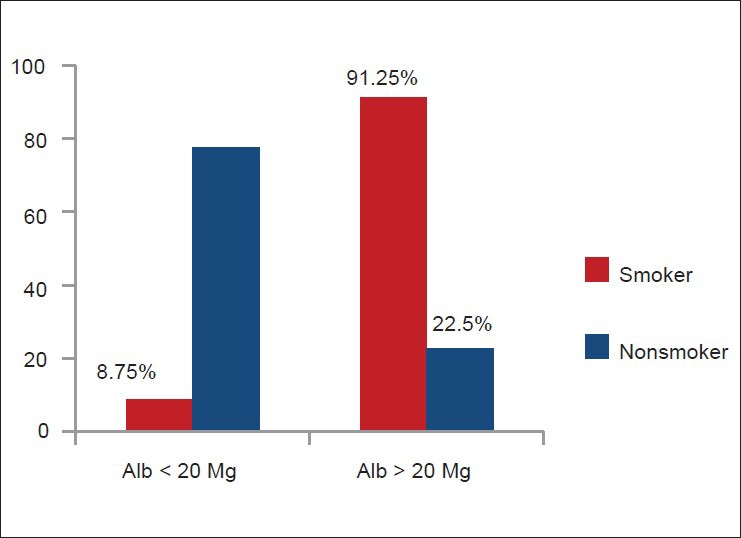
Comparison of smoker (n =80) and non-smoker (n =40) subjects for microalbuminuria
Smoker (n = 80) had higher mean urinary ACR (93.98 μg/mg) than non-smoker (n = 40, 18.99 μg/mg) (P < 0.001) [Figure 4]. Among smokers (n = 80), urinary ACR level was directly related to the amount of smoking (pack-years) [Figure 5]. Sixty four smokers (80%) and two non-smokers (5%) had urinary ACR level >30 μg/mg. Only 16 smokers (20%) and 38 non-smokers (95%) had urinary ACR <30 μg/mg [Figure 6].
Figure 4.
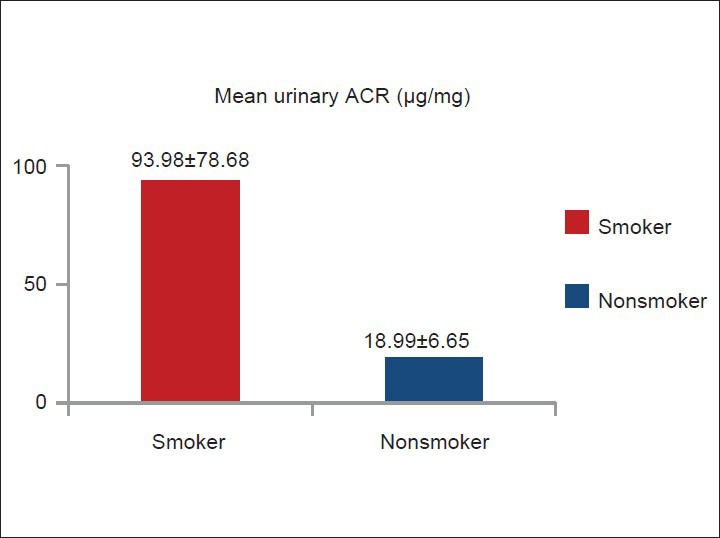
Comparison of mean urinary albumin creatinine ratio level between smoker (n =80) and non-smoker (n =40)
Figure 5.
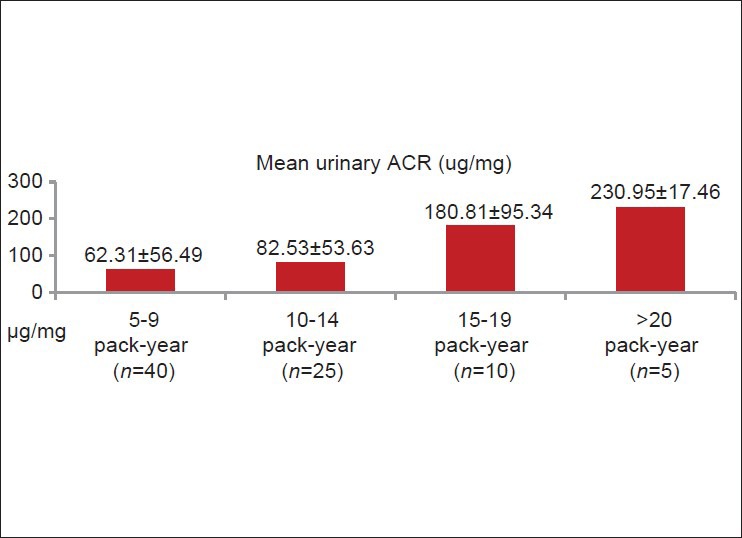
Relationship between quantity of smoking and urinary albumin creatinine ratio in smoker
Figure 6.
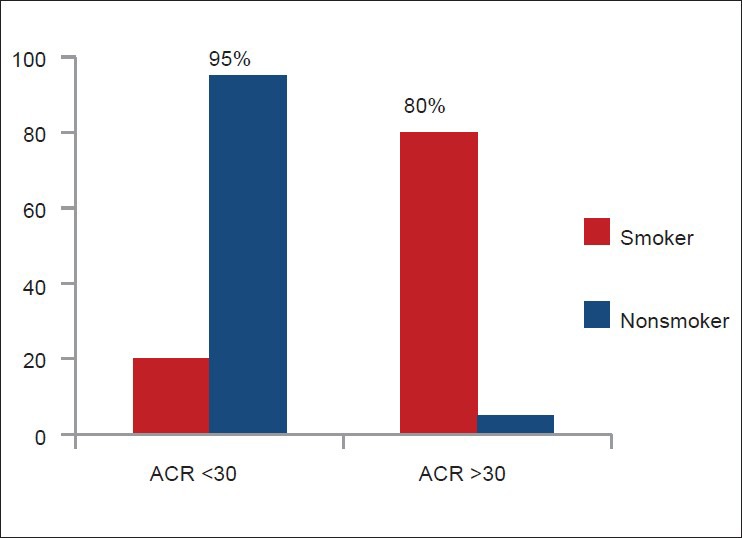
Comparison of smoker (n = 80) and non-smoker (n = 40) subjects for high urinary albumin creatinine ratio
Male and female smokers had no statistically significant distribution of urinary albumin, urinary creatinine and urinary ACR (p = NS).
Discussion
This study shows non-diabetic normotensive smoker had higher mean urinary albumin level, which is directly related to the amount of smoking (pack-years) among smokers. Also, more smokers had microalbuminuria, which signify smokers have 4-fold higher prevalence of microalbuminuria than non-smokers.
The heart outcome prevention evaluation study[7] documented that smoking was an independent determinant of microalbuminuria in all participants, i.e., non-diabetic and diabetic patients with a high cardiovascular risk profile. PREVEND study[8,9] showed statistically significant difference in urinary albumin excretion in non-smokers and smokers. A recent study[10] found that patients with hypertension and left ventricular hypertrophy smoking >20 cigarettes/day had a 1.6-fold higher prevalence of microalbuminuria and a 3.7-fold higher prevalence of macroalbuminuria than never-smokers. Several studies documented that smoking is an independent predictor of (micro) albuminuria in otherwise healthy hypertensive subjects. The prevalence of microalbuminuria is almost double in smoking than non-smoking lean patients with the primary hypertension.[3]
One of the underlying mechanisms by which smoking induces albuminuria and abnormalities in renal function is through advanced glycation end products (AGEPs). AGEPs are cross-linking moieties formed from the reaction of reducing sugars and the amino groups of plasma proteins, lipids and nucleic acids. It is known that AGEPs are responsible for enhanced vascular permeability[11,12] and that they accelerate vasculopathy of end-stage diabetic renal disease.[13,14,15] Recently, Cerami et al.,[16] have shown that both aqueous extracts of tobacco and cigarette smoke contain glycotoxins, highly reactive glycation products that can rapidly induce AGEP formation on proteins in vitro and in vivo. It is reasonable to expect that the AGEPs formed by the reaction of glycotoxins from cigarette smoke with serum and tissue proteins will have the same effect on the systemic and renal vasculature as mentioned. Another mechanism, based on the patho-physiological effect of smoking induced renal damage, is insulin resistance. Several investigators have described smoking to be causally related to insulin resistance in non-diabetic subjects.[17,18,19] Insulin resistance has been known to be related to both albuminuria[20] and abnormalities in renal function.[21,22]
Both mechanisms act through endothelial dysfunction that is by inducing an imbalance between the contracting and relaxing substances produced by the endothelium. The plasma concentration of endothelin 14 has shown to be increased in smokers as compared to non-smokers, also indirect evidence available for a disturbance of endothelin, prostacyclin or nitric oxide release on stimulation in smokers.[23,24,25]
This study shows non-diabetic normotensive smoker had significantly higher mean urinary ACR which is directly related to the amount of smoking (pack-years) among smokers. Amongst non-smokers, female had significantly higher urinary ACR. Females have low muscle mass compared with males; therefore, this sex specific difference in ACR is due to decreased urinary creatinine excretion in females. Warram et al., also proposed Sex-specific ACR cut points for microalbuminuria as ≥17 and ≥25 μg/mg for men and women, respectively.[26]
A smoker had serum creatinine and creatinine clearance comparable with non-smoker, similar to PREVEND study.[8,9]
Among smokers, both male and female had comparable urinary albumin, urinary creatinine and urinary ACR (P = NS), which signifies that the effect of smoking on renal function is not affected by gender.
In this study, subjects having hypercholesterolemia (total cholesterol >200 mg/dl) were excluded due to their known influence on renal function.[27] Smoker had significantly lower HDL than non-smokers, similar to several studies, which shows HDL is lower in smokers than in non-smokers.[28]
However, limitations of this study are a small number of subjects, single center data and screening with one timed urine sample, it is in comparison with one of the many ongoing prevention of renal and vascular end stage disease (PREVEND) group studies[8,9] (PREVEND), running in the city of Groningen, the Netherlands (n = 7476) with the mean age of smoker (47 ± 12 years who smoke <20 cigarette/day, 46 ± 10 years who smoke > 20 cigarette/day) and non-smoker (47 ± 13 years).
This area of research needs further attention of physicians and nephrologists, looking to the highly prevalent smoking addiction in Indian community as an independent risk factor and its impact on renal function, therefore; more such studies can be planned at various centers.
Conclusion
Non-diabetic normotensive smokers have significantly higher level of urinary albumin and urinary ACR than non-smoker, which is directly proportional to quantity of smoking. Similarly, smokers have a higher prevalence of 4-fold for microalbuminuria and 16-fold for increased urinary ACR than non-smokers. Smoking significantly reduces the HDL level; however, no significant effect on serum creatinine and creatinine clearance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87(3 Suppl):II56–65. [PubMed] [Google Scholar]
- 2.Orth SR, Ritz E, Schrier RW. The renal risks of smoking. Kidney Int. 1997;51:1669–77. doi: 10.1038/ki.1997.232. [DOI] [PubMed] [Google Scholar]
- 3.Mimran A, Ribstein J, DuCailar G, Halimi JM. Albuminuria in normals and essential hypertension. J Diabetes Complications. 1994;8:150–6. doi: 10.1016/1056-8727(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 4.Hörner D, Fliser D, Klimm HP, Ritz E. Albuminuria in normotensive and hypertensive individuals attending offices of general practitioners. J Hypertens. 1996;14:655–60. doi: 10.1097/00004872-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Biesenbach G, Janko O, Zazgornik J. Similar rate of progression in the predialysis phase in type I and type II diabetes mellitus. Nephrol Dial Transplant. 1994;9:1097–102. doi: 10.1093/ndt/9.8.1097. [DOI] [PubMed] [Google Scholar]
- 6.Graziani MS, Gambaro G, Mantovani L, Sorio A, Yabarek T, Abaterusso C, et al. Diagnostic accuracy of a reagent strip for assessing urinary albumin excretion in the general population. Nephrol Dial Transplant. 2009;24:1490–4. doi: 10.1093/ndt/gfn639. [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Mann JF, Pogue J, Dinneen SF, Hallé JP, Hoogwerf B, et al. Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the heart outcomes prevention evaluation study. The HOPE study investigators. Diabetes Care. 2000;23(Suppl 2):B35–9. [PubMed] [Google Scholar]
- 8.Pinto-Sietsma SJ, Mulder J, Janssen WM, Hillege HL, de Zeeuw D, de Jong PE. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med. 2000;133:585–91. doi: 10.7326/0003-4819-133-8-200010170-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–26. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 10.Wachtell K, Olsen MH, Dahlöf B, Devereux RB, Kjeldsen SE, Nieminen MS, et al. Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE study. J Hypertens. 2002;20:405–12. doi: 10.1097/00004872-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Esposito C, Gerlach H, Brett J, Stern D, Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989;170:1387–407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc Natl Acad Sci U S A. 1992;89:12043–7. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–21. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 14.Makita Z, Bucala R, Rayfield EJ, Friedman EA, Kaufman AM, Korbet SM, et al. Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet. 1994;343:1519–22. doi: 10.1016/s0140-6736(94)92935-1. [DOI] [PubMed] [Google Scholar]
- 15.Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–42. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 16.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94:13915–20. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–30. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 18.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance – A potential link with the insulin resistance syndrome. J Intern Med. 1993;233:327–32. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 19.Eliasson B, Attvall S, Taskinen MR, Smith U. The insulin resistance syndrome in smokers is related to smoking habits. Arterioscler Thromb. 1994;14:1946–50. doi: 10.1161/01.atv.14.12.1946. [DOI] [PubMed] [Google Scholar]
- 20.Forsblom CM, Eriksson JG, Ekstrand AV, Teppo AM, Taskinen MR, Groop LC. Insulin resistance and abnormal albumin excretion in non-diabetic first-degree relatives of patients with NIDDM. Diabetologia. 1995;38:363–9. doi: 10.1007/BF00400643. [DOI] [PubMed] [Google Scholar]
- 21.Park SK, Kang SK. Renal function and hemodynamic study in obese Zucker rats. Korean J Intern Med. 1995;10:48–53. doi: 10.3904/kjim.1995.10.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53:1343–7. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 23.Kiowski W, Linder L, Stoschitzky K, Pfisterer M, Burckhardt D, Burkart F, et al. Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced vasoconstriction to exogenously administered endothelin-1 in clinically healthy smokers. Circulation. 1994;90:27–34. doi: 10.1161/01.cir.90.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Nitenberg A, Antony I, Foult JM. Acetylcholine-induced coronary vasoconstriction in young, heavy smokers with normal coronary arteriographic findings. Am J Med. 1993;95:71–7. doi: 10.1016/0002-9343(93)90234-g. [DOI] [PubMed] [Google Scholar]
- 25.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–55. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 26.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–7. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 27.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: A meta-analysis. Kidney Int. 2001;59:260–9. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 28.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: An analysis of published data. BMJ. 1989;298:784–8. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]


