Abstract
Irritable bowel syndrome (IBS) is one of the most common conditions encountered in clinical practice but one of the least well understood. Symptoms of this disorder are chronic, sometimes severe and often respond poorly to treatment, resulting in reduced quality of life. There is no specific test for IBS, although diagnostic tests may be performed to rule out other problems. In present clinical trial 51 patients of IBS were registered out of which 46 patients completed the treatment. Bilvadileha was administered for the duration of 12 weeks. The therapy showed statistically significant improvement in all the clinical features of IBS as well as in the IBS severity score.
Keywords: Ayurvedic management, Bilvadileha, irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a vague term for a variety of diseases causing discomfort in the gastro-intestinal tract which causes great morbidity in the population. IBS is also called by many names, among them colitis, mucous colitis, spastic colon, or spastic bowel are few. It is a functional bowel disorder characterized by chronic abdominal pain, discomfort, bloating and alteration of bowel habits in the absence of any organic cause.[1] Certain psychological conditions are also more common in those with IBS. Diarrhea or constipation may predominate, or they may alternate (classified as IBS-D, IBS-C or IBS-A, respectively). IBS may begin after an infection (post-infectious, IBS-PI), a stressful life event, or onset of maturity without any other medical indicators.[2] IBS affects 15-20% of Indian population.[3] It occurs more often in women than in men and it begins before the age of 35 in about 50% of people.[4] Psychological factors like stress, anxiety etc., plays an important role in the manifestation of IBS.[5]
IBS is relatively a recent concept and as such is a new entry into the realm of clinical medicine itself. As there is no single disorder in Ayurveda which can be exactly co-relate with IBS because Ayurveda is based entirely on different basic principles. Some conditions in particular, quite practically similar with IBS in their clinical picture. These are Atisara,[6] Pravahika, Grahani[7] and Pakvashayagata Vat.[8] In Ayurvedic system of medicine, the concept of psychosomatic disorders has been widely discussed.[9] According to Charaka, Sharira and Manasika Doshas are interdependent on each other.[10] One follows other, so the diseases, which are derived from them, also cannot exist without one another. Hence while understanding the ”Samprapti” (pathogenesis) of IBS, one has to give importance to Annavaha, Purishava, Rasavaha and Manovaha Srotodushti.
In the management of IBS many formulations have been mentioned in modern medicine. Modern therapeutic molecules may provide instant relief in these cases, but are tend to develop a number of adverse drug reactions and no permanent cure is visible. Knowing this, the current suffering population is looking toward new remedies from other systems of medicines, which can provide relief without manifesting any inconveniency. Bilvadileha,[11] a promising herbal drug compound that is being successfully prescribed by Ayurvedic physicians without any side effect since centuries is evaluated for its clinical efficacy in the condition of IBS in the present study.
Aims and objective
The objective is to evaluate the efficacy of Bilvadileha in IBS.
Materials and Methods
Patients attending OPD of Department of Kayachikitsa and Rog Nidana and Vikruti Vijnana, fulfilling the criteria for inclusion were selected irrespective of sex, race, caste and religion, between the age group of 18-65 years.
Bilvadileha
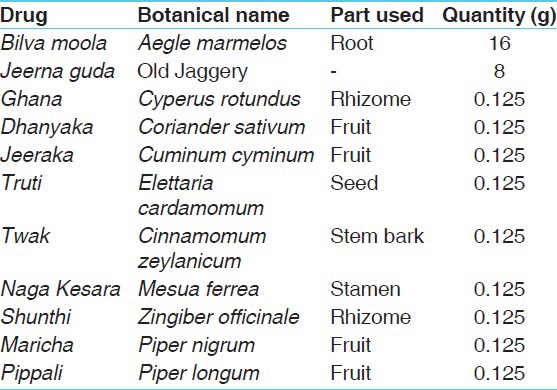
Bilvadileha was prepared by Arya Vaidya Sala, Kottakkal - 676 503, Kerala, India. The ingredients and the parts used are given in Table 1.
Table 1.
Each 10 g of Bilvadileha contains
Pattern of study
The study was an open label, single group and non-controlled clinical trial.
Timelines
Washout/preparatory period (if required): 4 weeks
Treatment period: 12 weeks
CTRI registration
This clinical trial has been registered under CTRI (Ref-CTRI/2012/01/002348).
Ethical clearance
The study was approved by Institutional Ethics Committee (Ref PGT/7-A/Ethics/210-11/1858).
Eligibility criteria
Patients of either sex with age between 18 and 65 years
-
Known case of IBS as per Rome III criteria:[12]
(Symptoms of recurrent abdominal pain or discomfort and a marked change in bowel habit for at least 6 months, with symptoms experienced on at least 3 days/month in the last 3 months associated with two or more of the following:
- Pain is relieved by defecation
- Onset associated with change of frequency of stools
- Onset associated with a change in form (appearance) of stools.
Willing and able to participate in the study.
Exclusion criteria
Patients with bleeding per rectum.
Mixed infection with parasites such as round worms, hook worms etc.
Patients with evidence of malignancy.
Patients with diabetes mellitus (B.S. [F] >126 mg% and/or B.S. [2 h. PP] >200 mg% or hemoglobin A1c (HbA1c > 6.5%).
Patient with poorly controlled hypertension (>160/100 mm Hg).
Patients on prolonged (>6 weeks) medication with corticosteroids, anti-depressants, anti-cholinergics, etc., or any other drugs that may have an influence on the outcome of the study.
Patients suffering from major systemic illness necessitating long term drug treatment Rheumatoid arthritis, tuberculosis, psycho-neuro-endocrinal disorders, etc.
Patients who have a past history of atrial fibrillation, acute coronary syndrome, myocardial infarction, stroke or severe arrhythmia in the last 6 months.
Symptomatic patient with clinical evidence of heart failure.
Patients with concurrent serious hepatic disorder (defined as aspartate amino transferase (AST) and/or alanine amino transferase (ALT), total bilirubin, alkaline phosphatase (ALP) >2 times upper normal limit) or renal disorders (defined as serum creatinine >1.2 mg/dL), severe pulmonary dysfunction (uncontrolled bronchial asthma and/or chronic obstructive pulmonary disease), or any other condition that may jeopardize the study.
Alcoholics and/or drug abusers.
History of hypersensitivity to the trial drug or any of its ingredients.
Pregnant/lactating woman.
Patients who have completed participation in any other clinical trial during the past 6 months.
Any other condition which the principal investigator thinks may jeopardize the study.
Investigation
Routine investigations were done, before (to rule out any other pathology) and after treatment (to assess any untoward effect of trial drug during the regimen).
Hematology
Hemoglobin, total leucocyte count, differential leucocyte count (neutrophil, eosinophil, basophil, lymphocytes, monocytes), erythrocyte sedimentation rate.
Bio-chemistry
Blood sugar (fasting, PP), HbA1c, blood urea, serum uric acid, mg/dl, serum creatinine, serum glutamic oxaloacetic transaminase (AST), serum glutamic-pyruvic transaminase. (ALT), total protein, serum albumin, serum globulin, A/G ratio, serum bilirubin, conjugated bilirubin, unconjugated bilirubin, serum ALP.
Stool examination (Microscopic)
Parasites (ova/cyst), mucous, vegetative cells, occult blood.
This disease being a functional disorder, rest of the biochemical and stool examination were conducted to rule out the exclusion criteria and after treatment the safety profile of the drug.
Interventions
Bilvadileha has been administered to the patients at the dose of 10 g twice a day orally after food with luke warm water for the duration of 12 weeks.
Methods of assessment
The assessment of IBS was done at the interval of 14 days on the basis of relief in chief complaints of IBS, disease specific Ayurvedic parameters and IBS severity score.[13]
Response at 12 weeks
Positive response to treatment-decrease in IBS severity score by >50%.
Partial response to treatment-decrease in IBS severity score by 30-50%.
No response to treatment-decrease in IBS severity score by <30%.
Observations and Results
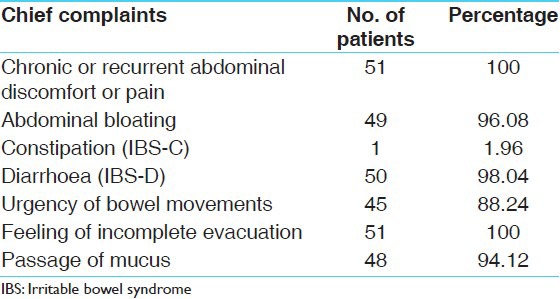
A total of 51 patients were registered. Out of which 5 were drop out (Out of 5 patients drop out, 4 got transferred at another station. Cause of drop out for one patient was unknown and 46 completed the treatment [Table 2]. All the patients were complaining about chronic and recurrent abdominal pain (100%). Abdominal bloating was found in 96.08%; IBS-C in 1.96%; IBS-D in 98.04%; Passage of mucus in 94.12%; Urgency of bowel movements in 88.24% patients [Table 3].
Table 2.
Total number of subjects registered for therapy

Table 3.
Chief complaint's wise distribution of 51 patients
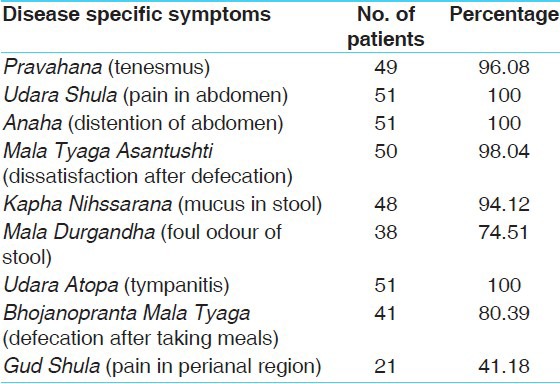
In the present study, Udara Shula (Pain in abdomen), Anaha (Distention of the abdomen) and Udara Atopa (Tympanitis) was found in 100% patients whereas Pravahana (Tenesmus) in 96.08% patients, Mala Tyaga Asantushti (dissatisfaction after defecation) in 98.04% patients, Mala Durgandha (Foul odor of stool) in 74.51% patients, Bhojanopranta Mala Tyaga (Defecation after taking meals) in 80.39% patients and Guda Shula (Pain in the perianal region) was found in 41.18% patients [Table 4].
Table 4.
Disease specific symptoms wise distribution of 51 patients
After completion of therapy, chronic and recurrent abdominal pain improved by 67.09%, Abdominal bloating by 100%; IBS-D by 86.96%, passage of mucus by 100%; Urgency of bowel movements by 97.50%; Feeling of incomplete evacuation by 89.13% [Table 5]. All these improvements were statistically highly significant (P < 0.001). Bivadi leha has shown 95.65% improvement in Pravahana. Udara Shula was improved by 67.39%; Anaha (Distention of the abdomen) by 93.48%; Mala tyaga Santushti (Satisfaction after defecation) by 89.13%; Kapha Nihssarana (Mucus in stool) by 100%; Udara Atopa by 97.83%; Mala Durgandha (Foul odour of stool) by 100%; Bhojanopranta Mala Tyaga by 100%; Guda Shula by 67.39% [Table 6]. All these improvements were also statistically highly significant (P < 0.001). Effect of therapy on IBS severity score showed a relief of 64.59% that was statistically highly significant (P < 0.001) [Table 7]. In stool, no any significant pathological changes were seen in before and after treatment stool examination.
Table 5.
Effect of therapy on chief complaints
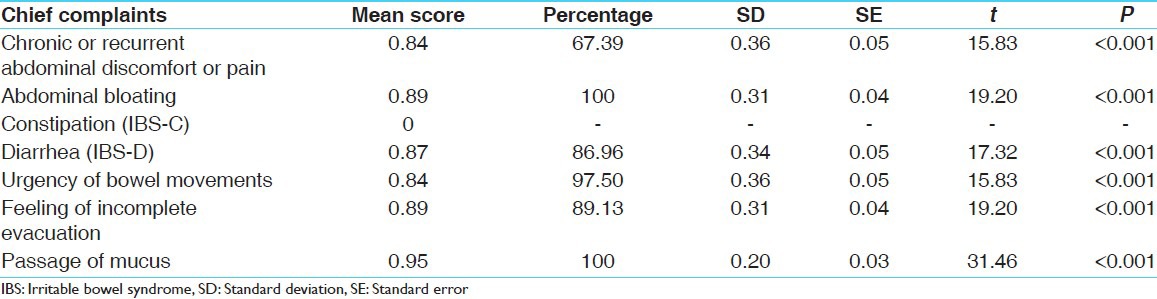
Table 6.
Effect of therapy on disease specific symptoms
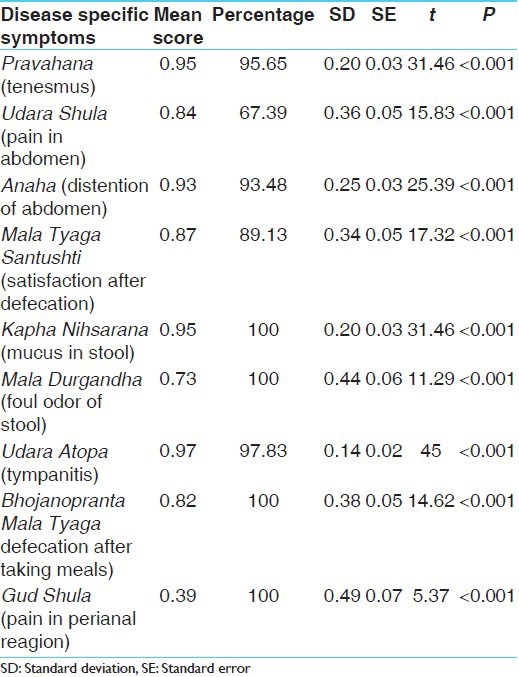
Table 7.
Effect of therapy on IBS severity score

Out of 46 patients, after the completion of treatment with Bilvadileha, positive response to treatment was found in 37 (80.43%) patients, 7 (15.21%) patients showed Partial response to treatment and 2 (4.34%) patients reported no response to the treatment [Table 8].
Table 8.
Overall effect of therapy

Discussion
All the cardinal symptoms and associated symptoms along with IBS severity score improved in a statistically highly significant manner (P < 0.001). Bilva roots are the main ingredient of the formulation Bilvadileha. The roots are sweet, astringent, bitter and febrifuge; useful in diarrhea, dysentery, dyspepsia, gastralgia, palpitation, seminal weakness, uropathy, vomiting, intermittent fever, swellings and gastric irritability in infants.[14]
Vitiation of Agni (Mandagni) is the main reason for IBS. This ultimately results in Ama formation and may lead to diarrhea or constipation. Bilva, due to its Kashaya, Tikta Rasa, Katu Vipaka and Laghu Guna acts as Agni Deepana and also Amapachaka. Kashaya Rasa and Ushna Virya help in reducing the colonic motility. Sangrahi property of Bilva is very useful to treat the increased frequency of defecation and the consistency of stool. In Bilvadileha in addition to Bilva, Prakshepa Dravyas like Dhanyak, Jirak, Ela, Keshar, Twak, Trikatu, Musta, have properties like: Deepana, Pachak, Kaphahara, Vedanasthapak, Rasayana.
Thus, due to different properties of its ingredients, Bilvadileha has properties like: Tridoshahara, Deepana, Pachana, Amanashaka, Grahi, Vibandhahara and Vatanulomana which checks the Samprapti (pathogenesis) and pacify the symptoms of IBS. Pharmacological activities such as antidiarrheal,[15] antidysentry, antibacterial, antiprotozoal, antispasmodic, antidepressant, antifungal, antigiardiasis, anthelmintic, antispasmodic, anti-inflammatory,[16] carminatives etc., check the IBS-PI. Having Rasayana effect, relieve the psychological factors such as anxiety fear etc., it may enhance the Bala of Deha and Indriya.
Conclusion
On the basis of this study, it can be concluded that the trial drug Bilvadileha is found to be effective in reliving symptoms of IBS. There was no adverse drug reaction seen during the period of trial. Further studies can be should be carried out with larger sample size in different places with a standard control drug in order to obtain more data the effect of this novel drug compound in management IBS.
References
- 1.Dorland WA. 28th ed. Philadelphia: Elsevier publication; 1997. Dorland's illustrated medical dictionary. [Google Scholar]
- 2. [Last retrieved on 2010 Jun 26]. Available from: http://www.en.wikipedia.org/wiki/Irritable_bowel_syndrome .
- 3.Kapur OP, Shah S. Irritable bowel syndrome in Indian patients. [Last retrieved on 2010 Jun 26]. Available from: http://www.bhj.org/journal/special_issue_tb/DPII_13.htm .
- 4. [Last retrieved on 2010 Jun 26]. Available from: http://www.digestive.niddk.nih.gov/ddiseases/pubs/ibs .
- 5.Masand PS, Keuthen NJ, Gupta S, Virk S, Yu-Siao B, Kaplan D. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11:21–5. doi: 10.1017/s1092852900024123. [DOI] [PubMed] [Google Scholar]
- 6.Agnivesha, Charaka, Dridhabala . Charaka Samhita, Chikitsa Sthana, Atisara Chikitsa Adhyaya, 19. In: Yadavji Trikamji Acharya., editor. reprint edition. Varanasi: Chaukhamba Surbharti Publication; 2001. p. 547. [Google Scholar]
- 7.Ibidem. Charaka Samhita, Chikitsa Sthana Grahnichikitsa Adhyaya 15. :511. [Google Scholar]
- 8.Ibidem. Charaka Samhita, Chikitsa Sthana Grahnichikitsa Adhyaya 15. :511. [Google Scholar]
- 9.Ibidem. Charaka Samhita, Vimana Sthana Trividhakukshiya Adhyaya 2/3. :252. [Google Scholar]
- 10.Ibidem. Charaka Samhita, Sutra Sthana, Ashtodariya Adhyaya 19/7. :112. [Google Scholar]
- 11.Anonymous. 1st ed. Part - II. Vol. 1. New Delhi: Dept. of Ayurveda, Yoga and Naturopathy, Unani, Siddha, and Homeopathy, Min. of Health and Family Welfare, Government of India; 2007. The Ayurvedic Pharmacopoeia of India; pp. 7–9. [Google Scholar]
- 12.Lin C. From Rome to Los Angeles-The Rome III criteria for the functional GI disorders, Medscape gastroenterology. [Last retrieved 2010 Jun 26]. Available from: http://www.cme.Medscape.com/gastroenterology .
- 13.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharma PC, Yelne MB, Dennis TJ, Joshi A. Vol. 1. New Delhi: Central Council for Research in Ayurveda and Siddha, Dept of ISM and H, Min. of Health and Family Welfare, Government of India; 2001. Database on Medicinal Plants Used in Ayurveda; p. 79. [Google Scholar]
- 15.Mazumder R, Bhattacharya S, Mazumder A, Pattnaik AK, Tiwary PM, Chaudhary S. Antidiarrhoeal evaluation of Aegle Marmelos (Correa) Linn. root extract. Phytother Res. 2006;20:82–4. doi: 10.1002/ptr.1804. [DOI] [PubMed] [Google Scholar]
- 16.Benni JM, Jayanthi MK, Suresha RN. Evaluation of the anti-inflammatory activity of Aegle marmelos (Bilwa) root. Indian J Pharmacol. 2011;43:393–7. doi: 10.4103/0253-7613.83108. [DOI] [PMC free article] [PubMed] [Google Scholar]


