Abstract
Background and Aims:
Acute neuropathic pain (ANP) is an under-recognised and under-diagnosed condition and often difficult to treat. If left untreated, it may further transform into persistent post-operative chronic pain leading to a disability.
Aims:
This prospective study was undertaken on 300 patients to identify the prevalence of ANP in the post-operative period by using a neuropathic pain detection questionnaire tool.
Methods:
This is an open-label study in which patients with six different types of cancer surgeries (Thoracic, gastro-intestinal, gynae/urology, bone/soft-tissue, head and neck and breast subgroups-50 each) were included for painDETECT questionnaire tool on the 2nd and 7th day surgery.
Results:
This study found a 10% point prevalence of ANP. Analysis showed that 25 patients had ‘possible’ ANP, the maximum from urological cancer surgery (6) followed by thoracic surgery (5). Five patients were found to have ‘positive’ ANP including 2 groin node dissection, 2 hemipelvectomy and 1 oesophagectomy.
Conclusion:
Significant relationship between severity of post-operative pain was found with the occurrence of ANP in the post-operative period requiring a special attention to neuropathic pain assessment. Larger studies are required with longer follow-up to identify accurately the true prevalence and causative factors of ANP after surgery.
Keywords: Acute neuropathic pain, cancer surgery, post-operative pain, prevalence
INTRODUCTION
Neuropathic pain is caused by a lesion or disease of the somatosensory nervous system. Acute neuropathic pain (ANP) occurs consequent to medical disease or surgery or trauma exhibiting pain with sensory dysfunction. Acute post-surgical neuropathic pain is characterised by the presence of signs and symptoms of neuropathic pain distinct from the typical post-operative nociceptive pain, which is usually localised to surgical incision, is sharp and self-limiting. Prevalence estimates indicate that 1-3% of the population in the developed world experience ANP. Persistent post-surgical neuropathic pain (NP) is mostly an unrecognised clinical problem. ANP may further progress to persistent post-operative pain and leading to permanent disability.[1] A large component of persistent pain after surgery can possibly be due to neuropathic component, caused from nerve injury during surgery and resultant pain can persist for months and years after the surgical wound has healed. The chronicity and persistence of post-surgical NP is often severely debilitating and impinges on the psychosocial, physical, economic and emotional well-being of patients. Prevention is better than waiting for post-surgical NP to become persistent and chronic.[2,3] Acute post-operative pain is followed by persistent pain in 10-50% of individuals after common operations, such as groin hernia repair, breast and thoracic surgery, leg amputation and coronary artery bypass surgery.[4] Since chronic pain can be severe in about 2-10% of these patients, persistent post-surgical pain represents a major, largely under diagnosed clinical problem. Iatrogenic neuropathic pain is probably the most important cause of long-term post-surgical pain. Consequently, surgical techniques that avoid nerve damage should be applied whenever possible. The objective of this study was to identify the prevalence of ANP after cancer surgery as well as intensity and characteristics of pain on the 2nd and 7th day in the post-operative period as assessed by using pain DETECT questionnaire.
METHODS
After Institutional Review Board approval, consecutive 300 patients in the age group of 18-70 years of either sex scheduled for elective surgeries were included in the study during October-December 2008. All patients were treated for pain as per the post-operative pain protocol laid down by the department. All patients received round the clock paracetamol. All thoraco-abdominal surgery patients received thoracic epidural analgesia and lumbar epidural for lower limb procedures. Non-steroidal anti-inflammatory drugs were administered, if patients required rescues, if there were no contraindications. This is an open-label non-randomised study which included consecutive patients who were scheduled for major cancer surgery during the defined period. Patient having a history of neuropathic pain before operation or who could not comprehend the questionnaire were excluded from the study. A specialised neuropathic pain questionnaire ‘painDETECT’ was used to assess neuropathic pain and to detect the prevalence of ANP. This questionnaire had been prepared by professionals and validated in various languages including Hindi and Marathi. Patients were explained regarding the pain questionnaire in their own language including English preoperatively and written informed consent was taken in the language best understood by them. Patients were followed up in the ward or out-patient department (OPD) as applicable on 2nd and 7th day morning in the post-operative period and the pain questionnaires were completed by patients themselves. A total of 50 patients were included each from thoracic, gynaecology/genitourinary (GU/GY), gastrointestinal, breast, bone and soft-tissue (BST) and head and neck (H and N) services. As the patients were discharged after breast surgery on the post-operative day 1 as per our hospital protocol, they were followed-up in the OPD on their first follow-up visit. Patients were assessed for post-operative pain score on 0-10 numerical scale on 2nd and 7th post-operative day. The study was aimed to determine the correlation of post-operative pain with the occurrence of ANP on 2nd and 7th post-operative day; assessment was planned on the 7th post-operative day. The pain DETECT questionnaire [Figure 1] was used for assessing neuropathic pain which consisted of three questions describing the intensity of pain on 2nd and 7th day, average pain and maximum pain during last 7 days. Seven neuropathic pain descriptors (each of these items were quantified on a 0-5 numerical rating scale [NRS]) and two descriptors assessing radiation and temporal characteristic of pain. A final scoring total based on items on the questionnaire was calculated: If the score was 0-12, neuropathic component was unlikely, if 13-18, neuropathic component ‘possible’/unclear, if the score was 19-38, neuropathic component was considered likely/‘positive’ (>90%). Mean post-operative pain score of last 24 h were assessed. Short surgery patients were discharged on 2nd post operative day and major surgery patients, by 7th post-operative day. Thereafter, pain was assessed either in OPD or during hospitalisation as applicable.
Figure 1.
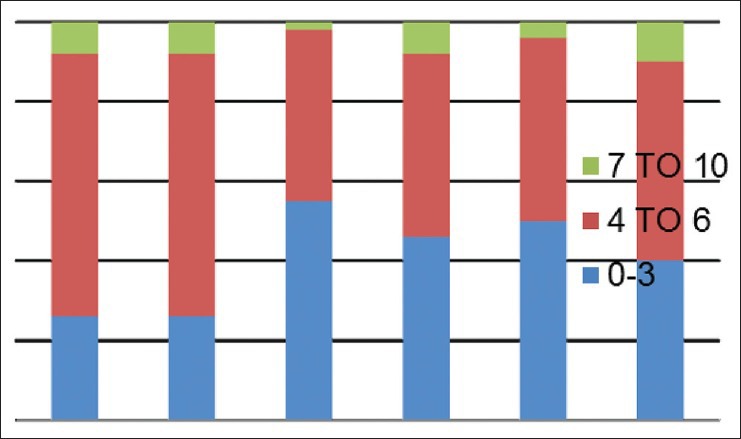
Mean pain scores (numerical rating scale) on day 2
Data were analysed as percentages in different services viz., thoracic, GU/GY, gastrointestinal, breast, BST and H and N services and a descriptive subgroup analysis was performed. Different severity of pain on 0-10 numerical rating pain scale was categorised as mild: 0-3, moderate 4-6, severe 7-10 in different services. painDETECT scores were categorised into two groups-unlikely (negative) as 1-12 and positive (13-38). Possible scores (13-18) were merged with positive (19-38) for convenience of statistical analysis. Chi-square test was used to analyse the relationship between post-operative pain scores and painDETECT score. P < 0.05 was taken as significant.
RESULTS
A total of 300 patients completed a painDETECT questionnaire on 2nd and 7th day in the post-operative period in their own language spoken or best understood by them. 50 patients each from thoracic, gynaecological/genitourinary, breast, gastrointestinal, H and N, BST surgery responded to the questionnaire. The prevalence of different severity of pain on 2nd and 7th post-operative day on 0-10 numerical rating pain scale were categorised as mild, moderate, severe in different services. Maximum pain perceived by the patient in first 7 days of the post-operative period was assessed on (NRS: 0-10) and results are shown [Figures 1 and 2] on day 2nd and 7th day as percentage occurrences in respective services. Table 1 shows the various descriptions of neuropathic pain as described by patients. Burning sensation was common in breast (32%) and thoracic (28%) and BST patients (22%). Persistent pain with slight fluctuation was the most common (40-60%) temporal characteristic in 1st week after surgery followed by pain attacks without pain in between in 20% and 30% in most surgeries. In between pain attack was the least common characteristic. Out of 300 patients assessed by questionnaire, 29 patients were found to have some characteristics of neuropathic pain. 24 patients had ‘possible’ ANP, which include thoracic surgery = 5, GU/GY surgery = 6, BST surgery = 5, breast surgery = 5, H and N surgery = 5 and none from gastrointestinal surgery. Five patients found to have positive NP (>90%), including 2 patients of groin node dissection, one patient each of hemicolectomy, open TTE (open transthoracic oesophagectomy) and hemipelvectomy. Neuropathic pain score. Table 2 shows the pain scores on day 2 and day 7 with concomitant painDETECT scores. Chi-square test shows that a significant relationship between severe post-operative pain scores (7-10) with positive painDETECT scores on day 2 and also a significant relation on day 7 with moderate to severe post-operative pain [Figure 3].
Figure 2.
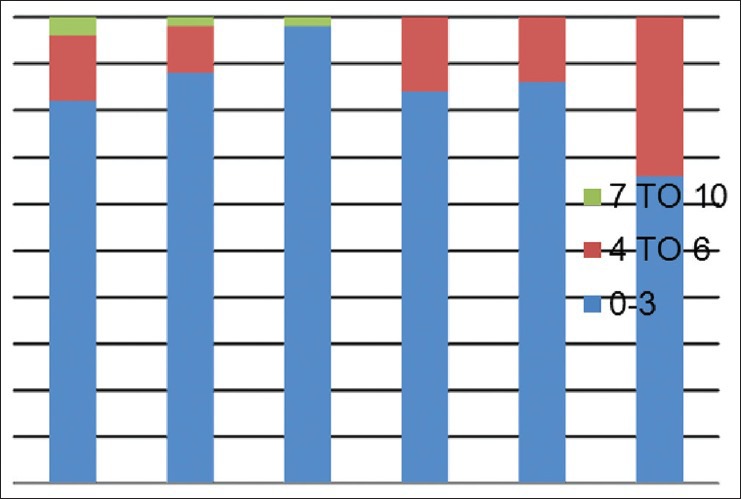
Mean pain scores (numerical rating scale) on day 7
Table 1.
Prevalence of neuropathic pain (sensory) descriptors
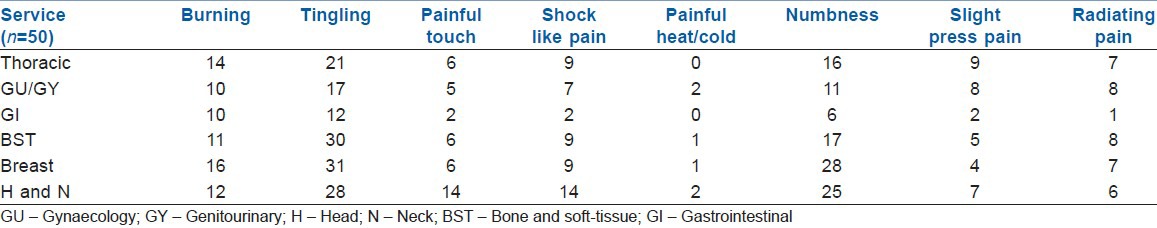
Table 2.
Patients with possible NP (13-18) or positive NP (19-38) as detected on painDETECT
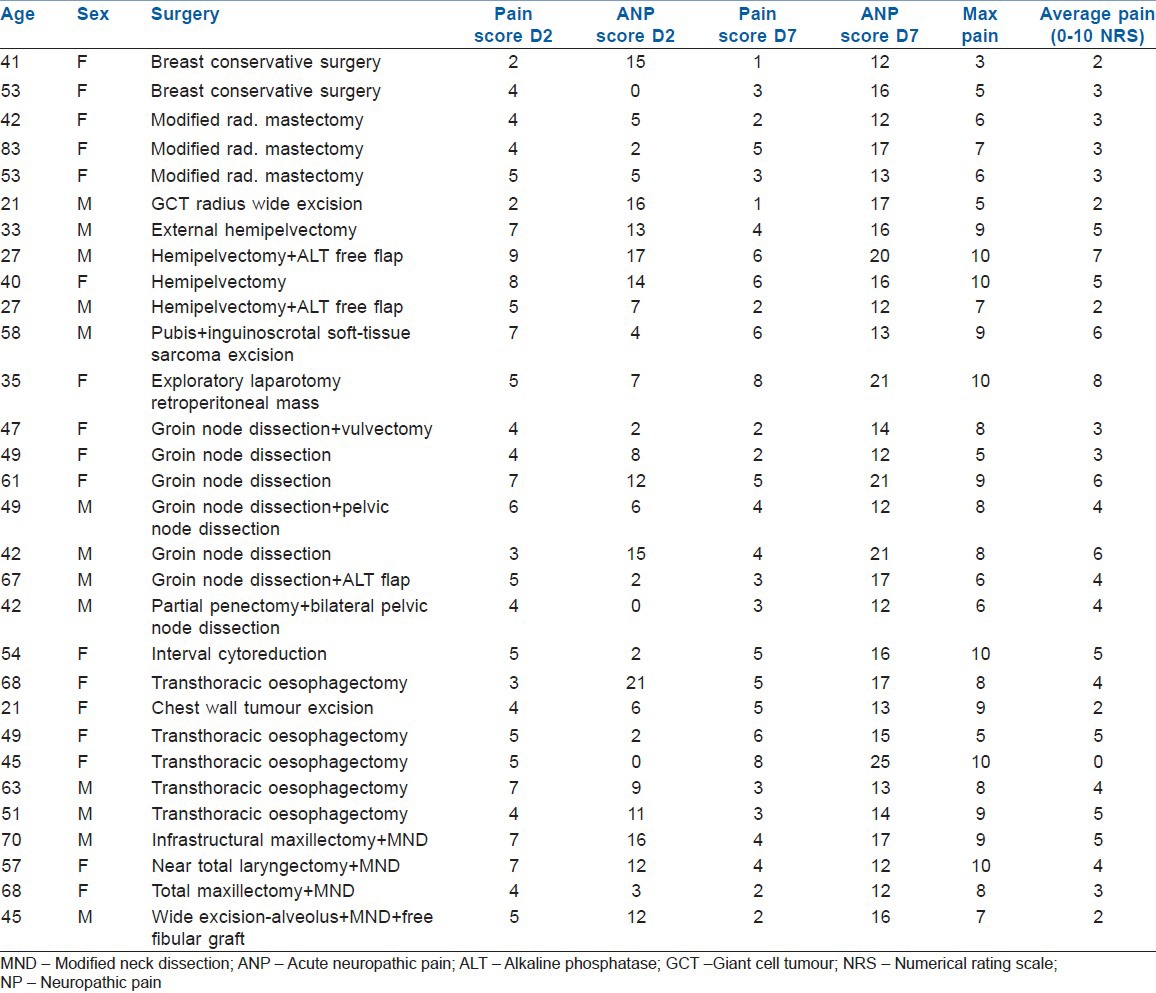
Figure 3.
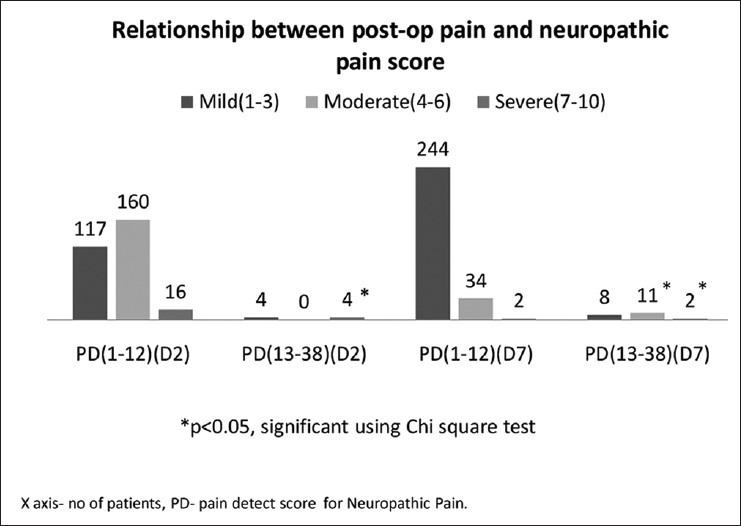
Relationship between post-operative pain and painDETECT score
DISCUSSION
This study found that approximately 10% patients had characteristics of ANP. A neuropathic pain assessment tool ‘painDETECT questionnaire’ showed 8.3% as ‘possible’ and 1.7% likely ‘positive’ prevalence of neuropathic pain in post-surgical patients during 1st week of the post-operative period in a tertiary care cancer centre. There was a strong correlation between post-operative pain score and neuropathic pain score. This study found a significant relationship between severity of post-operative pain with the occurrence of ANP in the post-operative period (P < 0.05). Among the neuropathic pain characteristics, tingling was found to be a common problem (25-60%) in different surgical subgroups. Numbness was reported in 15-55% radiating pain was found in 12-16% of patients in most surgical groups. Among spatial characteristics, 45-65% patients reported persistent pain with slight fluctuation and less than 10% revealed their pattern as ‘pain attack’ during a stable background pain.
The painDETECT questionnaire was developed and validated by Freynhagen et al.,[5] which incorporates easy to use patient based (self-report) pain questions including seven weighted sensory descriptor items and two items relating to spatial and temporal characteristics of individual pain pattern. This questionnaire was validated in a multicentre study of 392 patients with either neuropathic pain (n = 167) or nociceptive pain (n = 225) and it correctly classified 83% of patients to their diagnostic group with a sensitivity of 85% and specificity of 80%. The predetermined questions, which were written in lay languagewere related to the post-operative neuropathic pain experience.
The prevailing approach of focusing on established chronic pain implicitly assumes that information generated during the acute injury phase is not important to the subsequent development of chronic pain. However, a rarely appreciated fact is that every chronic pain was once acute. The transition of acute post-operative pain to chronic post-surgical pain is a complex and poorly understood developmental process, involving biological, psychological and social environmental factors.[3] An understanding of the post-operative pain experience from a patient's perspective is important, if health-care professionals are to identify ways to improve overall pain management.
The general prevalence of ANP in the post-operative patient has received little attention in the scientific literature. However, a prospective study in an Australian acute pain service identified 51 post-operative patients over a 2.5-years period with a history and examination suggestive of ANP, with overall incidence of 1.04%.[6] The investigators excluded those patients who were receiving an above or below knee amputation and therefore it could be assumed that the overall incidence would have been considerably higher. The striking finding of the study was that those identified to have ANP, 55% continued to have pain at 12 months with 30% of these patients describing no change or a worsening of pain since their hospital admission.[6]
The burden of post-surgical neuropathic pain is still large in the present era of sophisticated diagnostic facilities, advanced surgical and therapeutic techniques and effective pharmacotherapy. There are lapses in many places but the most important is neglect towards the neuropathic component of post-surgical pain. There is presently no consensus regarding early diagnosis and effective treatment protocol for post-operative neuropathic pain.
Post-operative neuropathic pain depends upon preoperative tumour pain with the extent of malignancy and its treatment, psychosocial issues, genetic factors. Tumour-related painful peripheral neuropathies and plexopathies including brachial and cervical lumbosacral plexopathies. The treatment-related pain etiologies include post-chemotherapies, post-radiotherapy and surgery-induced pain (i.e. amputation). Chemotherapy-induced neuropathic pain may due to drugs such as cisplatin, paclitaxel and vinca alkaloids. Post-radiotherapy pain syndromes include radiation myelopathy, radiation fibrosis of cervical, brachial or lumbosacral plexuses. Post-surgical neuropathic pain syndrome examples are post-mastectomy syndrome, post-thoracotomy syndrome, post-radical neck dissection, post-nephrectomy syndrome and different phantom pains. However, it is often difficult to assess and differentiate pain elements accurately between tumour or treatment related etiopathologies. Acute post-operative neuropathic pain may primarily be caused by nerve injury or inflammation during surgery compounded by various other aforementioned factors in the pre-operative period. Therefore, assessment and treatment of ANP is a complex issue.
This study did not take into account confounding factors other than surgery, which may be responsible for causing ANP. A total of 30 patients out of 300 who completed questionnaire survey had neuropathic pain characteristics, eight received chemotherapy and one received radiotherapy pre-operatively. In a prospective study[6] of 4888 (surgical and non-surgical) patients found ANP in 14 post-surgical and 37 non-surgical patients during 2.5 years duration showing overall incidence of 1.04%. They reported that patients who had ANP, 78% continued to have pain at 6 months.
In this study, 50 breast patients showed prevalence of ANP was 10% and 7% respectively after modified radical mastectomy (n = 20) and breast conserving therapy (n = 30). A breast study reported that in modified radical mastectomy (n = 53) and breast conservation surgery (n = 40) the prevalence of NP at 6 months was 23% and 35% respectively.[7] This study followed patients until 7th post-operative day only due to poor follow-up.
The prevalence of ANP after thoracic surgery was 10% (‘possible’) in 5 patients and 2% (‘positive’) in 1, out of 50 patients, which was in congruence with other studies.[8,9] A pain survey conducted on 100 patients undergoing video assisted thoracic surgery or open thoracotomy using Leeds assessment of neuropathic symptoms and signs scoring found that 8 patients (8%) developed ANP in the immediate post-operative period and out of that 5 (62.5%) developed chronic neuropathic pain (CNP) at 3 months.[7] Factors such as patient age, operating consultant and time since the operation had a significant effect on the development of ANP. This study did not follow patients beyond the 7th day post-operative day, due to general loss of patients for follow-up.
A study of post-thoracotomy patients (n = 150) reported that 60% had little pain and 35% considerable pain and rest 5% had excruciating pain during 1st post-operative week.[10] Persistent post-thoracotomy pain at 6 months was reported in 44%. Another study[11] (n = 85) on post-thoracotomy pain included 50 patients out of which 39 (45%) reported mild pain, 11 (13%) moderate pain 1 day after surgery. All 60 patients reported persistent pain (34 mild, 14 moderate, 12 severe) even a month after surgery. In our study, on 2nd post-operative day, 4% of patients had severe pain, 66% had moderate pain and the rest 30% had mild pain. The differences in results may be due to differences in the type of surgery conducted. The clinical time course and symptoms indicate that nerve impairment rather than simple nociceptive impact may be involved in this syndrome.[10,12] In our study, among thoracotomy patients (n = 50), 52% had mild pain (NRS: 0-3), 46% moderate pain (NRS: 4-6) and 2% had severe pain (NRS: 7/10 or above) during 1st post-operative week, which is in agreement to the above findings.
A study by Vecht et al. analysed neuropathic pain in 25 patients with H and N cancer at 3-6 months after surgery according to signs and symptoms and six (23%) had severe neuropathic pain secondary to the sequels of neck dissection.[13] We found the prevalence of ‘possible’ neuropathic pain in H and N region as 10%.
In a retrospective study of proximal major limb amputation surgery (n = 45), 14. Daigeler et al. reported 87% incidence of phantom pain at 3-6 months.[14] A questionnaire survey by Schley et al. for upper limb amputees (n = 96) revealed phantom pain in 8 patients (28% of phantom pain prevalence) in immediate post-operative period and 3 patients (10%) after 1 month.[15] Another study on amputation patients (n = 255) by a questionnaire survey reported phantom limb pain in 183 patients (72%) at 6 months. This study found that in BST surgery (n = 50) ANP was positive in 1 patient (2%) after hemipelvectomy and possible ANP was found in 5 patients (10%) including three hemipelvectomies.[16] In our study, ANP was higher in four out of seven proximal major amputations (six hemipelvectomies) and one hip disarticulation.
In this study, the ANP in GU and GY cancer surgery (n = 50) was positive in 2 patients (4%) and possible in 6 (12%). There are previous no studies to our knowledge on post-surgical ANP in GU/GY.
There are some limitations of this study that neither a preoperative neuropathic pain screening was performed, nor its relationship with cancer therapy (chemotherapy, radiation, or surgery-related nerve injury), administered anaesthesia and perioperative analgesia including epidural or other regional nerve blocks were studied. The presence of comorbidities also contributes significantly to persistent post-operative pain. These all factors need to be thoroughly considered in sufficiently sized future studies.
At present, there is no consensus concerning the optimal assessment and therapeutic strategy for post-operative neuropathic pain. The possible reasons are: non-recognition of neuropathic pain as a condition in its own right, difficulty in establishing unequivocal diagnosis, the scarcity of well-designed, large clinical trials to correctly identify ANP and offer opportunities to test interventions to reduce the development of CNP. However, it is important to note that the majority of patients with CNP may not have positive signs of neuropathic pain in the immediate post-operative period, although having significant neuropathic complaints.[8] Further larger size studies on ANP after different procedures with accountability of all contributing factors are needed.
CONCLUSION
Many patients suffer ANP during the post-operative period. This study found a significant relationship between severity of post-operative pain with the occurrence of ANP in the post-operative period requiring a special attention to neuropathic pain assessment and may need a definitive treatment to prevent its progression to persistent neuropathic pain. Early recognition by developing newer methods to diagnose acute post-operative neuropathic pain may enhance the potential to treat post-operative pain more successfully.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gray P. Acute neuropathic pain: Diagnosis and treatment. Curr Opin Anaesthesiol. 2008;21:590–5. doi: 10.1097/ACO.0b013e32830c900c. [DOI] [PubMed] [Google Scholar]
- 2.Shipton E. Post-surgical neuropathic pain. ANZ J Surg. 2008;78:548–55. doi: 10.1111/j.1445-2197.2008.04569.x. [DOI] [PubMed] [Google Scholar]
- 3.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: Risk factors and protective factors. Expert Rev Neurother. 2009;9:723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 5.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–20. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 6.Hayes CB, Browne SB, Lantry GR, Burstal R. Neuropathic pain in the acute pain service: A prospective survey. Acute Pain. 2002;4:45–8. [Google Scholar]
- 7.Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74:2024–31. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Searle RD, Simpson MP, Simpson KH, Milton R, Bennett MI. Can chronic neuropathic pain following thoracic surgery be predicted during the postoperative period? Interact Cardiovasc Thorac Surg. 2009;9:999–1002. doi: 10.1510/icvts.2009.216887. [DOI] [PubMed] [Google Scholar]
- 9.Kalso E, Perttunen K, Kaasinen S. Pain after thoracic surgery. Acta Anaesthesiol Scand. 1992;36:96–100. doi: 10.1111/j.1399-6576.1992.tb03430.x. [DOI] [PubMed] [Google Scholar]
- 10.Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5:89–96. doi: 10.1053/eujp.2001.0225. [DOI] [PubMed] [Google Scholar]
- 11.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29:800–5. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Vecht CJ, Hoff AM, Kansen PJ, de Boer MF, Bosch DA. Types and causes of pain in cancer of the head and neck. Cancer. 1992;70:178–84. doi: 10.1002/1097-0142(19920701)70:1<178::aid-cncr2820700128>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Daigeler A, Lehnhardt M, Khadra A, Hauser J, Steinstraesser L, Langer S, et al. Proximal major limb amputations – A retrospective analysis of 45 oncological cases. World J Surg Oncol. 2009;7:15. doi: 10.1186/1477-7819-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schley MT, Wilms P, Toepfner S, Schaller HP, Schmelz M, Konrad CJ, et al. Painful and nonpainful phantom and stump sensations in acute traumatic amputees. J Trauma. 2008;65:858–64. doi: 10.1097/TA.0b013e31812eed9e. [DOI] [PubMed] [Google Scholar]
- 16.Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81:1039–44. doi: 10.1053/apmr.2000.7583. [DOI] [PubMed] [Google Scholar]


