Abstract
Background:
Traditionally, midazolam has been used for providing conscious sedation in endoscopic retrograde cholangiopancreatography (ERCP). Recently, dexmedetomidine has been tried, but very little evidence exists to support its use.
Objective:
The primary objective was to compare haemodynamic, respiratory and recovery profile of both drugs. Secondary objective was to compare the degree of comfort experienced by patients and the usefulness of the drug to endoscopist.
Study Design:
Open-label Randomised Controlled Trial.
Methods:
Subjects between 18 and 60 years of age with American Society of Anaesthesiologist Grade I-II requiring ERCP were enrolled in two groups (30 each). Both groups received fentanyl 1 μg/kg IV at the beginning of ERCP. Group M received IV midazolam (0.04 mg/kg) and additional 0.5 mg doses until Ramsay Sedation Scale (RSS) score reached 3-4. Group D received dexmedetomidine at loading dose of 1 μg/kg over 10 min followed by 0.5 μg/kg/h infusion until RSS reached 3-4. The vital parameters (heart rate (HR), blood pressure (BP), respiration rate, SpO2), time to achieve RSS 3-4 and facial pain score (FPS) were compared during and after the procedure. In the recovery room, time to reach modified Aldrete score (MAS) 9-10 and patient and surgeon's satisfaction scores was also recorded and compared. Any complication during or after the procedure were also noted.
Results:
In Group D, patients had lower HR and FPS at 5, 10 and 15 min following the initiation of sedation (P<0.05). There was no statistically significant difference in BP and respiratory rate. The procedure elicited a gag response in 29 (97%) and 7 (23%) subjects in Group M and Group D respectively (P<0.05). MAS of 9-10 at 5 min during recovery was achieved in 27 (90%) subjects in Group D in contrast to 5 (17%) in Group M (P<0.05). Dexmedetomidine showed higher patient and surgeon satisfaction scores (P<0.05).
Conclusion:
Dexmedetomidine can be a superior alternative to midazolam for conscious sedation in ERCP.
Keywords: Conscious sedation, dexmedetomidine, endoscopic retrograde cholangiopancreatography, midazolam
INTRODUCTION
The indications for endoscopy have increased enormously as it has matured from a purely diagnostic procedure to a therapeutic subspecialty. There has been a considerable progress in practice of sedation and analgesia during endoscopic procedure. Endoscopic retrograde cholangiopancreatography (ERCP) plays a crucial role in diagnosis and treatment of pancreaticobiliary pathologies and its use has increased in recent years.[1]
Most endoscopic procedures like ERCP are performed with the patient under moderate sedation, a practice that is referred to as ‘conscious sedation’. Conscious sedation is required mainly to minimize patient anxiety, discomfort and pain and to enhance patient cooperation throughout the procedure and facilitate the performance of the procedure by the endoscopists.[2]
There are various agents available to provide conscious sedation. Current drugs include benzodiazepines[3] (most commonly midazolam and diazepam) with an opioid[4] (often fentanyl or remifentanil), with or without propofol.[5,6] Ketamine has also been used in low doses for moderate sedation. Newer agents such as dexmedetomidine[7,8] and fospropofol are also being used now a days.
Most endoscopists favour midazolam because of its fast onset and short duration of action and high amnestic properties. Midazolam is a water-soluble agent that causes sedation, anxiolysis and amnesia. It is the shortest-acting benzodiazepine available.[9] Common adverse effects of midazolam include prolonged recovery after long-term or high dose use, hypoxemia, hypotension and respiratory depression when paired with an opioid.[7,10,11]
In recent years, dexmedetomidine has been used as an alternative to midazolam in conscious sedation. It is a potent and highly selective α-2 adrenoceptor agonist with sympatholytic, sedative, amnestic and the analgesic properties[12,13] and has been described as a useful and safe adjunct in many clinical applications. It is the most recently developed drug of this class. It provides a unique ‘conscious sedation’ (patients appear to be asleep, but are readily aroused) and analgesia, without respiratory depression.
AIMS AND OBJECTIVES
Primary outcome
Changes in the heart rate (HR), respiratory rate, non-invasive blood pressure (BP) (systolic, diastolic and mean BP) and oxygen saturation during ERCP and recovery.
Achievement of modified Aldrete score (MAS) of 9-10 at 5 min after completion of the procedure during recovery.
Secondary outcome
Complications during ERCP.
Complications during recovery.
Pain evaluation during ERCP at 5 min, 10 min and 15 min by using facial pain score (FPS).
Need of rescue drug.
Patient's satisfaction score.
Endoscopists satisfaction score.
MATERIALS AND METHODS
After ethical committee approval and written informed consent from patients, an open-label, randomised controlled trial (RCT) was carried out on 60 patients of either sex, aged 18-60 years of age undergoing diagnostic and therapeutic ERCP, with American Society of Anaesthesiologist (ASA) Grade I and II. We excluded patients who had ASA physical status Grade III and more, baseline SpO2 <90%, mechanically ventilated patients, patients with comorbid conditions such as diabetes mellitus, hypertension (HTN) or hepatic or renal insufficiency to see the pure effect of both these drugs and to avoid any interaction with any simultaneous drug intake, which could have altered the results. We also excluded patients who had difficulty in communication (due to language problem or deafness), patients with history of operative intervention within the past 72 h, because we wanted to record their Ramsay Sedation Scale (RSS) and MAS which might not have been possible in these subsets of patients. Patients with a known allergy to these drugs and with a history of sulphite, egg or soya bean allergy and pregnant patients were also excluded.
A detailed pre-operative check-up including general examination and systemic examination of the patient was carried out. On the arrival of patient in Endoscopy Room, all vital parameters such as HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and oxygen saturation (SpO2 %) were recorded and thereafter, readings were taken following the loading dose and every 5 min until the completion of the procedure. Venous access was secured on on non-dominant hand of every patient by 18G/20G cannula and Ringer Lactate drip was started.
Subjects were randomised with a 1:1 allocation ratio. The allocated intervention was written on a slip of paper, placed in serially numbered, opaque envelopes and sealed. As consecutive eligible subjects got enrolled, the envelopes were serially opened and the allocated intervention was implemented. The chief investigator, medical and nursing personnel were not blinded as it was an open-label trial. Subjects were followed from the point of randomisation until complete recovery. The numbers of patients were equally distributed in both groups. The groups were also similar in respect to time of intervention by endoscopist, i.e., after achievement of RSS 3-4. The intervention was in the form that both group patients were given fentanyl 1 μg/kg at the beginning of the procedure. Subjects in the study Group D received dexmedetomidine at loading dose of 1 μg/kg IV over 10 min followed by 0.5 μg/kg/h infusion until RSS reached 3-4. Control Group M received a single dose of 0.04 mg/kg IV midazolam and additional 0.5 mg doses until RSS reached 3-4. We used these doses of midazolam and dexmedetomidine to preserve sufficient consciousness to allow communication, but provided the necessary degree of sedation to enable surgical comfort and an adequate quality of recovery with no negative effects on haemodynamics and respiratory parameters.
The following parameters were monitored and recorded[14,15,16] : (1) time to achieve RSS of 3-4, along with the total dose of drug needed, (2) the FPS 0-10 to evaluate pain (performed by anaesthesiologist at 5 min intervals throughout procedure) [Figure 1] and (3) in the recovery room, patient FPS noted till MAS reached 9-10.
Figure 1.
Facial Pain Rating Scale (Wong baker face scale)
During the procedure if patient required more than three episodes of personal restraint by the assistant or if either patient or endoscopist was uncomfortable, the rescue IV sedation was provided with propofol in top up incremental dose of 10 mg until patient reached RSS 3-4. The requirement of rescue sedative drug was also recorded. During procedure, any of the following complications were observed, recorded and treated accordingly. Oxygen desaturation was considered when SpO2 level dropped below 92% for more than 10 s. A HR under 50 beats/min or a 20% decrease from the baseline was labelled as bradycardia, whereas a HR over 110 or an increase of more than 20% from the baseline level was considered as tachycardia. MAP levels that were lower than 60 mmHg or 20% less than the baseline were regarded as hypotension and a MAP value of over 150 mmHg or a 20% increase from the baseline was regarded as HTN. Possible complications, such as respiratory depression, allergies, coughing, gagging, nausea and vomiting, were recorded. After the procedure, the satisfaction of the surgeon and patients was assessed using satisfaction score [Table 1].
Table 1.
Satisfaction score

In the recovery room, MAS and vital parameters of patients were recorded every 5 min by Anaesthesiologist along with any adverse effect such as restlessness, shivering, nausea, vomiting, abdominal discomfort and respiratory depression. On achieving MAS of 9-10, patients were discharged. The duration of stay in the recovery room was also recorded. In case of any adverse events in the recovery room such as nausea, vomiting, abdominal discomfort respiratory depression, the patients were observed in hospital for at least 12 h.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics. Dichotomous outcomes were compared by Chi-square test with continuity correction or Fisher's exact test as applicable. Numerical variables were compared by the Student's t-test or Mann-Whitney U-test, depending on the distribution. Intra-group comparison was performed using repeated measure ANOVA.
Analysis was the intention to treat, i.e., all subjects who were randomized were included in analysis, irrespective of degree of compliance. Analysis was performed using SPSS version 17 (233 South Wacker Drive, 11th Floor, Chicago, IL 60606-6412). The results were considered significant when the P<0.05.
RESULTS
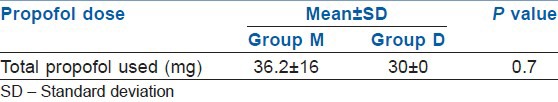
There were no statistically significant differences in either the demographic data [Table 2] or the baseline vitals between the two groups [Table 3]. There was no significant difference in SBP, DBP, MAP and RSS, respiration rate (RR) and SpO2 between the two groups during the procedure and during recovery [Table 3]. Patients in Group D had statistically significant (P<0.05) lower HR after infusion of loading dose, at 5, 10, 15 and 20 min during ERCP and during recovery [Table 3 and Figure 2]. Mean time to achieve RSS 3-4 was 3(±0.8) min in M group and 12 (±1.1) min in D group (P<0.001) [Table 4]. There was a trend towards lesser requirement of rescue drug (propofol) during the procedure in the Group D [Table 5].
Table 2.
Description of baseline characteristics in two groups
Table 3.
Comparison of vitals between two groups
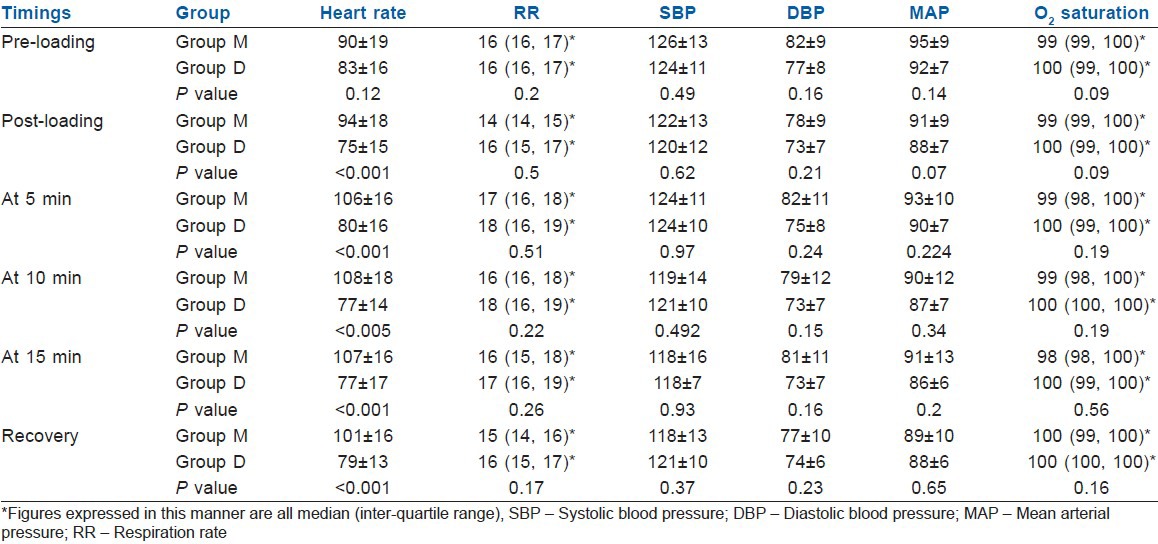
Figure 2.
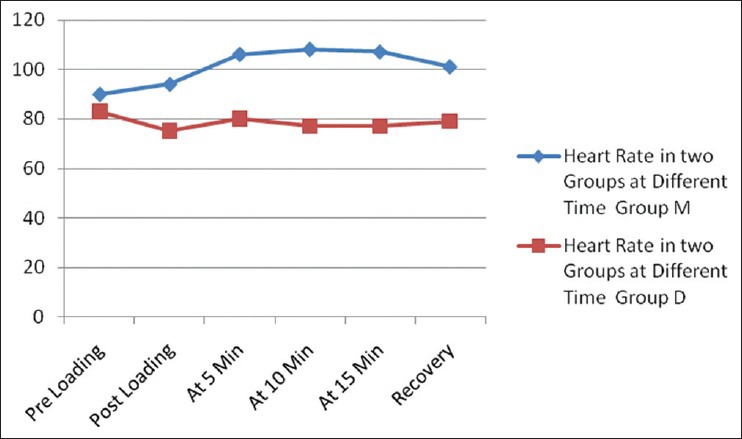
Heart rate in two groups at different time
Table 4.
Time to achieve desired RSS of 3-4

Table 5.
Dose of propofol in two groups
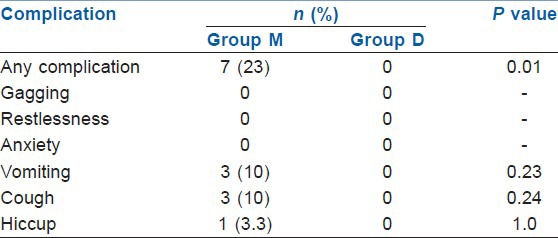
In Group M patients had significantly more incidences of gagging (97%) and restlessness (60%) during the procedure compared with the Group D (23% and 3% respectively) (P<0.001) [Table 6]. During recovery also the incidence of complications were significantly higher in Group M compared with Group D (P<0.01) [Table 7]. The overall incidence of complications was significantly higher in Group M compared to Group D both during the procedure and during recovery.
Table 6.
Complications during ERCP in two groups
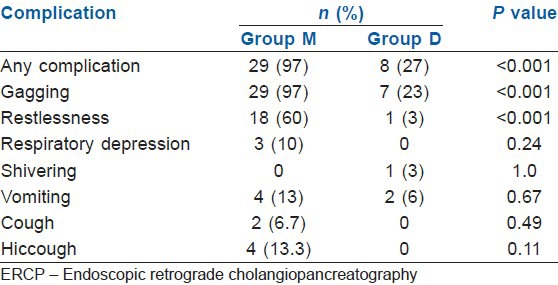
Table 7.
Complications during recovery in two groups
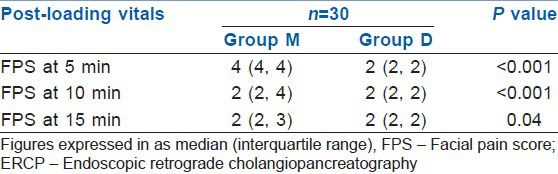
The recovery was quicker in the Group D and more number of patients (90%) achieved MAS of 9-10 at 5 min during recovery compared with the Group M (17%) [Table 8]. Both, the patient and the endoscopist satisfaction scores were higher in Group D as compared with Group M (P<0.05) [Table 9]. During recovery, the FPS was significantly better in Group D at 5 and 10 min (P<0.001). At 15 min of procedure, both groups had similar FPS (P>0.05) [Table 10].
Table 8.
Achievement of modified Aldrete score of 9-10 at 5 min during recovery
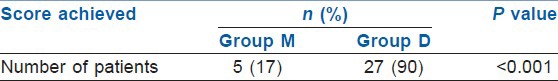
Table 9.
Satisfaction score in two groups
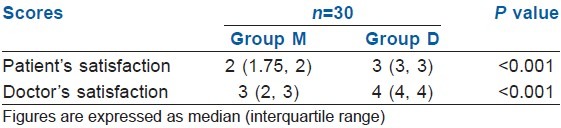
Table 10.
FPS in two groups during ERCP
DISCUSSION
Sedation and analgesia allow patient to tolerate unpleasant procedure by reliving anxiety, discomfort or pain and also can expedite the conduct of procedure and avoid complications such as duodenal perforation, pancreatitis that results from poor patient cooperation.
We conducted a prospective randomized controlled trial to compare the efficacy and safety of IV dexmedetomidine and IV midazolam, for conscious sedation in ERCP. The dose regimen of both dexmedetomidine and midazolam used in our study were similar to that used by Kilic et al.[17] and Arain and Ebert.[18] In Group D induction dose was given by infusion in 10 min followed by maintenance, but in Group M drug was given as a bolus. This explains the faster onset of sedation (RSS 3-4) in Group M compared with Group D (mean time 3 min vs. 12 min; P<0.001) [Table 3].
Our study showed no significant difference in SBP, DBP, MAP, RSS, RR and SpO2 in the Group D when compared with Group M. Patients in Group D had statistically significant lower HR (P<0.05) after infusion of loading dose and at 5, 10, 15 and 20 min during ERCP [Figure 1]. These results are in accordance with previous studies by Kilic et al.[17] Alhashemi[19] and Dere et al.,[20] where they also found statistically significant lower HRs in dexmedetomidine group compared with midazolam group. Hypotension and bradycardia are recognized as two major adverse effects associated with α2-agonist agents. It has been suggested that these effects are mediated by activation of α2-adrenoceptors, imidazoline preferring receptors or both in the ventrolateral medulla and especially in the solitarius nucleus tract.[21,22] In the present study, we observed decrease in HR and comparatively stable BP values in Group D. In Group M, HR and BP were found to be higher during the operation in subsequent measurements compared with both baseline values and Group D. These findings suggest that dexmedetomidine has clinical advantages over midazolam with regard to controlling hemodynamic variability.
In our study, MAS during recovery was statistically different between two groups in the 5th min (P<0.001). About 90% of Group D patients achieved MAS of 9-10 in 5th min and only 17% in Group M. There was no significant difference in MAS at 10 min of recovery. Kilic et al.[17] concluded that 24% in Group M and 80% in Group D reached an MAS value of 10 in the 5th min. By the 10th min, 64% in M group and 96% in Group D patients attained an MAS value of 10. There were statistically significant differences between the two groups at 5 and 10 min (P<0.001 and P<0.05 respectively).
Complication rate was higher in Group M which was statistically significant (P<0.001). Almost 97% patients had gagging and 60% had restlessness during the procedure in Group M. In study by Abdellatif et al.[23] and Arain and Ebert[18] no intraoperative or post-operative adverse effects were reported in dexmedetomidine group. In contrast, few patients in the midazolam group had oxygen desaturation, lost capnography wave form, nausea and vomiting while no cases of hypotension or bradycardia was reported. In the recovery room too, the total complications were significantly more in the Group M. About 23% patients in Group M had complications, in which 10% experienced cough and vomiting and 3% patients had hiccough. None of the patients in Group D suffered from any complications in the recovery room.
In our study, we did FPS throughout the procedure, to measure the comfort of patient during procedure. Results were statistically different at 5 and 10 min in both groups and Group D showed better FPS score. At 15 min of procedure, both groups had similar FPS. Kilic et al.[17] in his study concluded that there were no significant differences in FPS values between groups during the procedures. Though, there was no significant difference between two groups regarding the requirement of propofol as rescue drug, but there was an increasing trend of using it in Group M.
In our study, we compared patients and endoscopist satisfaction by a scoring system and there was statistically significant difference between two groups (P<0.001). Group D had higher satisfaction scores both for patients and endoscopist. Our findings were similar to findings of Kilic et al.[17] and Karaaslan et al.[24] Midazolam sedation in our study was associated with lower patient satisfaction, higher pain scores and more use of rescue analgesic. The method of sedation was described as excellent in most of patients in Group D (77.7%) versus 7.4% of patients in Group M (P<0.001). Poor satisfaction was reported in 25.9% of patients in Group M but not in Group D. Alhashemi[19] and Dere et al.[20] found similar results for patient's satisfaction score and endoscopist satisfaction score.
The main limitation of the study is that it is an open labelled RCT so there is always an inherent risk of bias towards intervention group.
Implication
Our study is able to demonstrate that the use of dexmedetomidine for conscious sedation during short invasive procedure as ERCP could be superior alternative to midazolam. Dexmedetomidine is a safe drug with good recovery profile and very few studies are published regarding its use in ERCP. However, there is need for further multicentric RCT to confirm the findings of our study. So that dexmedetomidine can become standard of care for conscious sedation in ERCP patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chen WX, Lin HJ, Zhang WF, Gu Q, Zhong XQ, Yu CH, et al. Sedation and safety of propofol for therapeutic endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2005;4:437–40. [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists. Continuum of Depth of Sedation: Definition of General Anesthesia and Levels of Sedation/Analgesia. 2004. [Last accessed on 2011 Nov 12]. Available from: http://www.asahq.org/publications and services/standards/20.pdf .
- 3.Froehlich F, Schwizer W, Thorens J, Köhler M, Gonvers JJ, Fried M. Conscious sedation for gastroscopy: Patient tolerance and cardiorespiratory parameters. Gastroenterology. 1995;108:697–704. doi: 10.1016/0016-5085(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 4.Zakko SF, Seifert HA, Gross JB. A comparison of midazolam and diazepam for conscious sedation during colonoscopy in a prospective double-blind study. Gastrointest Endosc. 1999;49:684–9. doi: 10.1016/s0016-5107(99)70282-8. [DOI] [PubMed] [Google Scholar]
- 5.Muller S, Borowics SM, Fortis EA, Stefani LC, Soares G, Maguilnik I, et al. Clinical efficacy of dexmedetomidine alone is less than propofol for conscious sedation during ERCP. Gastrointest Endosc. 2008;67:651–9. doi: 10.1016/j.gie.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: A risk factor analysis. Scand J Gastroenterol. 2008;43:368–74. doi: 10.1080/00365520701679181. [DOI] [PubMed] [Google Scholar]
- 7.Aantaa R, Jaakola ML, Kallio A, Kanto J, Scheinin M, Vuorinen J. A comparison of dexmedetomidine, and alpha 2-adrenoceptor agonist, and midazolam as i.m. premedication for minor gynaecological surgery. Br J Anaesth. 1991;67:402–9. doi: 10.1093/bja/67.4.402. [DOI] [PubMed] [Google Scholar]
- 8.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard AR. Sedation and analgesia in intensive care. Medications attenuate stress response in critical illness. Postgrad Med. 2002;111:59–60. doi: 10.3810/pgm.2002.02.1107. 63. [DOI] [PubMed] [Google Scholar]
- 10.Harvey MA. Managing agitation in critically ill patients. Am J Crit Care. 1996;5:7–16. [PubMed] [Google Scholar]
- 11.Shafer A. Complications of sedation with midazolam in the intensive care unit and a comparison with other sedative regimens. Crit Care Med. 1998;26:947–56. doi: 10.1097/00003246-199805000-00034. [DOI] [PubMed] [Google Scholar]
- 12.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 13.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 14.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 15.Talu GK. Evaluation of the patient's pain. In: Erdine S, editor. Pain. 3rd edition. İstanbul: Nobel publishing houses: 2007. pp. 61–9. [Google Scholar]
- 16.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilic N, Sahin S, Aksu H, Yavascaoglu B, Gurbet A, Turker G, et al. Conscious sedation for endoscopic retrograde cholangiopancreatography: Dexmedetomidine versus midazolam. Eurasian J Med. 2011;43:13–7. doi: 10.5152/eajm.2011.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 19.Alhashemi JA. Dexmedetomidine vs. midazolam for monitored anaesthesia care during cataract surgery. Br J Anaesth. 2006;96:722–6. doi: 10.1093/bja/ael080. [DOI] [PubMed] [Google Scholar]
- 20.Dere K, Sucullu I, Budak ET, Yeyen S, Filiz AI, Ozkan S, et al. A comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedation. Eur J Anaesthesiol. 2010;27:648–52. doi: 10.1097/EJA.0b013e3283347bfe. [DOI] [PubMed] [Google Scholar]
- 21.Maze M, Tranquilli W. Alpha-2 adrenoceptor agonists: Defining the role in clinical anesthesia. Anesthesiology. 1991;74:581–605. [PubMed] [Google Scholar]
- 22.Kuhar MJ, Unnerstall JR. Mapping receptors for alpha 2-agonists in the central nervous system. J Cardiovasc Pharmacol. 1984;6(Suppl 3):S536–42. [PubMed] [Google Scholar]
- 23.Abdellatif AA, Elkabarity RH, Hamdy TA. Dexmedetomedine vs. midazolam sedation in middle ear surgery under local anesthesia: Effect on surgical field and patient satisfaction. Egypt J Anaesth. 2012;28:117–23. [Google Scholar]
- 24.Karaaslan K, Yilmaz F, Gulcu N, Colak C, Sereflican M, Kocoglu H. Comparison of dexmedetomidine and midazolam for monitored anesthesia care combined with tramadol via patient-controlled analgesia in endoscopic nasal surgery: A prospective, randomized, double-blind, clinical study. Curr Ther Res Clin Exp. 2007;68:69–81. doi: 10.1016/j.curtheres.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


