Abstract
Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy plays a considerable role as a treatment modality in surgical oncology in western countries. The advantage of this procedure is that the heated chemotherapeutic agent is circulated in the abdominal cavity and achieves high peritoneal concentration with limited systemic absorption. This procedure is complex not only to the surgical team, but also to the anaesthetist because apart from managing the usual physiologic changes associated with major surgery, one should also be prepared to manage the physiologic changes during the hyperthermic phase. Here, we present our experience with our first case.
Keywords: Acute renal failure, cytoreductive surgery, end tidal carbon dioxide, hyperthermic intraperitoneal chemotherapy, pseudomyxoma peritonei
INTRODUCTION
Pseudomyxoma peritonei is a disorder with low malignant potential, originates from the appendix and ovaries and spreads to the abdominal cavity causing mucinous ascites.[1] This condition is treated by cytoreduction and in recent times by the addition of hyperthermic intraperitoneal chemotherapy (HIPEC). This was first described by Spratt et al., in the year 1980 followed by Sugarbaker et al. who did extensive work in this field.[2,3]
CASE REPORT
A 52-year-old postmenopausal lady weighing 74 kg, diagnosed to have pseudomyxoma peritonei was planned for cytoreductive surgery (CRS) with HIPEC presented to the pre-anaesthesia clinic. She had gross abdominal distension [Figure 1] and bilateral pitting pedal oedema. Vital signs were within the normal range. Her haemoglobin (Hb) was 7.6 g% and blood group A negative. Echocardiography was normal. Pre-operatively, she was prepared with incentive spirometry and packed cell transfusion to bring her Hb to the optimal level. Our hospital has a policy of improving the Hb to at least 9 g% when major surgery with massive blood loss is anticipated. Prior to surgery anti-aspiration prophylaxis was administered and nasogastric tube was inserted. Intraoperative monitoring included non-invasive blood pressure, electrocardiogram, pulse oximetry, invasive arterial pressure, central venous pressure (CVP), capnography and temperature monitoring. Due to the grossly distended abdomen modified rapid sequence induction was conducted using injection propofol and injection scoline. Following intubation ventilation was difficult with airway pressure reaching 40-42 cm of H2O. We could achieve a tidal volume of only 150 ml, at this point the end tidal carbon dioxide (ETCO2) ranged from 50 to 56 mm of Hg and saturation fell to 76% with a fraction of inspired oxygen (FiO2) 100%. However, bilateral air entry was equal. The surgeons were requested to open the abdomen and do decompression. Over a period of time slowly ventilation improved. Anaesthesia was maintained with air, oxygen, sevoflurane, atracurium and morphine.
Figure 1.
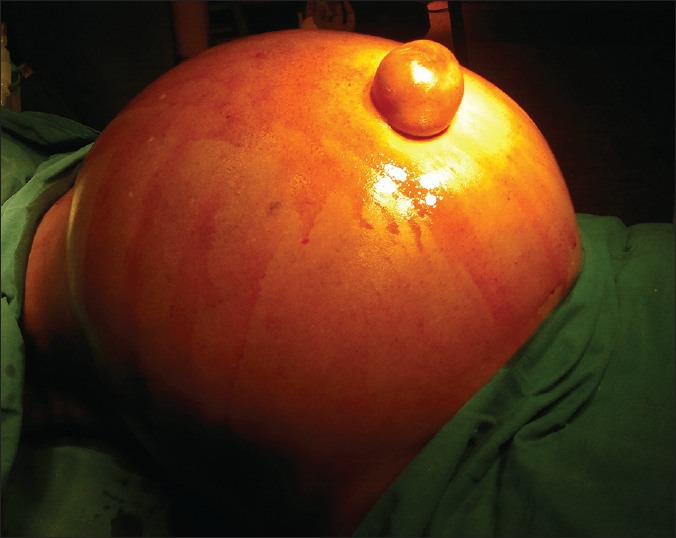
The distended abdomen before cytoreduction
After partial removal of mucinous material, airway pressure improved to less than 20 cm H2O and we were able to come down on the FiO2-40% and saturation came up to 100%.
Mucinous material weighing 21 kg was removed from the abdomen. Pan hysterectomy and anterior abdominal peritonectomy was done. After 10 h of surgery with blood loss of around 5500 ml as more than 50% of the procedure was still pending, decision was taken by the team to do the rest of the surgical procedure and HIPEC as a staged procedure. Patient received 8 units of packed cells along with blood products, adequate crystalloids and colloids. Intraoperative arterial blood gas (ABG) was normal with Hb 10 g%. Patient was shifted to post-operative intensive care unit (ICU) for elective ventilation on intravenous morphine analgesia. She was extubated the following day.
The second stage was planned after 2 weeks and meanwhile patient was given high protein parentral nutrition, deep vein thrombosis prophylaxis and was advised spirometry. On the day of surgery following an uneventful anaesthesia, total peritonectomy with complete omentectomy was done. HIPEC was initiated by a CLOSED technique [Figure 2], five drainage tubes (two inlet and three outlet tubes) were inserted into the abdomen and skin closed. Perfusate was peritoneal dialysis fluid along with chemotherapy agent mitomycin C at a temperature of 42°C. The solution was circulated for 70 min in the abdominal cavity by tilting the operation table in different directions and by shaking the patient to facilitate the circulation of the solution. During the HIPEC period, a raise in body temperature above 42 should be alerted to the perfusionist so temperature should be recorded at 15 min interval, similarly, urine output should also be measured at 15 min interval as most of the chemotherapeutic agents are nephrotoxic, blood sugar and lactate were monitored every 15 min.[4,5] ABG was checked at 30 min interval. Throughout, the procedure patient was haemodynamically stable with adequate urine output. The surgery lasted for 7 h with blood loss of 3500 ml. Six units of blood and other blood products along with adequate crystalloids and colloids were replaced. At the end of the procedure, cooling measures were adopted by switching our warming blanket (Geratherm Operation System) to the cooling mode at 34°C, we used cooled saline to replace the lost volume and icepacks wrapped in sterile sheets were placed in the groin and axilla by the surgeons. Our observation was that temperature gradually came down to normal within 30 min. Patient was shifted to ICU for elective ventilation, the next day she was extubated without complications.
Figure 2.
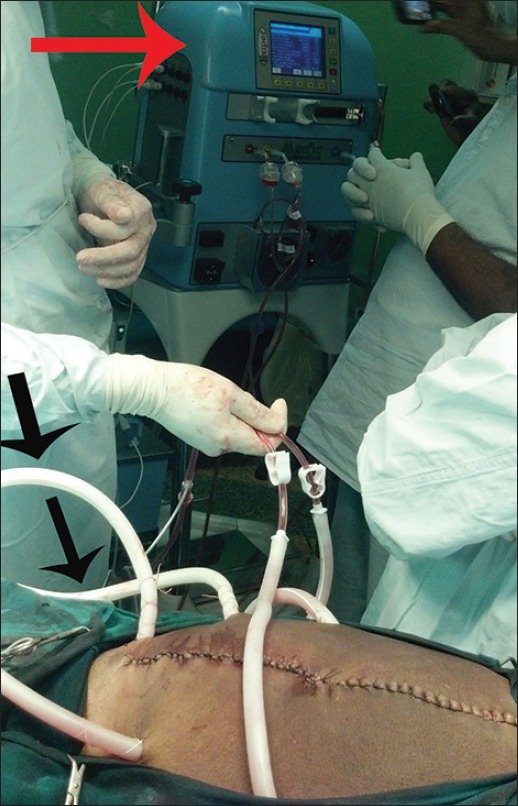
Hyperthermic intraperitoneal chemotherapy using closed technique. The figure shows HIPEC being initiated after cytoreduction. The two tubes the surgeon is holding are the inlet tubes and the other tubes marked with black arrows are the outlet tubes. The red arrow point to the HIPEC machine
DISCUSSION
Pseudomyxoma peritonei occur 2 in 10,000 laprotomies. The sites of origin are the ovaries and the appendix. A definite diagnosis is made when jelly like material is encountered. CRS with HIPEC aims for loco-regional disease control and long-term survival. The main anaesthetic goals of this surgical procedure are to maintain normovolemia, normothermia, correcting metabolic derangements and also a thorough knowledge about the adverse effects of chemotherapeutics used during HIPEC. Pre-operative evaluation of airway along with adequate preparation to manage the airway resistance problem should be made because they have an increased abdominal girth pushing the abdominal contents against the diaphragm and reducing the functional residual capacity leading to rapid desaturation.[6] Although routine use of H2 blocker is not recommended, these patients with increased abdominal girth are at a risk of aspiration of gastric contents after induction of anaesthesia,[7] so premedication with H2 blocker and a prokinetic is desirable in this subset of patients.[8] Major fluid shifts are expected requiring administration of large volume of intravenous fluids leading to dilution of platelets and coagulation factors;[9] hence, we avoided epidural analgesia in both stages, but in studies conducted by Schmidt et al., patients received epidural analgesia,[10] invasive arterial line was placed was placed for continuous monitoring and for blood gas analysis. Central line was established for venous access and for inotropes if needed. We did not use invasive monitors for cardiac output monitoring. Many centres where they do such cases more often have stopped monitoring CVP and pulmonary artery pressure because they do not reflect the volume status or volume responsiveness, such centres use minimally invasive pulse contour analysis and non-invasive bio-reactance devices.[4] In the cytoreductive stage, the goal was to replace the substantial fluid loss and maintain normothermia despite the propensity for hypothermia due to drainage of the mucus and large area of exposure for a long period of time. The temperature was maintained by using warming blankets and infusing warm fluids. Crystalloids at the rate of 10-15 ml/kg/h were infused[4] and urine output was used as a guide to administer fluids. After cytoreduction before initiation of HIPEC, we ensured that fluid warmers and warming blankets were turned off. HIPEC was performed using a closed technique, the advantage being that heated chemotherapy achieves high peritoneal concentration and increased tissue penetration because of increased intra-abdominal pressure with limited systemic absorption. HIPEC produces hyperdynamic, vasodilated state leading to increase in heart rate and increase in intra-abdominal pressure, especially in the closed technique with reduction in the cardiac output.[11] Most of the chemotherapeutic agents used are nephrotoxic, so it is advisable to have a steady urine output. Output was checked every 15 min during HIPEC. Our patient had an output of 50-70 ml every 15 min.[4] Volume replacement was the goal so that systemic haemodynamic complication such as acute renal failure can be avoided during the hyperthermic phase.[12] Studies carried out by Shime et al., Esquivel et al. showed that during HIPEC, there is an increase in metabolic rate, oxygen consumption, cardiac output, heart rate, ETCO2 with metabolic acidosis and elevated arterial lactate levels.[5,10] In our patient, there was an increase in heart rate from 100 to 120 beats/min,increase in blood lactate from 0.89 to 2.9 mmol/L and a temperature increase from 34.6°C to 37.4°C. It was noticed that there were also changes in the ETCO2 and CVP [Figures 3 and 4] possibly due to the increased intra-abdominal pressure. There was no compromise in the urine output. The change in vitals was only transient as after cessation of HIPEC, the body temperature started dropping to normal levels. It was also noticed that metabolic acidosis and an increase in arterial lactate levels were only transient In view of the extensive and long duration of the surgery; we electively ventilated the patient overnight.
Figure 3.
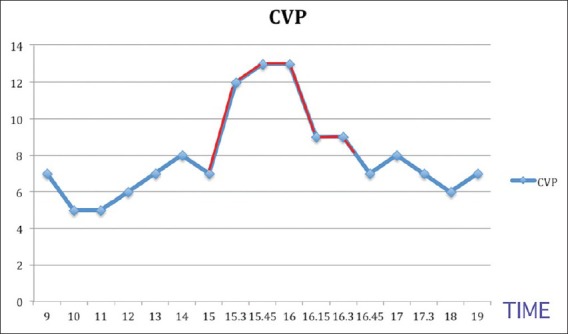
Graph depicting the central venous pressure changes during the procedure
Figure 4.
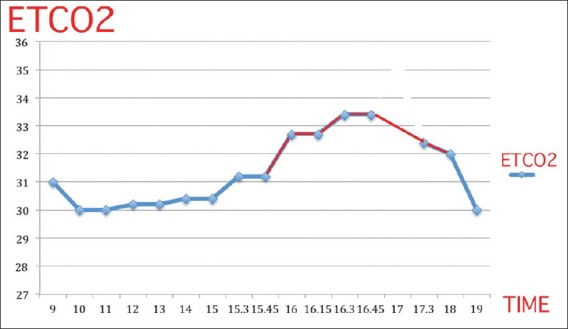
Depicting changes in end tidal carbon dioxide during the procedure
CONCLUSION
Cytoreduction with HIPEC is becoming an important adjunct in the therapy of peritoneal surface malignancies. These cases are challenging to the anaesthetist because there is massive blood loss, fluid shifts, acid base disturbances and hypothermia during the resection with hyperthermia related changes during HIPEC. Adequate preparation, pre-operative optimisation, closes monitoring and timely intervention by the anaesthesiologist is crucial for effective management of these patients.
ACKNOWLEDGMENT
The authors wish to acknowledge the encouragement and support of our Adviser Prof. Dr. Hemanth Raj.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P. Systematic review of the Sugarbaker procedure for pseudomyxoma peritonei. Br J Surg. 2005;92:153–8. doi: 10.1002/bjs.4862. [DOI] [PubMed] [Google Scholar]
- 2.Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–60. [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE, Hull WE, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: Pharmacological studies. Cancer Res. 1990;50:5790–4. [PubMed] [Google Scholar]
- 4.Rothfield KP, Crowley K. Anesthesia considerations during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Surg Oncol Clin N Am. 2012;21:533–41. doi: 10.1016/j.soc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Shime N, Lee M, Hatanaka T. Cardiovascular changes during continuous hyperthermic peritoneal perfusion. Anesth Analg. 1994;78:938–42. doi: 10.1213/00000539-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Morgan GE, Mikhail MS. A Lange Medical Book. 2nd ed. Ch 22. Stamford: Appleton and Lange, A Simon and Schuster Company; 1992. Clinical Anaesthesiology; p. 415. [Google Scholar]
- 7.Engelhardt T, Webster NR. Pulmonary aspiration of gastric contents in anaesthesia. Br J Anaesth. 1999;83:453–60. doi: 10.1093/bja/83.3.453. [DOI] [PubMed] [Google Scholar]
- 8.Clark K, Lam LT, Gibson S, Currow D. The effect of ranitidine versus proton pump inhibitors on gastric secretions: A meta-analysis of randomised control trials. Anaesthesia. 2009;64:652–7. doi: 10.1111/j.1365-2044.2008.05861.x. [DOI] [PubMed] [Google Scholar]
- 9.Aitkenhead AK, Rowbotham DJ, Smith G. 4th ed. Ch 23. Edinburgh: Churchill Livingstone, Harcourt Publishers Limited; 2001. Textbook of Anaesthesia; p. 268. [Google Scholar]
- 10.Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia. 2008;63:389–95. doi: 10.1111/j.1365-2044.2007.05380.x. [DOI] [PubMed] [Google Scholar]
- 11.Esquivel J, Angulo F, Bland RK, Stephens AD, Sugarbaker PH. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open “coliseum technique”. Ann Surg Oncol. 2000;7:296–300. doi: 10.1007/s10434-000-0296-2. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg. 2005;241:219–26. doi: 10.1097/01.sla.0000151791.93571.70. [DOI] [PMC free article] [PubMed] [Google Scholar]


