Abstract
Introduction:
Despite the advances in medical sciences, the morbidity and mortality due to sepsis in severe trauma patients remains high; hence the need for early and accurate diagnosis. Very few prospective studies are available in a country like India, which tried to analyze the prediction of sepsis using serum procalcitonin (PCT) in such a large scale among trauma patients. This study explores the role of the biomarker PCT in early diagnosis of sepsis and prediction of outcomes in severe trauma cases.
Materials and Methods:
We studied the patient population prospectively in two different groups. One with acute trauma but no clinical evidence of sepsis and the second group with clinical evidence of sepsis and are followed. Bronchoalveolar lavage, tracheal aspirates, pus, urine, body fluids from sterile body sites, etc., were collected including blood for culture and serum for PCT assays. Such assays were done on samples collected on days 1 and 4 and then compared. Additionally, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels were also tested. Antimicrobial sensitivity tests were carried out for all the isolates from the clinical samples and correlated with the clinically suspected cases of sepsis. Outcomes of the patients were noted.
Results:
Patients with high initial PCT levels (>2 ng/ml) in severe trauma cases had poor outcomes and risk of developing complications. Its correlation with severe outcomes was better marked as compared with CRP and ESR levels. The difference in PCT levels between days 1 and 4 in group two patients was statistically significant (P = 0.006) but were not statistically significant for CRP (P = 0.646) and ESR (P = 0.935). The study also shows that PCT levels fall in response to appropriate antimicrobial treatment.
Conclusion:
PCT is a useful biomarker for early and accurate prediction of sepsis in severe trauma patients. If used in adjunct to clinical findings, it proves to be a good biomarker for early diagnosis, treatment and for monitoring response to therapy in confirmed cases of sepsis. It will prove to be a good supportive indicator of sepsis in early stages for the trauma patients in a low resource country like India.
Keywords: Biomarkers, procalcitonin, prognosis, sepsis, trauma
INTRODUCTION
Severe trauma, a potent cause of systemic inflammatory response syndrome (SIRS),[1] is on a rise due to the increasing number of vehicles plying on the roads thereby leading to a surge in road traffic associated accidents. Such patients require long-term hospital care and are at high risk for developing sepsis. Sepsis is defined as a host response to infection and it is an important cause of morbidity and mortality all around the world.[2] Recent data have suggested that 18 million of new sepsis cases occur each year worldwide, with a mortality rate of almost 30%.[3] The diagnosis of sepsis is difficult, because clinical signs of sepsis often overlap with other noninfectious causes of systemic inflammation.[1,4] SIRS is very common in critically ill patients, being found in various conditions including trauma, surgery, and hypoxic injuries. Though SIRS might be present in ≥90% of the surgical intensive care unit (ICU) patients, its definitive diagnostic criteria might not always be clearly evident or absent.[5] Hence, parameters like body temperature, heart rate, blood cell count, and respiratory rate can be measured and noted, but they give variable results, which causes a problem in arriving at an early and accurate diagnosis. For such critically ill patients, early diagnosis and treatment is the cornerstone for the management.
In developing countries like India, trauma is a major problem, due to a very high incidence of vehicular accidents (6% of global vehicular accidents), other accidental injuries, crime and violence.[6] Rising population, urbanization, industrialization, and a drastic rise in vehicular transport has contributed to an annual increase in road traffic accidents by 3%.[6,7] An accident death is reported every 1.9 minutes in India;[7] in 1997, 10.1% of all deaths in India were due to accidents and injuries.[6] Although India contributes to a significant burden of global trauma morbidity, studies on complications of trauma are very scarce.
With an increasing population of young adults being admitted with severe trauma, studies are urgently needed to develop and evaluate rapid diagnostic assays for sepsis, which is one of the most important contributors of posttrauma mortality. Rapid diagnosis of this primarily treatable condition will be extremely beneficial for preventing morbidity and mortality of traumatized patients.
The currently available continuously monitoring blood culture systems make it possible to detect bacterial growth within 2-10 hours after the blood sample has been taken.[8,9] Full identification and sensitivity requires time (12-24 hours). As a result, the outcome may be worse and the length of hospital stay longer.[10] New parameters are being explored, which will help in opening doors to an early diagnosis and better outcomes. Serial measurements of various biomarkers of body organ function and inflammatory responses of the patients in conditions of trauma or associated sepsis are being explored. Parameters, which have been tried, ranges from procalcitonin (PCT), C-reactive protein (CRP), interleukin-6 (IL-6), sepsis related or sequential organ failure assessment (SOFA) score,[11] and white blood cell (WBC) count. Abnormal levels or specific algorithms patterns of these biomarkers often hints at the revision of the therapeutic management.
These biomarkers have certain short-comings while interpreting the results and hence should be interpreted with caution. CRP is one of the nonspecific acute phase reactants and may increase in response to an inflammatory reaction irrespective of its causative agent. Similarly, white cell count also lack specificity for bacterial infection.[12]
To solve this dilemma, PCT has been evaluated with great interest as a potential and more accurate diagnostic marker of sepsis. PCT, a 116-amino acid peptide involved as a precursor in calcium homeostasis, has been studied as a marker to differentiate sepsis from other noninfectious causes of SIRS. PCT is produced ubiquitously in response to endotoxin or mediators released in response to bacterial infections [i.e. IL-1b, tumor necrosis factor (TNF)-α, and IL-6] and strongly correlates with the extent and severity of bacterial infections.[13] Since, upregulation of PCT is attenuated by interferon (INF) γ, a cytokine released in response to viral infections; PCT is more specific for bacterial infections and may help to distinguish bacterial infections from viral illnesses.[14,15,16,17,18,19] The PCT plasma level in healthy individuals is low; usually <0.1 ng/ml. The levels have been shown to rise with the severity of sepsis. PCT shows a favorable kinetic profile for use as a clinical marker: It promptly increases within 6-12 hours upon stimulation and circulating PCT levels halve daily when the infection is controlled by the host immune system or antibiotic therapy.[19] A number of studies have signified the role of PCT in diagnosing infections like community acquired and ventilator associated pneumonia (VAP), pancreatitis, neonatal infections, and septicemia. About 90% of the studies are from developed countries, where PCT is studied with CRP and IL6.[17] However, very few studies have been done on the role of PCT in predicting sepsis and outcome in multiple trauma patients in a low resource country like India on a large scale prospectively.
Therefore, this study was conducted with the aim to evaluate the utility of serum PCT levels as a diagnostic marker for sepsis in trauma patients and to compare the PCT assay with blood culture for prognostication of response to treatment.
MATERIALS AND METHODS
Study design
This study was conducted prospectively for a period of 4 years from January 2008 to December 2011 at a level – I Apex trauma center covering most of the north Indian population. Patients admitted with acute trauma care and who have survived the initial 24 hours of trauma was included consecutively in the study within the said time period. To avoid discrepancy and confounding between the groups, each patient was enrolled only once in the study. Patients with existing comorbidities like diabetes mellitus, hypertension, liver diseases, renal dysfunction, COPD, etc., were excluded from the study. Each of the included patients or next of kin received written information and gave informed consent. The study protocol was approved by the institute's ethics committee. The patients were divided into two groups for studying the two objectives.
Group 1: A total of 200 patients of fresh physical trauma due to an accident, admitted to the emergency and ICUs of Jai Prakash Narayan Apex Trauma Centre, AIIMS were included in this group. Out of these, 92 were from various ICUs and 108 were from emergency department. Being a trauma care center, participating ICUs were surgical, orthopedic, and neurosurgery. The inclusion criteria applied to this group were (1) a patient should be aged more >18 years, (2) who have survived the initial 24 hours of trauma, and (3) who had no clinical evidence of sepsis. Patients who had suffered from chemical or burn trauma were not included in this study group. Referred patients of trauma who had been treated outside the JPN Apex Trauma Center for more than 24 hours were not included. Thus patients enrolled in group 1 of this study were those who were 24 hours posttrauma and admitted due to severe injuries.
Group 2: A total of 75 patients of physical trauma admitted to the ICUs, in whom a clinical suspicion of sepsis was present, were included in this group. Sepsis was defined as per the recommendations of the consensus conference committee of the American College of Chest Physician's criteria/Society of critical care Medicine.[1,20] The presence of two or more of the following in a patient suspected or proven to have infection was taken as diagnostic of sepsis. The criteria includes temperature >38°C or <36°C, heart rate >90 beats/minute, respiratory rate >20 breaths/minute or PaCO2 <32 mmHg and WBC count >12,000 cells/mm3 or < 4000 cells/mm3 or > 10% immature (band) forms.
Sepsis was classified as microbiologically documented when microorganisms were recovered from the infection site or blood, and as clinically documented when objective signs and symptoms of infection were found but cultures were negative. The patients in this group were exclusive of those in group 1. Patients who had suffered from chemical or burn trauma were excluded.
Work-up of patients
Work up of patients in Group 1: A detailed history and clinical examination were done for all the patients. Data like age, gender, chronic conditions if any, severity of trauma according to the injury severity score (ISS)[11,20,21] and number and type of blood products infused within the first 24 hours were recorded and then every day thereafter for the duration of the ICU stay. The patients underwent surgical interventions whenever necessary as per accepted treatments for blood loss, wound treatment, bone fracture, or organ damage.
Results of routine investigations like hemogram, liver and kidney function tests, and any other test conducted during the initial 24 hours were recorded. In this study, the following samples were collected at 24-48 hours posttrauma for all these patients. Two consecutive blood samples for culture were collected on days 1 and 4 from the date of the trauma in both the groups. Five to ten milliliter of peripheral blood sample was collected in blood culture bottles before the start of the antimicrobials for bacterial (Bact T Alert, Organon Teknika, Durham, CA, USA) and fungal cultures. Apart from this, 2 ml of peripheral blood sample was collected in a plain vial for the PCT assay.
Sample processing: The blood sample for culture was processed in the laboratory using standard methods[22] and cultured using Bact T Alert (Organon Teknika, Durham, CA, USA). The identification and sensitivity of bacterial/fungal isolates in case of positive culture was done using both conventional microbiological methods and VITEK 2-compact (bioMérieux Inc. Durham, NC, USA) identification and sensitivity cards.
The PCT assay was done by quantitative technique using mini VIDAS system (bioMérieux Inc. RCS, Lyon, France). The tests were performed and interpreted according to manufacturer's instructions. Serum samples from confirmed cases of malaria and candidemia were used as positive control.
A CRP assay was done and its concentration was measured by the turbidometric method using SYNCHRON CX9 PRO system (Beckman Coulter Inc. Fullerton, CA, USA).
Clinical follow-up: All the patients were clinically followed up in the ICU till 21 days or discharge, which ever was earlier and the data was analyzed each day for 7 days and on days 14 and 21 of treatment in the ICU. Clinical evidence and laboratory data of infection, microbiological findings and the duration of treatment in the ICU as well as the data necessary to evaluate the Sepsis-related Organ Failure Assessment (SOFA)[11,23] were recorded in a prescribed performa. The trauma-related complications like sepsis, blood transfusions, and organ dysfunction as described by the SOFA score, duration of stay in ICU, and overall outcome was recorded for each patient.[24] At the end of the study, a correlation was made between the initial PCT level at 24-48 hours posttrauma and the clinical course of the patients.
Work up of patients in Group 2
The enrollment of trauma patients in this group was done whenever there was any clinical suspicion of sepsis during their ICU stay. A detailed clinical history, examination findings from their day of ICU admission including the blood products transfused, the type of surgery and subsequent follow up, laboratory findings and results of any microbiological culture were recorded at the time of inclusion of the study. The time period of ICU admission and the first clinical suspicion of sepsis were also noted. Using very high sensitive PCT immunoassay, a cut of 0.06μg/l was used to guide successful antibiotic stewardship in lower respiratory tract infections.[17] Based on these specific cut-off ranges, initiation or continuation of antibiotics was more or less discouraged (< 0.1μg/l or < 0.25 μg/l) or encouraged (> 0.5μg/l), respectively.[17]
Five to ten milliliters of peripheral blood sample was collected in blood culture bottles for culture on days 1 and 4 of inclusion using standard protocols. Two milliliters of peripheral blood sample was collected in a plain vial for PCT assay. Other specimens (bronchoalveolar lavage [BAL]/tracheal aspirate/pus/urine/fluids from sterile body sites/any other sample) were sent by the treating physician for routine diagnostic microbiological cultures at their clinical discretion if there was a suspicion of infection at any site. Similarly, results of other investigation like hemogram, biochemical analysis, or any other tests, sent by the clinicians for routine diagnostic purposes, were also recorded.
Clinical and laboratory follow up: The patients are initially given empiric antibiotics based on the ICU protocols. The antibiotics were appropriately changed if the blood culture or any other cultures were positive. The patients were followed up clinically after initiation of specific antibiotic treatment. On the fourth day of specific antibiotic treatment in confirmed cases of sepsis, a PCT assay was repeated from the serum sample. Simultaneously, a blood culture was also repeated.
In patients of group 2, the difference in PCT levels at days 1 and 4 was noted to assess the response to treatment. All these patients were clinically followed up till their final outcome in the hospital.
Statistical analysis
The sensitivity, specificity, positive predictive value, negative predictive value of the rapid PCT assays was calculated as per standard protocols. Comparison between the PCT levels and the outcome of the sepsis was done. The level of significance was tested by using the P value. A value of P < 0.5 was considered significant.
RESULTS
Patients and methods
The prospective study was conducted on acute trauma patients, who had survived the initial 24 hours after trauma. The patients in group 1 were admitted in ICU (n = 92, 46%) or the casualty (n = 108, 54%) at the time of inclusion in the study. Out of these, 88 (44%) had head injuries; 47 (24%) had blunt trauma to abdomen (BTA); 16 (8%) had blunt trauma to chest; 17 (9%) had blunt trauma to both chest and abdomen; 7 (4%) each had spinal injury, gun-shot injuries, and pelvic fracture; 6 (3%) had pan-facial injuries; 4 (2%) had neck injuries; and 1 patient had fracture femur with liver laceration.
A total of 75 trauma patients with suspected sepsis were included in the group 2 over the study period. All the patients in this group were admitted in the ICU at the time of the inclusion. Among these group of patients, 28 (37%) had head injury; 15 (20%) had blunt trauma to chest with hemothorax; 12 (16%) patients had spinal injury; 11 (15%) patients had blunt trauma to abdomen; 4 (5%) had gunshot injuries; 3 (4%) each were admitted with degloving injuries, pan-facial injury with cerebrospinal fluid (CSF) rhinorrhea; 2 (3%) had perineal injury with fracture of right humerus; and 1 patient had pelvic fracture.
The mean duration of stay of the patients of group 2 in the ICU was 11 days (range 2-35). There was no statistical difference in PCT levels between the specific types of trauma (P > 0.5); however, patients with abdominal trauma of both groups 1 and 2 presented with a little higher PCT levels.
Demographic characteristic of patients (study population)
There was no significant difference in the mean age of either of the two groups but a male predominance was seen in both the groups [Table 1].
Table 1.
Demographic characteristic, clinical and laboratory findings of patients (study population)
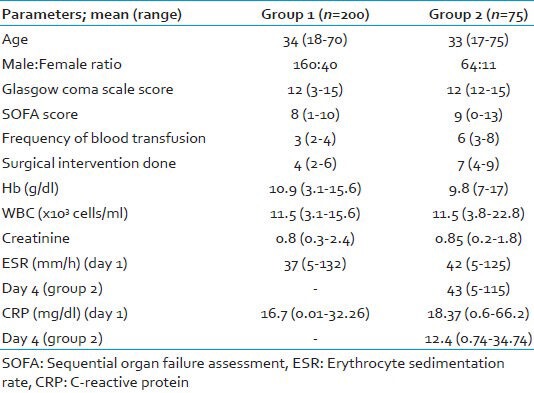
Clinical and laboratory findings of patients
The results of the laboratory parameters are shown in Table 1. There was no statistically significant difference (P = 0.935) in the mean erythrocyte sedimentation rate (ESR) levels between the samples collected on days 1 (42 mm/h) and 4 (43 mm/h) among the patients of the group 2. Similarly, the difference in values between days 1 (18.37 mg/dl) and 4 (12.4 mg/dl) mean CRP levels was not statistically significant (P = 0.646) in patients of group 2. Both ESR and CRP showed not much significant difference between the 2 days, which does not provide much help in predicting the outcome of such patients compared with the PCT findings. However, the CRP results obtained on day 4 in group 2 patients was lower compared with that on day 1, which is an unusual finding in absence of bacterial sepsis. This might have given shape to more concrete and definitive results if CRP was done on samples taken on day 7, which we could not in this study.
Procalcitonin levels in study population and its diagnostic/prognostic application
Group 1: Out of the 200 patients in group 1, 78 (39%) had negative PCT results and the remaining 122 (61%) had positive results. The PCT levels in the 122 patients who showed positive results exhibited varied levels [Figure 1].
Figure 1.
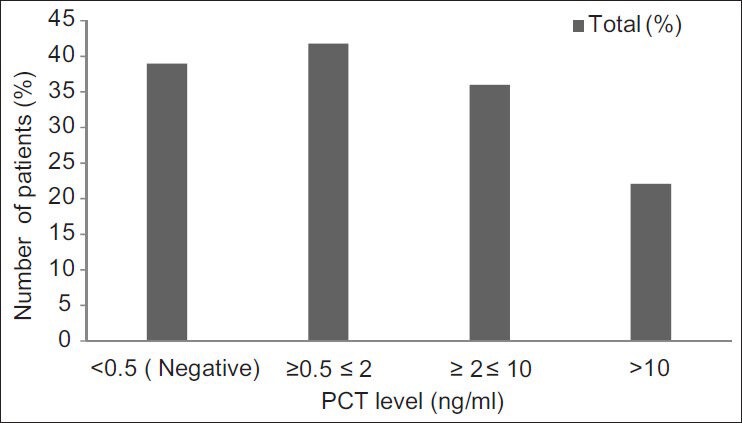
PCT assay results in group 1 patients showing the distribution of various levels
Of the 78 patients who had PCT value of < 0.5 ng/ml in group 1, 73 were discharged following treatment, without any complications. They did not develop any infection till their final follow-up. The remaining five developed infections within 4-7 days of admission. Of these, three patients had multiple infections (blood stream infection [BSI], along with VAP and urinary tract infection [UTI]) and two developed an episode of BSI. Of these five patients who developed hospital-acquired infections, one died. Although the clinical cause of death was mentioned as cardiac arrest, he also had multiple infections (BSI with VAP and UTI), contributing to the fatal outcome.
Of the 51 patients having PCT levels of 0.5-2 ng/ml, 1 developed a surgical site infection (caused by Acenitobacter baumannii); 1 developed VAP (caused by Acinetobacter baumannii); 2 patients developed BSI along with VAP; and 2 had VAP along with UTI. Of these six infected patients, four died (due to various causes like hypotension, polytrauma, renal failure, cardiac arrest, and sepsis).
Of the 44 patients who had PCT levels in the range 2-10 ng/ml, 4 (9%) patients developed BSI, 2 patients each (5%) developed VAP and BSI with VAP; 1 patient each (2%) developed surgical site infection (SSI), BSI along with UTI. Of these 10 patients, 6 (60%) died due to severe head injury, cardiac arrest, hypotension, and sepsis.
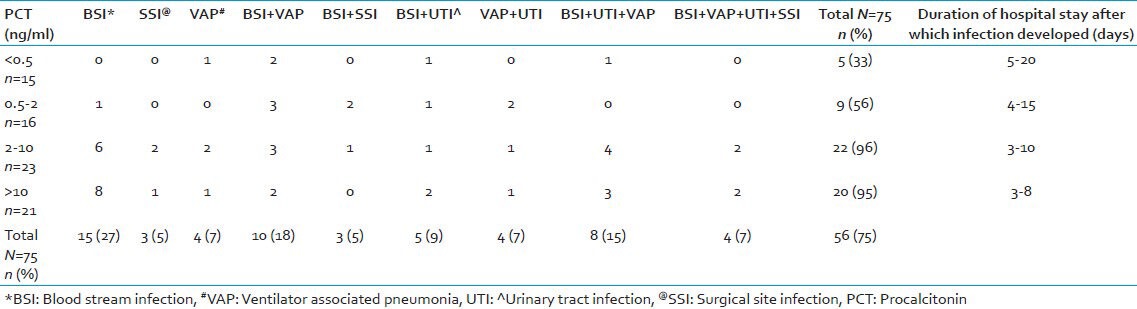
Of the 27 patients who had PCT levels in the range > 10, 6 (22%) developed BSI; 2 (7%) developed VAP; 3 (11%) developed BSI with VAP, and 1 patient each (4%) developed BSI with UTI, VAP with UTI and BSI with VAP and UTI. Of these, five (19%) patients died due to severe head injury and clinical sepsis [Tables 2 and 3].
Table 2.
Correlation between PCT results and microbiological finding in group 1
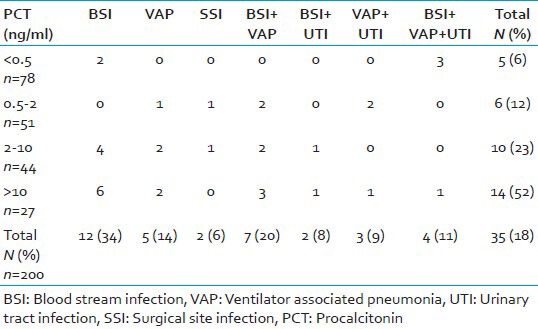
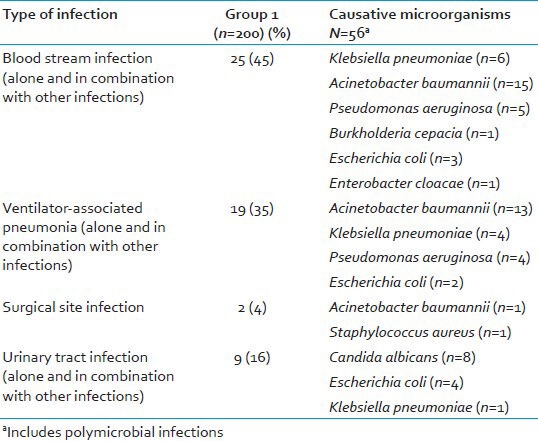
Table 3.
Microbiological etiology of culture positive patients of group 1
Group 2: Of the 75 patients in group 2 who had clinical evidence of sepsis, 52 (69.3%) were found to have a positive PCT assay. The difference in mean PCT levels, between days 1 and 4 samples in group 2 patients were statistically significant (P = 0.006) [Figure 2].
Figure 2.
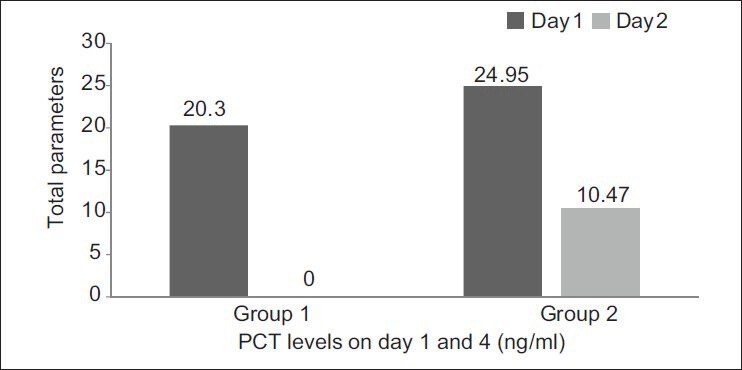
The difference of PCT levels (ng/ml) taken on days 1 and 4 between groups 1 and 2 and the changes seen over days in relation to the patient's condition
For all the patients in group 2, a simultaneous sample for blood culture and other relevant samples depending on the clinical presentation were sent for microbiological culture. Of the 75 patients, 48 (77%) had one or more microbiologically confirmed infections. Correlation of PCT values with microbiologically confirmed infections in group 2 are shown in Table 4.
Table 4.
Correlation between PCT results and microbiological findings in group 2
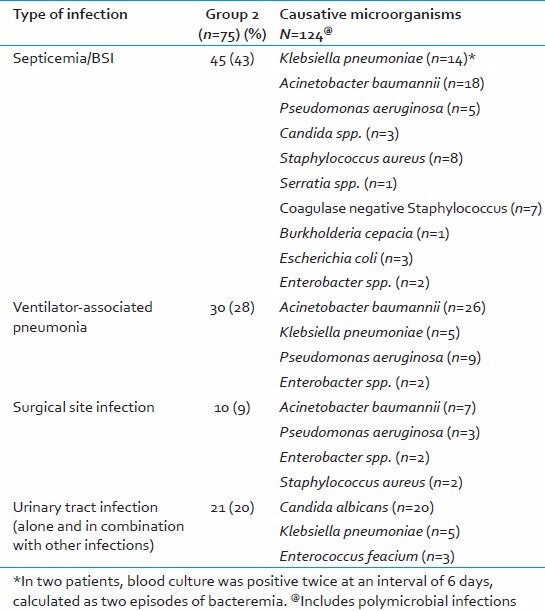
There was a correlation between an initial high level of PCT (>2 ng/ml) in severe trauma cases and their risk for development of complications, in the form of infections, sepsis, and consequent prolonged ICU stay. All patients who had PCT levels >2 ng/ml had an episode of microbiologically confirmed infection. Individually, BSI was the most common infection. The microbiological etiology in group 2 was varied with Acinetobacter baumannii being the most common isolated pathogen [Table 5].
Table 5.
Microbiological etiology of culture positive patients of group 2
It was seen that, those patients in which a PCT level of 2-10 μg/dl was more likely to develop BSIs or BSIs in combination with other hospital-acquired infections like VAP, UTI, SSIs, etc., within 5-7 days of the achieved level, however, those patients in whom PCT >10 μg/ml were seen to develop infection after 1 week on admission or measurement of PCT level, whichever is earlier.
The initial ESR and CRP concentrations (days 1-4) did not significantly differ in patients developing a microbiologically proven infection versus others. Although a high PCT level was associated with a higher likelihood of developing sepsis, the initial PCT and CRP concentration did not correlate with the severity of organ dysfunction, measured as maximum SOFA score values during the study period.
Appropriateness of antibiotic therapy and PCT levels
Appropriate antibiotic therapy administered according to antimicrobial susceptibility profile of the isolated pathogen resulted in a significant decrease in PCT between days 1 and 4 in 54 of the 56 cases with microbiological evidence of infection. One patient (who had septicemia due to Klebsiella pneumoniae) died after 2 days of initiating treatment and another succumbed to polymicrobial infections. Thus, the PCT kinetics within the first 48 hours of management of sepsis could be significantly different according to the appropriateness of the antibiotic therapy.
Final outcome
Of the 200 patients in group 1, 6% (12) died, whereas in group 2, out of 75 patients, 20% (15) died. All the patients who had a fatal outcome in group 2 had positive PCT result. The highest mortality rates were seen in patients having PCT >10 ng/ml. In group 1, mortality rates were same in patients having PCT in the range of 0.5-2 and 2-10 ng/ml. Of the 78 patients having a PCT level <0.5 ng/ml in group 1, there was only one case of fatal outcome; this patient died due to cardiac arrest and mixed infection (BSI along with VAP and UTI). In group 2, of the 15 patients who had a fatal outcome, 7 had PCT levels >10 ng/ml and 4 each had PCT levels between 0.5-2 ng/ml and 2-10 ng/ml, respectively. All these patients died due to polytrauma with signs of clinical sepsis [Figure 3].
Figure 3.
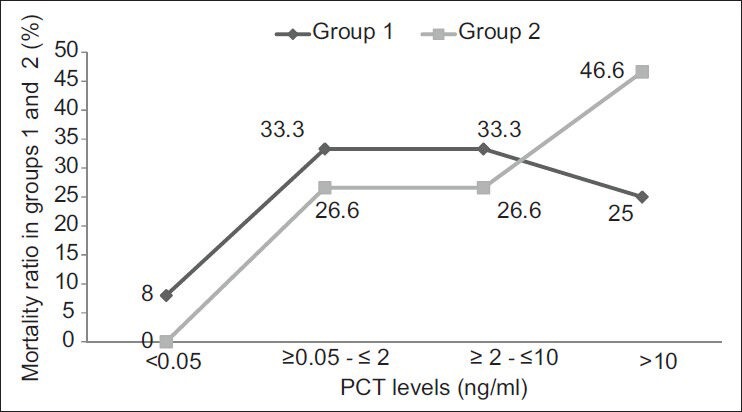
The mortality ratio between groups 1 and 2 in relation to the difference in PCT levels, which clearly shows that mortality rate is higher in the group with PCT > 10 ng/ml especially in group 2 patients
Sensitivity and specificity of the assay
The sensitivity and specificity of the PCT assay was 91% and 66%, respectively. The positive predictive value of PCT was 81% and negative predictive value is 83%.
DISCUSSION
Sepsis is a major complication in trauma patients requiring long in-patient stay. Almost 1.5 million people in northern America and another 1.5 people in northern Europe present annually with severe sepsis and/or septic shock with an estimated mortality of 35-50%.[25] So, an early and reliable method of diagnosis as well having a prognostic capability is the need of the hour. The answer lies in using a biomarker produced by the body, which correlates well with the body's changing response to traumatic condition and well as serves as a diagnostic marker. Hence PCT was used as a predictive biomarker in our prospective study.
The present study showed that there was a correlation between an initial high level of PCT (>2 ng/ml) in severe trauma cases and their risk for development of complications, in the form of infections, sepsis, and consequent prolonged ICU stay. This findings was supported by another study in trauma patients where complications such as sepsis, infection, blood transfusion, prolonged ICU treatment, and poor outcome were more frequent in patients with initially high PCT (>1 ng/ml), whereas CRP shows no positive correlation.[26,27] Favorable outcome was seen in patients with low PCT levels. This finding is consistent with the result seen in another study where decrease of serum PCT within the first 48 hours by 30% or its persistence at levels lower than 0.25 ng/ml was an indicator of favorable outcome. On the contrary, any increase of serum PCT or any decrease lower than 30% should alarm about the need for a change of therapeutic strategy.[28] In patients with multiple trauma due to an accident, the PCT level provides more information than the CRP level since only moderate amounts of PCT are induced, and higher concentrations correlate with more severe trauma and a higher frequency of various complications, including sepsis and infection.[26,27] This is highlighted by our study done in trauma patients, where not many studies are available to observe the role of PCT in the Indian context.
Our findings also show that PCT levels fall in response to appropriate antimicrobial treatment and thus could be used as a marker of treatment response in blood-stream and other infections. In a previous study, PCT of day 1 greater than 1 ng/ml is an alert signal of bad outcome.[28] In patients with PCT of day 1 lower than 1 ng/ml, a steady increase during the follow up was associated with bad prognosis and reverse with good prognosis.[27] This finding was similar to what is observed in our study.
In our patients with clinical sepsis, 75% of cases had microbiological evidence of infection. A predominance of Gram-negative pathogens was observed over Gram-positive bacteria with A. baumannii as the most common isolate in both the groups. In our study, blood cultures were positive in 41 (85%) of the 48 cases having a high PCT levels. A detailed microbiological investigation revealed other foci of infection in patients having a high PCT, in the presence of sterile blood cultures. Thus, a good correlation between elevated PCT levels and sepsis was observed. This finding is also supported by other studies, which showed similar elevated PCT levels in patients who later developed sepsis.[23,24] Table 6 summarizes some of the studies done by various workers on PCT.
Table 6.
A review of various studies done on procalcitonin in various patients
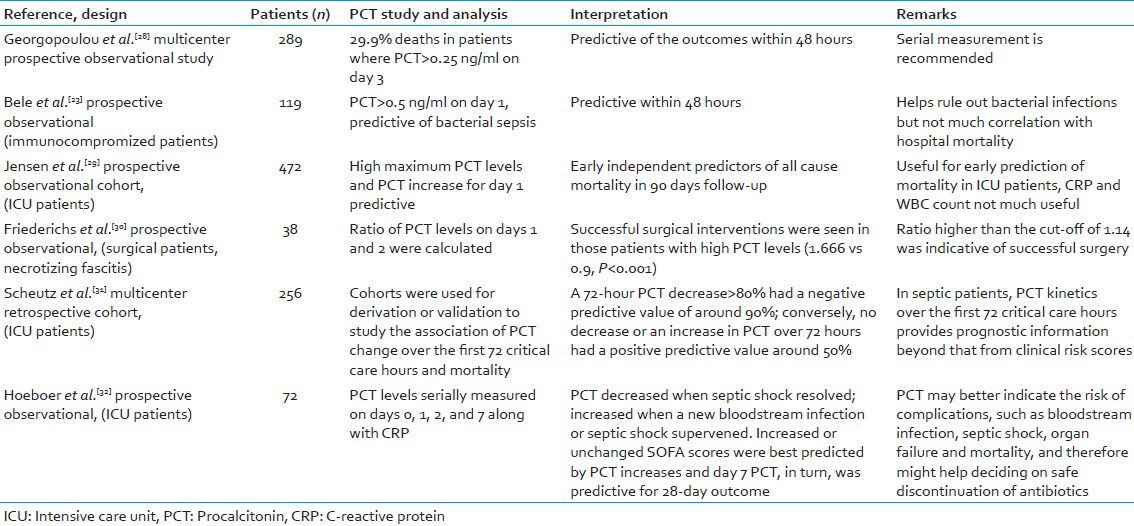
Along with the clinical findings, the predictive value of PCT can be used to guide the clinicians regarding their decision making, especially with regard to their antibiotic therapy. However, the PCT values should not be a substitute to clinical examinations or findings; but should be used as supportive evidence in decision making.
Strengths
The population was from a heterogeneous group of patients coming with various traumas. Our study is a prospective study done on a large scale in trauma patients for which few studies are available in India and hence its increased significance as most of the studies were from developed countries. Patients with coexisting morbidities, referred patients or patients with less than 24 hours trauma were not included to prevent the confounding factors.
Limitations
Though, control serum samples from confirmed cases of malaria and candidemia were used for positive control to rule out nonspecificity, other diseases like visceral leishmaniasis, viral fevers, tuberculosis, enteric fever, and dengue could not incorporated. Further tests are required with patients coming with minor or trivial trauma to see the PCT profile in such patients and whether it mirrors the findings seen in severe trauma patients.
CONCLUSION
Based on our data, PCT can be used as a surrogate marker of early diagnosis of sepsis and outcome after major trauma. It can also be used as a marker for monitoring response to treatment in confirmed cases of BSIs. PCT is superior to other biomarkers like CRP. So, it should be considered for inclusion in the diagnostic work-up for sepsis and in clinical practices in trauma care ICUs along with the support of clinical findings.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 2.De La Rosa GD, Valencia ML, Arango CM, Gomez CI, Garcia A, Ospina S, et al. Towards an operative diagnosis in sepsis: A latent class approach. BMC Infect Dis. 2008;19:8–18. doi: 10.1186/1471-2334-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slade E, Tamber PS, Vincent JL. The surviving sepsis campaign: Raising awareness to reduce mortality. Crit Care. 2003;7:1–2. doi: 10.1186/cc1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26:S64–74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210–7. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 6.Joshipura MK, Shah HS, Patel PR, Divatia PA, Desai PM. Trauma care systems in India. Injury. 2003;34:686–92. doi: 10.1016/s0020-1383(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 7.Radjou AN, Balliga DK, Pal R, Mahajan P. Injury-related mortality audit in a regional trauma center at Puducherry, India. J Emerg Trauma Shock. 2012;5:42–8. doi: 10.4103/0974-2700.93111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marra AR, Edmond MB, Forbes BA, Wenzel RP, Bearman GM. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J Clin Microbiol. 2006;44:1342–6. doi: 10.1128/JCM.44.4.1342-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez JA, Soto S, Fabrega A, Almela M, Mensa J, Soriano A. Relationship of phylogenetic background, biofilm production, and time to detection of growth in blood culture vials with clinical variables and prognosis associated with Escherichia coli bacteremia. J Clin Microbiol. 2006;44:1468–74. doi: 10.1128/JCM.44.4.1468-1474.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beekman SE, Diekema DJ, Chapin KC, Doern GV. Effects of rapid detection of bloodstream infection on length of hospitalisation and hospital charges. J Clin Microbiol. 2003;41:3119–25. doi: 10.1128/JCM.41.7.3119-3125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 12.Muller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. doi: 10.1186/1471-2334-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–80. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 14.Christ-Crain M, Müller B. Procalcitonin in bacterial infections–hype, hope, more or less? Swiss Med Wkly. 2005;135:451–60. doi: 10.4414/smw.2005.11169. [DOI] [PubMed] [Google Scholar]
- 15.Christ-Crain M, Müller B. Biomarkers in respiratory tract infections: Diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30:556–73. doi: 10.1183/09031936.00166106. [DOI] [PubMed] [Google Scholar]
- 16.Linscheid P, Seboek D, Zulewski H, Keller U, Müller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146:2699–708. doi: 10.1210/en.2004-1424. [DOI] [PubMed] [Google Scholar]
- 17.Schuetz P, Christ-Crain M, Müller B. Procalcitonin and other biomarkers to improve assessment and antibiotic stewardship in infections-hope for hype? Swiss Med Wkly. 2009;139:318–26. doi: 10.4414/smw.2009.12584. [DOI] [PubMed] [Google Scholar]
- 18.Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: Past, present and future. BMC Med. 2011;9:107. doi: 10.1186/1741-7015-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker KL, Nylén ES, White JC, Müller B, Snider RH., Jr Clinical review of 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: A journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–25. doi: 10.1210/jc.2002-021444. [DOI] [PubMed] [Google Scholar]
- 20.Munford RS. Severe sepsis and septic shock. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18th ed. New York: Mc Graw Hill; 2012. pp. 2223–31. [Google Scholar]
- 21.Civil ID, Schwab CW. The abbreviated injury scale, 1985 revision: A condensed chart for clinical use. J Trauma. 1988;28:87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. pp. 131–45. [Google Scholar]
- 23.Bele N, Darmon M, Coquet I, Feugeas JP, Legriel S, Adaoui N, et al. Diagnostic accuracy of procalcitonin in critically ill immunocompromised patients. BMC Infect Dis. 2011;11:224. doi: 10.1186/1471-2334-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castelli GP, Pognani C, Cita M, Stuani A, Sgarbi L, Paladini R. Procalcitonin, C reactive protein, white blood cells and SOFA score in ICU: Diagnosis and monitoring of sepsis. Minerva Anestesiol. 2006;72:69–80. [PubMed] [Google Scholar]
- 25.Becker JU, Theodosis C, Jacob ST, Wira CR, Groce NE. Surviving sepsis in low income and middle-income countries: New directions for care and research. Lancet Infect Dis. 2009;9:577–82. doi: 10.1016/S1473-3099(09)70135-5. [DOI] [PubMed] [Google Scholar]
- 26.Meisner M, Adina H, Schmidt J. Correlation of procalcitonin and C-reactive protein to inflammation, complications, and outcome during the intensive care unit course of multiple-trauma patients. Crit Care. 2006;10:R1. doi: 10.1186/cc3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakran JV, Michetti CP, Sheridan MJ, Richmond R, Waked T, Aldaghlas T, et al. The utility of procalcitonin in critically ill trauma patients. J Trauma Acute Care Surg. 2012;73:413–8. doi: 10.1097/TA.0b013e31825ff5b7. [DOI] [PubMed] [Google Scholar]
- 28.Georgopoulou AP, Savva A, Giamarellos-Bourboulis EJ, Georgitsi M, Raftogiannis M, Antonakos N, et al. Early changes of procalcitonin may advise about prognosis and appropriateness of antimicrobial therapy in sepsis. J Crit Care. 2011;26:331. doi: 10.1016/j.jcrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596–602. doi: 10.1097/01.CCM.0000239116.01855.61. [DOI] [PubMed] [Google Scholar]
- 30.Friederichs J, Hutter M, Hierholzer C, Novotny A, Friess H, Bühren V, et al. Procalcitonin ratio as a predictor of successful surgical treatment of severe necrotizing soft tissue infections. Am J Surg. 2013;206:368–73. doi: 10.1016/j.amjsurg.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Schuetz P, Maurer P, Punjabi V, Desai A, Amin D, Gluck E. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care. 2013;17:R115. doi: 10.1186/cc12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoeboer SH, Groeneveld AB. Changes in circulating procalcitonin versus C-reactive protein in predicting evolution of infectious disease in febrile, critically ill patients. PLoS One. 2013;8:e65564. doi: 10.1371/journal.pone.0065564. [DOI] [PMC free article] [PubMed] [Google Scholar]


