Abstract
Background:
The prevalence of nondiabetic renal disease (NDRD) among type 2 diabetics varies widely depending on the populations being studied and the selection criteria. Also, for patients found to have NDRD different predicting factors have been identified by different studies.
Objectives:
To determine: (i) Frequency and spectrum of NDRD in type 2 diabetics with atypical clinical renal disease, in our set up and (ii) common clinical markers that are associated with NDRD in our local population.
Materials and Methods:
Ninety-three type 2 diabetic patients with atypical clinical renal disease who had undergone renal biopsy to rule out NDRD were recruited. Patients were grouped into Group 1 with isolated NDRD, Group 2 with NDRD superimposed on diabetic nephropathy (DN), and Group 3 with isolated DN; and their clinical and biochemical parameters were statistically analyzed using analysis of variance, Kruskal-Wallis test, and Chi-square tests of statistical significance.
Results:
68.8% of the patients had NDRD with or without concurrent DN. Patients with isolated NDRD had shorter duration of diabetes compared to the other groups. Absence of retinopathy and presence of microscopic hematuria and active urinary sediment had positive predictive value of 79.24, 81, and 100%, respectively, for NDRD in type 2 diabetics. Chronic interstitial nephritis was the commonest NDRD and membranous glomerulonephritis was the commonest glomerular NDRD in our setup.
Interpretation and Conclusions:
The frequency of NDRD in type 2 diabetics with atypical clinical renal disease is high in our setup thereby making the renal biopsy procedure imperative to rule out the same. Shorter duration of diabetes, absence of retinopathy, presence of microscopic hematuria, and active urinary sediment are markers associated with NDRD in type 2 diabetes with clinical renal disease.
Keywords: Diabetic nephropathy, hematuria, nondiabetic renal disease, retinopathy
INTRODUCTION
Diabetic renal disease develops in about 20-40% of patients suffering from diabetes mellitus (DM).[1,2] Unlike type 1 DM patients where >95% of renal disease is the result of diabetic nephropathy (DN) (diabetic glomerulosclerosis (DGS)), the renal disease in type 2 DM patients is more complex and heterogeneous. In addition to classic DN, 12-81% of type 2 DM patients develop nephropathy unrelated to DM known as nondiabetic renal disease (NDRD).[3,4] A wide spectrum of NDRD, including both glomerular and tubulointerstitial lesions are reported, the precise diagnoses of which require histologic examination and immunofluorescence study.
The early appearance of overt proteinuria (< 5 years duration), rapid decline in renal function, impaired renal function without significant proteinuria, and active urinary sediment are inconsistent with natural history of DN and suggest NDRD.[4] Different factors/clinical markers including age of onset of DM, absence of retinopathy, microhematuria, subnephrotic proteinuria, and shorter duration of DM have been found to predict NDRD in type 2 diabetics.[3] However, these factors were found to have variable predictive values in different series.[1,3,4,5,6,7,8]
The high prevalence of NDRD in some studies underscores the need for clinicians to consider renal biopsy in diabetic patients with an atypical clinical course, since additional disease-specific therapies may be helpful for this subset of population other than the standard angiotensin receptor blockers and angiotensin converting enzyme inhibitors commonly used in typical DGS.[1,5] Since diabetic patients with NDRD have significantly better renal outcomes compared to patients with biopsy proven DN (DGS), it is important to suspect, identify, and manage NDRD as early as possible.[9]
Hence, the present study was conducted; (i) to determine the frequency and spectrum of NDRD in type 2 diabetics with atypical clinical renal disease, in our set up and (ii) to determine the common clinical markers that are associated with NDRD in our local population where little data exists.
MATERIALS AND METHODS
Type 2 DM patients with atypical clinical renal disease (sudden and rapid onset of proteinuria, atypical presentation without transition through usual stages, hematuria, active urinary sediment, rapid deterioration in renal function, and renal dysfunction without significant proteinuria) who underwent renal biopsy to rule out NDRD at M. S Ramaiah Hospitals, Bangalore, from January 2009 to June 2012 were included in the study. DM had been diagnosed in these patients using the criteria of American Diabetes Association.
Examination of renal biopsy tissue was done by light microscopy (hematoxylin and eosin (H and E), periodic acid Schiff (PAS), and methenamine silver staining) and immunofluorescence microscopy using fluorescein isothiocyanate (FITC) conjugated rabbit anti-human immunoglobulin (Ig) G, IgM, IgA, and C3 antibodies from Biogenex. The biopsies were interpreted blindly and independently by two renal pathologists. DN was diagnosed when at least three of the following features were present; (i) global mesangial sclerosis with or without Kimmelstiel-Wilson nodule or nodular mesangial sclerosis, (ii) uniform glomerular capillary basement membrane thickening, (iii) exudative lesions such as “fibrin cap” or “capsular drop”, or (iv) glomerular hyaline arteriolosclerosis.[3,10,11]
Clinical details and laboratory parameters including age, age at onset (the time when DM was first diagnosed), duration of diabetes (the period between the age of onset and renal biopsy), blood urea nitrogen (BUN), serum (S.) creatinine, S. albumin, urine microscopy, 24 h proteinuria, presence or absence of retinopathy, and hypertension were recorded at the time of renal biopsy.
Microscopic hematuria was defined as greater than two red blood cells per high power field on two microscopic urinalysis without recent exercise, menses, sexual activity, or instrumentation.[12] Active urine sediment was defined as >5 red blood cells per high power field and/or cellular casts.[13]
Based on the biopsy and direct immunofluorescence findings, patients were grouped as Group 1, isolated NDRD; Group 2, NDRD with underlying DN; and Group 3, isolated DN.
Statistical analysis
Data was analyzed using Statistical Package for Social Sciences (SPSS) version 18. Descriptive statistics for quantitative variables were summarized using mean ± standard deviation (SD) and median with interquartile range as appropriate. Qualitative parameters were expressed in terms of percentage.
Differences of various parameters between the three groups were assessed by analysis of variance (ANOVA) test, Kruskal-Wallis test, and Chi-square test of statistical significance. P < 0.05 was considered as significant.
RESULT
A total of 98 patients were identified of which 5 were excluded as these patients had advanced renal failure at the time of renal biopsy which showed end-stage nephrosclerosis, the primary cause of which could not be clearly delineated as DN or NDRD. The remaining 93 patients were included in the study with the mean age ± SD being 56.31 ± 11.05 years. Out of these, 65 (69.89%) patients were males and 28 (30.11%) were females. Twenty-three patients (24.73%) had isolated NDRD (Group 1), 41 patients (44.08%) had NDRD with underlying DN (DGS) (Group 2), and 29 patients (31.18%) had isolated DN (DGS) (Group 3). Thus, 64 (68.8%) of the patients had NDRD with or without DN (DGS).
The male:female ratio was 1.09:1, 3.6:1, and 2.6:1 in Group 1, 2, and 3, respectively with greater proportion of females in the group 1.
Indications for renal biopsy included: Proteinuria not fitting to classical DN (i.e., patients with a short diabetes duration (<10 years) who did not have the classic picture of gradually evolving nephropathy (from normal to microalbuminuria to overt proteinuria over a period of years) or patients with a long duration of diabetes with normal renal function and without complications developing rapidly progressive proteinuria) in 34 (36.6%); unexplained rapid deterioration in renal function in 43 (46.2%); microscopic hematuria in 11 (11.8%); and acute nephritic syndrome with active urinary sediment in five (5.4%) patients. Table 1 shows the distribution of the above clinical presentations among the three groups. 29.4 and 19.8% of patients with NDRD (Groups 1 and 2) presented respectively with microscopic hematuria and active urine sediment as compared to 6.9 and 0% in patients with isolated DN (Group 3).
Table 1.
Clinical presentation and distribution of retinopathy
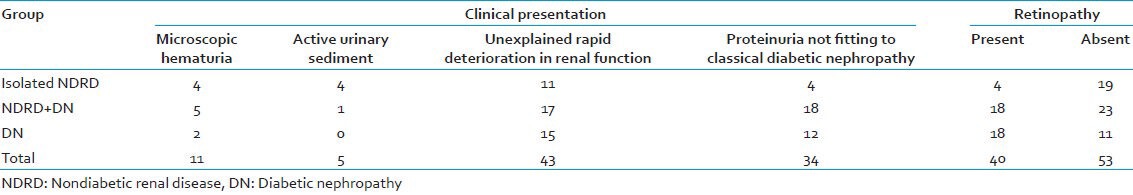
Retinopathy was present in 40 (43.0%) of the cases; 19 (82.61%) of Group 1, 23 (56.1%) of Group 2, and 11 (37.90%) of Group 3 cases did not show retinopathy at the time of renal biopsy, as depicted in Table 1. Absence of retinopathy was statistically significant between the three groups (P-value from Chi-square test = 0.005).
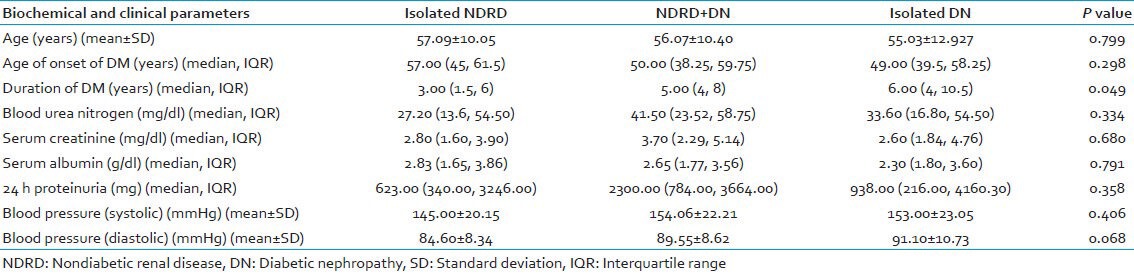
Table 2 depicts the clinical and biochemical parameters of the three groups of patients. There was no statistically significant difference in age of onset of DM, BUN, S. creatinine, 24 h proteinuria, S. albumin, and systolic and diastolic blood pressures among the three groups, (P > 0.05). Statistically significant difference was present amongst the groups for the duration of diabetes. Patients with isolated NDRD (Group 1) had shorter duration of diabetes compared to the other groups (P = 0.049).
Table 2.
Biochemical and clinical parameters
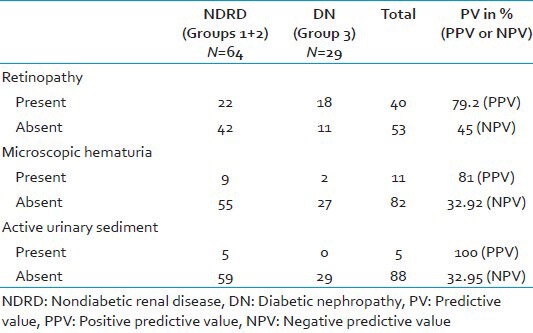
Clinical markers associated with NDRD: Absence of retinopathy had a sensitivity of 65.62%, specificity of 62.1%, and positive predictive value (PPV) of 79.24%; microscopic hematuria had a sensitivity of 14.1%, specificity of 93.1%, and PPV of 81%; and active urinary sediment had a sensitivity of 7.8%, specificity of 100%, and PPV of 100% for NDRD in type 2 diabetics with atypical clinical renal disease, as depicted in Table 3.
Table 3.
Clinical markers associated with NDRD
The most common lesion found in NDRD with or without concurrent DN (Group 1 + 2) was chronic interstitial nephritis [Figure 1] in 24 (37.50%) cases (seven in Group 1 and 17 in Group 2) followed by acute interstitial nephritis in 15 (23.44%) cases (six in Group 1 and nine in Group 2), acute on chronic interstitial nephritis in eight (12.50%) cases (three in Group 1 and five in Group 2), membranous glomerulonephritis with granular membranous immune deposits of IgG (+++) and C3(++) in five (7.81%) cases (two in Group 1 and three in Group 2), minimal change disease in three (4.68%) cases (one in Group 1 and two in Group 2), membranoproliferative glomerulonephritis in three (4.68%) cases (three in Group 1 and zero in Group 2) with granular membranous immune deposits of C3(+++) and Ig G(++), IgA nephropathy with dominant mesangial deposits of IgA in three (4.68%) cases (one in Group 1 and two in Group 2) and diffuse proliferative (postinfectious) glomerulonephritis with granular membranous and mesangial deposits of C3(++) and IgG(++) in three (4.86%) cases (zero in Group 1 and three in Group 2).
Figure 1.
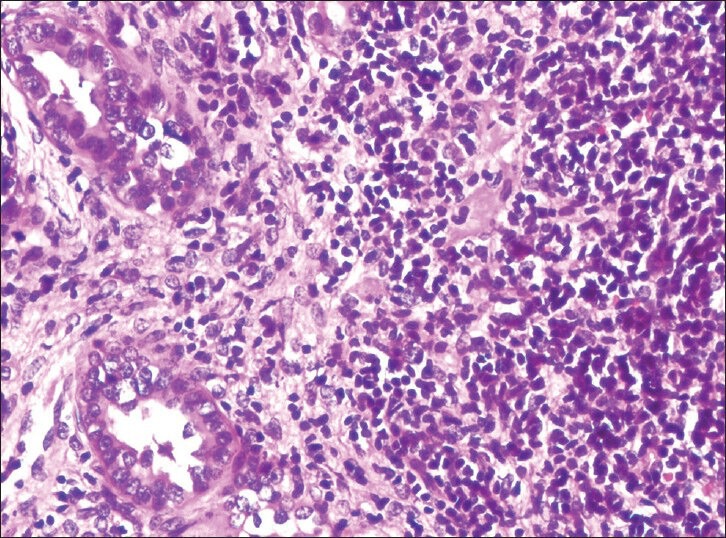
Chronic interstitial nephritis (H and E, ×400)
All the patients in Group 3 exhibited features of DGS [Figure 2] with global mesangial sclerosis with or without Kimmelstiel-Wilson nodule and uniform glomerular capillary basement thickening.
Figure 2.
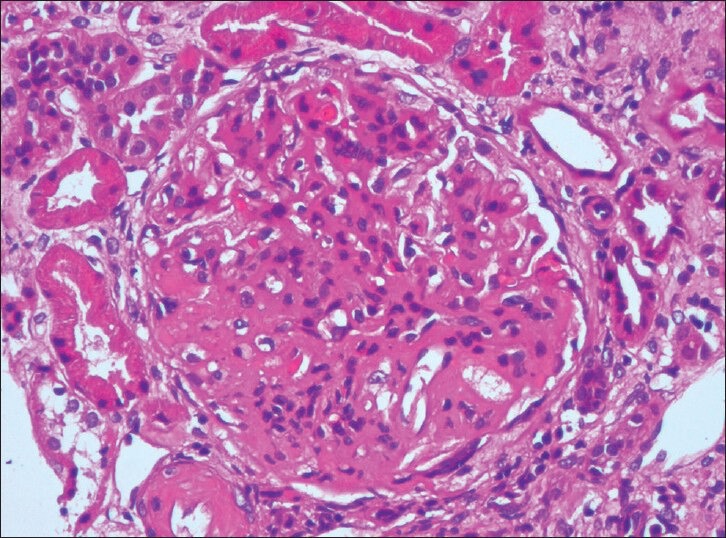
Diabetic glomerulosclerosis with global mesangial sclerosis (H and E, ×400)
DISCUSSION
The prevalence of NDRD among patients with type 2 DM in the literature varies widely from 12 to 18%, depending on the populations being studied.[1,3] In the current study, NDRD was detected in 68.81% of type 2 DM. This was in accordance with previous studies conducted by Soni et al., south India (72.5%);[6] Pham et al., USA (72.5%);[8] Chang et al., Korea (63.9%);[9] and Li et al., China (75.5%)[14] but different from other studies where the prevalence of NDRD was around 12.3–33.3%.[2,15] The large variation is presumably due to different selection criteria for doing renal biopsy in these patients. Some studies recruited patients with proteinuria ≥ 1 g/24h[3,10] others included proteinuria irrespective of the level.[1] Some included patients with absent retinopathy[4,9] and others recruited patients irrespective of the ophthalmological findings.[1,3] Although indications for renal biopsy to rule out NDRD in diabetics vary between institutions, patients with renal dysfunction not readily ascribed to diabetes alone are usually selected to undergo the procedure.
Unlike certain studies[1,3,10,16] where gender was comparable between NDRD and DN groups, in the present study female sex was more common in diabetics with isolated NDRD rather than diabetics with isolated DN, and shorter duration of DM was found in the NDRD group, in accordance with previous studies by Soni et al.,[6] and Chang et al.[9] Similar to Ghani et al.,[1] and Mak et al.,[3] no statistically significant difference was found for age, age at onset of diabetes, blood urea nitrogen levels, serum creatinine, serum albumin, and systolic and diastolic blood pressures among the NDRD and DN groups in our study.
Proteinuria is an independent predictor for adverse renal outcome not only in type 2 DM patients, but also in patients with superimposed or isolated NDRD.[10] The present study found comparable levels of 24 h proteinuria among the different groups. This was in accordance with results of Li et al.,[14] and Castellano et al.,[16] whereas other studies[1,3,7] reported significantly lower levels of proteinuria in NDRD cases as compared with diabetics with DN.
The present study showed that retinopathy was absent in 82.61% of the type 2 diabetics with isolated NDRD and present in 62.1% of type 2 DM with isolated DN, thereby confirming the commonly accepted view that absence of retinopathy is a significant predictor of NDRD in diabetics with renal disease. This result was in accordance with previous studies conducted by Ghani et al.,[1] Prakash et al.,[5] Soni et al.,[6] Pham et al.,[8] Chang et al.,[9] and Castellano et al.;[16] all of which demonstrated significantly less associated retinopathy in patients with NDRD. However, unlike our study, there was no correlation between NDRD and the absence of retinopathy in the studies conducted by Mak et al.,[3] Lin et al.,[7] and Li et al.[14] The shorter duration of diabetes and seemingly low frequency of retinopathy in the NDRD group in the present study are correlations that cannot be simply attributed to a selection bias as the patients underwent renal biopsy irrespective of the ophthalmological status and disease duration. Thus absence of retinopathy in diabetics with renal disease necessitates renal biopsy to rule out potentially treatable nondiabetic causes. Castellano et al.,[16] found that retinopathy had a predictive value of 100% in predicting DN and concluded that its existence makes renal biopsy procedure to rule out NDRD unnecessary. However, as even 17.39% of isolated NDRD patients in the current study had retinopathy, its existence does not obviate the need for renal biopsy, especially if the clinical presentation is atypical. Retinopathy strongly correlates with presence of DN, however discordance in the occurrence of the two complications has been reported and dissimilar genetic predispositions have been suggested.[10] 37.9% of patients with isolated DN did not have retinopathy. Retinopathy is considered as an important predictor for adverse renal outcome and disease progression.[10] In the present study, isolated DN patients with retinopathy had higher levels of proteinuria and serum creatinine compared to those with absent retinopathy.
Microscopic hematuria and active urinary sediment had a diagnostic specificity of 93.1 and 100% and positive predictive value of 81 and 100%, respectively for NDRD in diabetics. The strong correlation of the latter with NDRD is presumably related to the occurrence of proliferative glomerulonephritis (membranoproliferative glomerulonephritis, IgA nephropathy, and diffuse proliferative glomerulonephritis) in the NDRD group.
In the present study, 17 (26.6%) of the NDRD group had glomerular disease and 47 (73.4%) had tubulointerstitial disease. Membranous glomerulonephritis was the commonest glomerular NDRD and in accordance with Prakash et al.,[5] chronic tubulointerstitial nephritis was the commonest NDRD detected in the studied biopsies. Various studies in different populations have demonstrated crescentic glomerulonephritis,[1] IgA nephropathy,[3,14] acute interstitial nephritis,[6,7] focal segmental glomerulosclerosis,[8] membranous glomerulonephritis,[15] and proliferative glomerulonephritis[17] as the most frequent causes of NDRD.
Information regarding the mechanisms implicated in the development of NDRD in diabetes remains suboptimal and speculative.[10,18] Current knowledge suggests that hyperglycemia, advanced glycation end products, immune complexes, and other biochemical alterations in the diabetic milieu activate renal cells via stress-activated protein kinase signaling culminating in the upregulation of cell adhesion molecules and release of proinflammatory cytokines with consequent glomerular leukocyte recruitment and activation.[18] A number of modified proteins, like oxidized low density lipoproteins that develop in diabetes are potentially immunogenic resulting in immune complex generation and inflammation. Circulating immune complexes and glomerular IgG deposits especially the proinflammatory subtypes IgG1 and IgG3 isotypes are recognized in diabetic experimental models. Enhanced exposure of antigenic cellular components and preexisting glomerular alterations might favor an immune reaction in the subepithelial space.[1,6,10,19] However, some authors found no difference in the prevalence of NDRD between patients with and without diabetes and suggest that the coexistence of a different glomerulonephritis in the diabetic kidney may be merely coincidental.[10,20,21]
CONCLUSION
The study demonstrates that renal complications in type 2 diabetics may be due to heterogeneous nondiabetic disease. 68.81% of our type 2 diabetics with atypical clinical renal disease had NDRD, emphasizing the importance of kidney biopsy in this group of population. Chronic interstitial nephritis is the commonest NDRD and membranous glomerulonephritis is the commonest glomerular NDRD in our setup. Shorter duration of diabetes, absence of retinopathy, presence of microscopic hematuria, and active urinary sediment are markers associated with NDRD in type 2 diabetes with atypical clinical renal disease and are strong indicators for biopsy. Female gender is more common in type 2 DM with NDRD rather than type 2 DM with DN.
Footnotes
Source of Support: ICMR for funding part of this project under Short Term Studentship (STS) Scheme (Reference ID: 2011.03452).
Conflict of Interest: None declared.
REFERENCES
- 1.Ghani AA, Al Waheeb S, Al Sahow A, Hussain N. Renal biopsy in patients with type 2 DM: Indications and nature of the lesions. Ann Saudi Med. 2009;29:450–3. doi: 10.4103/0256-4947.57167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck MJ, Evans BJ, Quarry-Horn JL, Kerrigan JR. Type 2 Diabetes Mellitus: Issues for the medical care of pediatric and adult patients. South Med J. 2002;95:992–1000. [PubMed] [Google Scholar]
- 3.Mak SK, Gwi E, Chan KW, Wong PN, Lo KY, Lee KF, et al. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent Diabetes mellitus. Nephrol Dial Transplant. 1997;12:2588–91. doi: 10.1093/ndt/12.12.2588. [DOI] [PubMed] [Google Scholar]
- 4.Prakash J, Lodha M, Singh SK, Vohra R, Raja R, Usha Diabetic retinopathy is a poor predictor of type of nephropathy in proteinuric type 2 diabetic patients. J Assoc Physicians India. 2007;55:412–6. [PubMed] [Google Scholar]
- 5.Prakash J, Sen D, Usha, Kumar NS. Non-diabetic renal disease in patients with type 2 Diabetes mellitus. J Assoc Physicians India. 2001;49:415–20. [PubMed] [Google Scholar]
- 6.Soni SS, Gowrishankar S, Kishan AG, Raman A. Non-diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11:533–7. doi: 10.1111/j.1440-1797.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin YL, Peng SJ, Ferng SH, Tzen CY, Yang CS. Clinical indicators which necessitate renal biopsy in type 2 diabetes mellitus patients with renal disease. Int J Clin Pract. 2009;63:1167–76. doi: 10.1111/j.1742-1241.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 8.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of non diabetic renal disease in Diabetic patients. Am J Nephrol. 2007;27:322–8. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 9.Chang TI, Park JT, Kim JK, Kim SJ, Oh HJ, Yoo DE, et al. Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract. 2011;92:198–204. doi: 10.1016/j.diabres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Choi PC, Szeto CC, To KF, Tang NL, Chan AW, et al. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care. 2002;25:900–5. doi: 10.2337/diacare.25.5.900. [DOI] [PubMed] [Google Scholar]
- 11.Zelmanovitz T, Gerchman F, Balthazar AP, Thomazelli FC, Matos JD, Canani LH. Diabetic nephropathy. Diabetol Metab Syndr. 2009;1:10. doi: 10.1186/1758-5996-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollin T, Laroche B, Psooy K. Canadian guidelines for the management of asymptomatic microscopic hematuria in adults. Can Urol Assoc J. 2009;3:77–80. doi: 10.5489/cuaj.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson KL, Gipson DS, Massengill SA, Dooley MA, Primack WA, Ferris MA, et al. Predictors of relapse and end stage kidney disease in proliferative lupus nephritis: Focus on children, adolescents and young adults. Clin J Am Soc Nephrol. 2009;4:1962–7. doi: 10.2215/CJN.00490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Li XW, Huang QY, Ye WL, Duan L, Li Y. Non-diabetic renal disease in type II diabetes mellitus. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:101–4. [PubMed] [Google Scholar]
- 15.Chawarnkul O, Vareesangthip K, Ongajyooth L, Cheunsuchon B, Parichatikanond P. Non-diabetic glomerular disease in Type II DM: 10 years experience. J Med Assoc Thai. 2009;92(Suppl 2):S57–60. [PubMed] [Google Scholar]
- 16.Castellano I, Covarsí A, Novillo R, Gómez-Martino JR, Ferrando L. Renal histological lesions in patients with type II diabetes mellitus. Nefrologia. 2002;22:162–9. [PubMed] [Google Scholar]
- 17.John GT, Date A, Korula A, Jeyaseelan L, Shastry JC, Jacob CK. Nondiabetic renal disease in noninsulin-dependent diabetics in a South Indian Hospital. Nephron. 1994;67:441–3. doi: 10.1159/000188019. [DOI] [PubMed] [Google Scholar]
- 18.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm 2012. 2012 doi: 10.1155/2012/146154. 146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orfila C, Lepert JC, Modesto A, Pipy B, Suc JM. IgA nephropathy complicating diabetic glomerulosclerosis. Nephron. 1998;79:279–87. doi: 10.1159/000045050. [DOI] [PubMed] [Google Scholar]
- 20.Lai FM, Li PK, Pang SW, Suen MW, Lui SF, To KF, et al. Diabetic patients with IgA nephropathy and diabetic glomerulosclerosis. Mod Pathol. 1993;6:684–90. [PubMed] [Google Scholar]
- 21.Waldherr R, Ilkenhans C, Ritz E. How frequent is glomerulonephritis in diabetes mellitus type II? Clin Nephrol. 1992;37:271–3. [PubMed] [Google Scholar]


