Abstract
Aim:
The study aimed to evaluate the utility of various commonly used fluoroquinolones against Staphylococcus aureus isolates.
Materials and Methods:
A total of 250 isolates of S. aureus were studied from different clinical specimens like blood, pus, wound swabs, sputum, ear swabs, and body fluids between November 2009 and December 2011. All the isolates were tested for their susceptibility to fluoroquinolones and other antimicrobial agents by Kirby-Bauer disc diffusion method using criteria of standard zone of inhibition. Methicillin-resistant S. aureus (MRSA) detection was done by cefoxitin disk diffusion method. The MRSA isolates were tested for minimum inhibitory concentration (MIC) to vancomycin by E-test strips. All the MRSA strains were sent to National Staphylococcal Phage-typing Centre, Maulana Azad Medical College, New Delhi for phage typing.
Results:
A total of 107 strains of S. aureus (42.8%) were detected as MRSA. Multidrug resistance was observed among the MRSA strains more commonly than among the MSSA stains. Among the fluoroquinolones, maximum resistance in MRSA was seen to ciprofloxacin (92.5%), followed by ofloxacin (80.4%). None of the S. aureus isolates showed resistance to vancomycin and linezolid. The MICs of vancomycin for the MRSA tested ranged from 0.5 to 2 μg/ml. Phage typing pattern of 107 MRSA isolates revealed that 37 (34.6%) MRSA isolates were nontypeable and 70 (65.4%) were typeable.
Conclusion:
Ciprofloxacin can no longer be used in empirical therapy against MRSA infections. Use of other members of fluoroquinolone should be limited only to those strains that show laboratory confirmation of their susceptibility. Vancomycin remains the drug of choice to treat MRSA infections.
Keywords: Fluoroquinolones, methicillin-resistant S. aureus, phage typing, Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus has been recognized as one of the most devastating persistent human pathogen that contributes toward hospital infection worldwide. It causes variety of infections, ranging from minor skin diseases to life-threatening endocarditis.[1] With the emergence of methicillin resistance among S. aureus during the 1960s, the effectiveness of therapy of staphylococcal infections with penicillins and cephalosporins became questionable.[2]
Methicillin-resistant S. aureus (MRSA) is difficult to treat and has very limited treatment options. Vancomycin is the only drug of choice.[2] However, its high cost, difficultly in supervising intravenous (IV) administration, and several serious adverse drug reactions limit its routine use. In addition, there have been many reports of development of low grade to absolute resistance even to vancomycin from many parts of the globe.[3,4]
The quinolones antibiotics have been proposed as a possible alternative to parenteral vancomycin therapy on the basis of several in vitro[5] and in vivo animal model data.[6] Not much data is available concerning quinolone resistance in S. aureus from this region. Therefore, the present study was undertaken to evaluate the utility of various commonly used fluoroquinolones against S. aureus.
MATERIALS AND METHODS
The study was performed between November 2009 and December 2011 in the Department of Microbiology at our tertiary care hospital. A total of 250 isolates of S. aureus were isolated from 2850 different clinical specimens like blood, pus, wound swabs, sputum, ear swabs, and body fluids. Only one isolate per patient was included in the study. All the isolates were tested for their susceptibility to ciprofloxacin (5 μg), ofloxacin (5 μg), levofloxacin (5 μg), gatifloxacin (5 μg), moxifloxacin (5 μg), sparfloxacin (5 μg), penicillin (10 unit), tetracycline (30 μg), cotrimoxazole (25 μg), erythromycin (15 μg), gentamicin (10 μg), pristinamycin (15 μg), vancomycin (30 μg), and linezolid (30 μg) by Kirby-Bauer disc diffusion method using criteria of standard zone of inhibition. MRSA detection was done by cefoxitin disk diffusion method.
The MRSA isolates were tested for minimum inhibitory concentration (MIC) to vancomycin by E-test strips (Hi-media laboratories Pvt., Ltd., Mumbai).
All the MRSA strains were sent to National Staphylococcal Phage-typing Centre, Maulana Azad Medical College, New Delhi for phage typing.
RESULTS
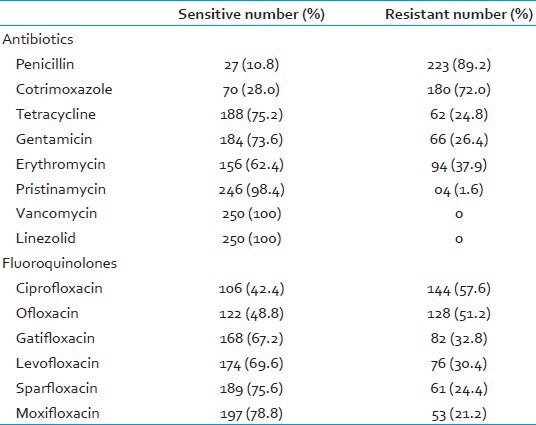
Out of the 250 clinical isolates of S. aureus, maximum resistance was noted to penicillin (89.2%), followed by co-trimoxazole (72.0%) and ciprofloxacin (57.6%). None of the S. aureus isolates showed resistance to vancomycin and linezolid [Table 1]. Maximum susceptibility among the fluoroquinolones tested was to moxifloxacin (78.8%), followed by sparfloxacin (75.6%). Also, 94 (37.6%) isolates of S. aureus were sensitive to all the fluoroquinolones and 33 (13.2%) isolates were resistant to all the fluoroquinolones tested.
Table 1.
Antimicrobial susceptibility pattern of S. aureus on disk diffusion (n=250)
In the present study, 107 strains (42.8%) were detected as MRSA. Most of the MRSA strains were isolated from pus/wound swabs and the others from blood and sputum.
Majority of the MRSA (58.8%) were from surgical specialty, followed by orthopedics (40%), and 87.9% MRSA isolates were obtained from inpatient wards and 12.1% from OPDs.
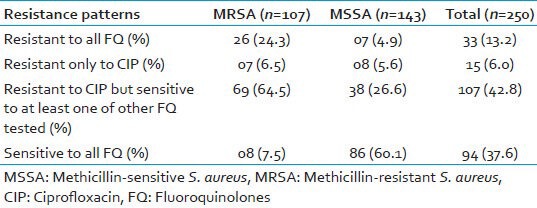
Among the fluoroquinolones, maximum resistance in MRSA was seen to ciprofloxacin (92.5%), followed by ofloxacin (80.4%), gatifloxacin (53.3%), levofloxacin (49.5%), sparfloxacin (45.8%), and moxifloxacin (39.3%). Of the 107 MRSA isolates, 26 (24.3%) MRSA isolates were found to be resistant to all the six fluoroquinolones tested. Only eight (7.5%) MRSA isolates were susceptible to all the six fluoroquinolones [Tables 2 and 3].
Table 2.
Comparison of antibiotic resistance pattern among MRSA and MSSA isolates
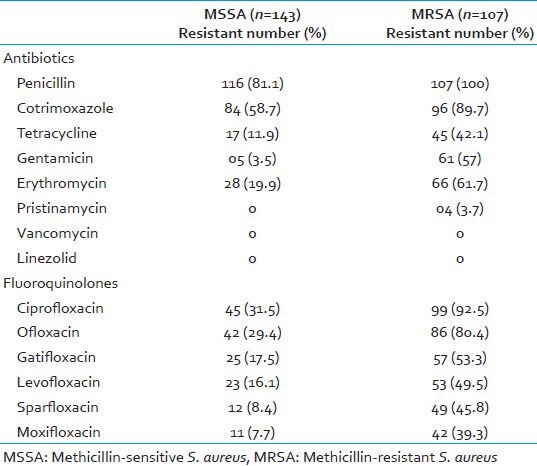
Table 3.
Phenotypic resistance patterns of S. aureus for six fluoroquinolones
The MICs of vancomycin for the MRSA tested ranged from 0.5 to 2 μg/ml [Figure 1].
Figure 1.
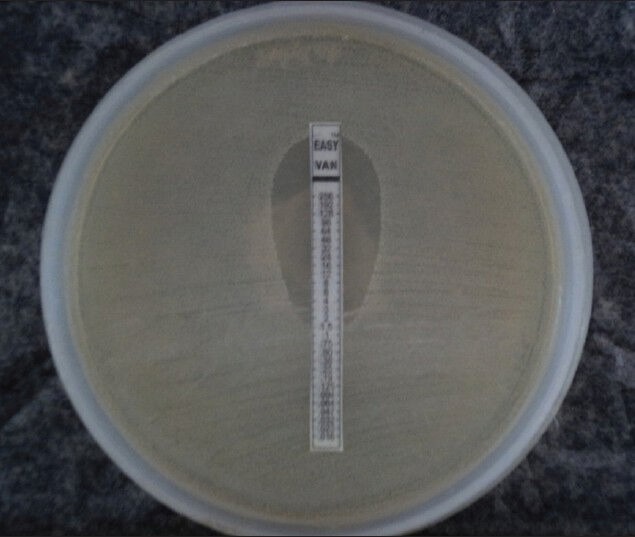
Vancomycin MIC by E-test
Phage-typing pattern of 107 MRSA isolates revealed that 37 (34.6%) MRSA isolates were nontypeable and 70 (65.4%) were typeable. Among the typeable isolates, 26 (24.3%) belonged to group III, 17 (15.9%) to group I, 5 (4.7%) to group II, 15 (14.0%) to mixed group, 06 to group III and the miscellaneous group, and 1 to the miscellaneous group.
DISCUSSION
Over the last four decades, MRSA has spread throughout the world, and its prevalence is soaring worldwide, as evident from many studies; however, there are considerable differences between countries.[7] In this study, 42.8% of the total isolates of the S. aureus were MRSA. Other studies have also shown such a high MRSA prevalence from various parts of the country ranging from 31-44%.[8,9] The present study showed an alarmingly high percentage of MRSA infection in this hospital. Such a high prevalence of MRSA may be due to several factors. The indiscriminate use of antibiotics, sub-therapeutic dosage, improper monitoring in the administration of various antibiotics, patient's compliance, and unethical treatment before visiting the hospital might have been contributing factors.[8]
Comparison of antibiotic resistance pattern among MRSA and methicillin-sensitive S. aureus (MSSA) isolates showed that resistance to fluoroquinolones as well as to other antibiotics tested was significantly higher in MRSA isolates than in MSSA isolates (P < 0.0001). Resistance of MRSA to penicillin (100%), cotrimoxazole (97%), and erythromycin (61.7%) was marked. High resistance to these drugs has also been reported in other studies.[8,10,11]
The development and spread of multiple antibiotic-resistant MRSA have gained much attention over the years.[2,10] Fluoroquinolone compounds such as ciprofloxacin and norfloxacin, first synthesized in the 1980s, were found to have extended antimicrobial spectra that included gram-positive bacteria, and were hoped to be useful in eradicating MRSA.[6] However, since these compounds became available for clinical use, resistance among MRSA has been observed in different parts of the world.[10,11]
In the present study, significantly higher percentage (92.5%) of MRSA isolates showed resistance to ciprofloxacin. Similar results of over 90% resistance have been reported in some studies from India[12] and Pakistan.[13] Mehta et al.,[14] reported that resistance to ciprofloxacin had steadily increased from 39% in 1992 to 68% in 1996.
If such resistance is found in healthcare units, ciprofloxacin may not be useful as a first-line antibiotic. It has been reported that ciprofloxacin resistant isolates tend to show increased resistance to other antibiotics, including aminoglycosides.[15,16]
In the present study, 80.4% MRSA showed resistance to ofloxacin, 53.3% to gatifloxacin, 49.5% to levofloxacin, and 45.8% to sparfloxacin. Lower resistance (39.3%) was noted to moxifloxacin. Different pattern of quinolone resistance was found among the ciprofloxacin resistant and susceptible isolates, and 24.3% MRSA isolates were found to be resistant to all six fluoroquinolones tested.
This different patterns and levels of resistance may arise following exposure to different quinolones, and different strains may produce different types of resistance.[17]
In the present study, linezolid and vancomycin were found to be useful drugs in treating MRSA infections. None of the MRSA isolates showed resistance to vancomycin as well as to linezolid. The MICs values of vancomycin for all the MRSA ranged from 0.5 to 2 μg/ml in our study.
In the present study, 3.7% MRSA isolates were resistant to pristinamycin. Many studies reported different range of resistance to pristinamycin ranging from 0% to 44%.[18]
Typing of MRSA strains is necessary for thorough epidemiological investigations of sources and modes of spread of these strains in hospitals and to design appropriate control measures.[19] In the present study, of 107 MRSA isolates, 37 (34.6%) isolates were nontypeable, and 65.4% were typeable. Among the typeable isolates, most strains belonged to group III. In this study, the affinity of MRSA strains to phages of group III was observed, although there were variations in their specific phage pattern. In view of high percentage of nontypeability among MRSA, there is a need for newer set of phages for MRSA typing.
CONCLUSION
In conclusion, S. aureus showed resistance to most of the antimicrobials in varying proportion, except to vancomycin and linezolid, to which the isolates were 100% sensitive. The percentage of MRSA among all S. aureus isolates was 42.8%. MRSA strains showed 100% resistance to penicillin. The study revealed that MRSA with associated multidrug resistance is common in this region. Most strains of MRSA were nontypeable using routine phages. There is a need to develop a local set of MRSA phages for improvement of typeability.
From the data, it appears that, over the period of last 15 years, MRSA have also acquired resistance to many commonly used fluoroquinolones. Ciprofloxacin can no longer be used as an empirical therapy against MRSA infections. Other members of quinolones may be used in S. aureus infections empirically in less serious selected cases. They may be continued after they show laboratory confirmation of their susceptibility.
Vancomycin is the mainstay of therapy in MDR MRSA infections and should be used judiciously. Looking at the possibility of emergence of resistance to the drug, newer agents like linezolid and pristinamycin may provide a valuable option for the treatment of MRSA infections.
ACKNOWLEDGMENT
The authors acknowledge the help provided by National Staphylococcal Phage-typing Centre, Maulana Azad Medical College, New Delhi for phage typing.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiwari HK, Das AK, Sapkota D, Sivarajan K, Pahwa VK. Methicillin resistant Staphylococcus aureus: Prevalence and antibiogram in a tertiary care hospital in western Nepal. J Infect Dev Ctries. 2009;3:681–4. doi: 10.3855/jidc.86. [DOI] [PubMed] [Google Scholar]
- 3.Robert J, Bismuth R, Jarlier V. Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: A 20 year study in a large French teaching hospital, 1983-2002. J Antimicrob Chemother. 2006;57:506–10. doi: 10.1093/jac/dki486. [DOI] [PubMed] [Google Scholar]
- 4.Bal M, Saha B, Singh AK, Ghosh A. Identification and characterization of a vancomycin-resistant Staphylococcus aureus isolated from Kolkata (South Asia) J Med Microbiol. 2008;57:172–9. doi: 10.1099/jmm.0.47144-0. [DOI] [PubMed] [Google Scholar]
- 5.Foster JK, Joseph R, Lentino RS, Divincenzo C. Comparison of in vitro activity of quinolone antibiotics and vancomycin against gentamicin- and methicillin-resistant Staphylococcus aureus by time-kill kinetic studies. Antimicrob Agents Chemother. 1986;30:823–7. doi: 10.1128/aac.30.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert MJ, Boscia A, Kobasa WD, Kaye D. Enoxacin compared with vancomycin for the treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1986;29:461–3. doi: 10.1128/aac.29.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randrianirina F, Soares JL, Ratsima E, Carod JF, Combe P, Grosjean P, et al. In vitro activities of 18 antimicrobial agents against Staphylococcus aureus isolates from the Institute Pasteur of Madagascar. Ann Clin Microbiol Antimicrob. 2007;6:5. doi: 10.1186/1476-0711-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anbumani N, Kalyani J, Mallika M. Prevalence of methicillin-resistant Staphylococcus aureus in a Tertiary Referral Hospital in Chennai, South India. Indian J Pract Doct 2006-08-2006-09. :3. [Google Scholar]
- 9.Tyagi A, Kapil A, Singh P. Incidence of methicillin resistant Staphylococcus aureus (MRSA) in pus samples at a tertiary care hospital, AIIMS, New Delhi. J Indian Acad Clin Med. 2008;9:33–5. [Google Scholar]
- 10.Tiwari HK, Sapkota D, Sen RM. High prevalence of multidrug-resistant MRSA in a tertiary care hospital of northern India. Infect Drug Resist. 2008;1:57–61. doi: 10.2147/idr.s4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai V, Rao VI, Rao SP. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus [MRSA] isolates at a tertiary care hospital in Mangalore, South India. J Lab Physicians. 2010;2:82–4. doi: 10.4103/0974-2727.72155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarma JB, Ahmed GU. Characterization of methicillin resistant S. aureus strains and risk factors for acquisition in a teaching hospital in northeast India. Indian J Med Microbiol. 2010;28:127–9. doi: 10.4103/0255-0857.62489. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AH, Rafi S, Qureshi SM, Ali AM. The current susceptibility patterns of methicillin resistant Staphylococcus aureus to conventional anti-staphylococcus antimicrobials at Rawalpindi. Pak J Med Sci. 2004;20:361–4. [Google Scholar]
- 14.Mehta AP, Rodrigues C, Sheth K, Jani S, Hakimiyan A, Fazalbhoy N. Control of methicillin resistant Staphylococcus aureus in a tertiary care Centre: A five-year study. J Med Microbiol. 1998;16:31–4. [Google Scholar]
- 15.Fernandez CJ, Ackerman VP. In vitro studies of ciprofloxacin and survey of resistance patterns in current isolates. Diagn Microbiol Infect Dis. 1990;13:79–91. doi: 10.1016/0732-8893(90)90090-i. [DOI] [PubMed] [Google Scholar]
- 16.Tsering DC, Pal R, Kar S. Methicillin-resistant Staphylococcus aureus: Prevalence and current susceptibility pattern in Sikkim. J Glob Infect Dis. 2011;3:9–13. doi: 10.4103/0974-777X.77289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maple P, Hamilton JM, Brumfitt W. Ciprofloxacin resistance in methicillin- and gentamicin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1989;8:622–4. doi: 10.1007/BF01968141. [DOI] [PubMed] [Google Scholar]
- 18.Deep A, Goel N, Sikka R, Chaudhary U, Yadav S, Gupta A, et al. Quinpristin dalfopristin resistance in gram positive bacteria: Experience from a tertiary care referral center in North India. J Infect Dis Antimicrob Agents. 2008;25:117–21. [Google Scholar]
- 19.Mehndiratta PL, Bhalla P. Typing of Methicillin resistant Staphylococcus aureus: A technical review. Indian J Med Microbiol. 2012;30:16–23. doi: 10.4103/0255-0857.93015. [DOI] [PubMed] [Google Scholar]


