Abstract
Context:
GLP-1 receptor agonists (GLP-1 RA) are unique antidiabetic agents that have the ability to lower blood glucose without causing hypoglycemia, while at the same time promoting weight loss. Information on the efficacy and safety of GLP-1 RA in the Indian diabetic population is limited.
Aims:
(1) To evaluate the effect of GLP-1 RA, Liraglutide on glycemic control, and weight in obese Indian patients with type 2 diabetes. (2) To study the adverse event profile of Liraglutide in these patients in real-world clinical setting.
Settings and Design:
Observational study conducted in a tertiary care hospital.
Materials and Methods:
Liraglutide was prescribed to 196 obese patients with type 2 diabetes who had poor glycemic control on oral medications ± insulin. The initial dose of Liraglutide was 0.6 mg, which was up-titrated to 1.2 mg after 1 week; further up-titration to 1.8 mg was done based on tolerance. Dipeptidyl peptidase-IV (DPP-IV) inhibitors were discontinued and dose of other medications adjusted according to clinical judgment during the study period.
Results:
Mean age of patients was 49.9 ± 9.6 years. Three month data were available for 175 patients out of a total of 196. At 3 months, glycosylated hemoglobin (HbA1c) was 7.6 ± 0.9% vs. 9.2 ± 1.9% at baseline (P = 0.007) and mean body weight was 96.0 ± 16.5 kg vs. 100.1 ± 17.5 kg at baseline (P < 0.001). Most common adverse events were nausea, burping, and eructation (10%).
Conclusion:
Liraglutide significantly improves glycemic control with low risk of hypoglycemia and is associated with significant weight loss in obese Indian patients with type 2 diabetes mellitus.
Keywords: GLP-1 receptor agonists, India, obesity, type 2 diabetes
INTRODUCTION
India, a country experiencing rapid socioeconomic progress and urbanization, carries a considerable share of the global diabetes burden. Recently, results of phase 1 of Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study estimated that 62.4 million people live with diabetes in India.[1] In spite of numerous interventions and medications existing to treat diabetes, only approximately 50% of patients with diabetes could reach the target HbA1c level.[2,3] Moreover, weight gain and hypoglycemia are two most common adverse effects of currently available antidiabetic medications, which limit the ability to achieve good glycemic control without adverse effects. Recent advances in diabetes research indicate a major role for the incretin hormone in the pathogenesis of type 2 diabetes. Glucagon like peptide-1 (GLP-1) is one such incretin hormone, which contributes to the maintenance of circulating glucose levels by various mechanisms, such as glucose-dependent stimulation of endogenous insulin secretion, inhibition of glucagon secretion, increase in satiety, reduction of food intake, delay in gastric emptying, and possible stimulation of islet growth, differentiation, and regeneration.[4] Liraglutide is a promising new drug, which is a modified form of human GLP-1 with 97% homology. The addition of the C16 acyl chain allows for noncovalent binding to albumin, contributing to a prolonged half-life and duration of action.[5] The efficacy and safety of Liraglutide was best evaluated in LEAD (Liraglutide Effect and Action in Diabetes) trials. A higher percentage of patients in the Liraglutide-treated groups reached a target of HbA1c <7.0% in all of the LEAD studies as compared with active comparators. Significant weight reduction, systolic blood pressure reduction, and improved beta cell function were other beneficial outcomes with Liraglutide use in the LEAD trials.[6] Throughout the trials, gastrointestinal adverse events were most frequently reported with Liraglutide monotherapy and combination therapy and were often dose-related. Liraglutide received FDA approval for use in the US in January 2010 in adults with type 2 diabetes. According to American Diabetes Association (ADA) consensus algorithm, Liraglutide and other GLP-1 RA, are recommended early in the course of type 2 diabetes after metformin.[7]
Phase 3 trials on efficacy of Liraglutide in management of type 2 diabetes were largely conducted in populations of European descent, although few other countries have published their data recently on efficacy and safety of Liraglutide in type 2 diabetes.[8,9,10] Information on the use of Liraglutide in the management of type 2 diabetes in India is limited and has not been published yet. In this article, we have presented observational data on effectiveness and tolerability of Liraglutide in Indian patients with type 2 diabetes in a real-life clinical practice setting.
MATERIALS AND METHODS
Liraglutide (Victoza®; Novo Nordisk Inc., Bagsvaerd, Denmark) was prescribed to patients with type 2 diabetes attending endocrinology OPD of a tertiary care center, who were obese (BMI >25 kg/m2) and had suboptimal glycemic control [HbA1c >7% (53 mmol/mol)] on oral medications ± insulin. Patients with deranged renal function tests, liver function tests, and history of thyroid malignancy were excluded. Informed written consent was obtained from all the patients. Ethical clearance was obtained from institutional review board.
In this 3 months observational data, initial dose of Liraglutide was 0.6 mg once daily, which was up-titrated to 1.2 mg once daily after 1 week; further up-titration to 1.8 mg once daily was done based on tolerance and adverse event profile. DPP-IV inhibitors were discontinued and dose of other medications adjusted according to clinical judgment on initiation of Liraglutide. Following parameters were recorded both at baseline and at each visit (every 4 weeks from baseline); self-monitoring of blood glucose (SMBG) record, fasting plasma glucose (FPG), postprandial plasma glucose (PPG), body weight, and body mass index (BMI). HbA1c was determined at baseline and at subsequent 3 month interval. Fasting lipid profile was measured in all patients. Adverse events including patient-reported hypoglycemic episodes (plasma glucose <70 mg/dl) were noted at each visit. Minor hypoglycemic episodes were defined as those that could be self-treated; major episodes were defined as requiring third-party assistance or medical intervention. Nausea was patient reported. HbA1c was analyzed by high performance liquid chromatography (HPLC) method using Bio-Rad D10 analyzer.
Statistical methods
Data are presented as mean ± SD. Data analysis was done using SPSS software (SAS 9.1.3). Comparison between mean pre-treatment and post-treatment value of various parameters was done using paired t-test. The significance level was set at P ≤ 0.05.
RESULTS
Liraglutide was prescribed to 196 patients. Mean age of patients was 49.9 ± 9.6 years and the mean duration of diabetes was 7.56 ± 4.5 years. Baseline parameters of patients are given in Table 1. At baseline, mean HbA1c was 9.2 ± 1.9% and mean body weight was 100.1 ± 17.5 kg. Also, 127 patients were hypertensive and 106 patients had dyslipidemia. Baseline treatment modalities were metformin alone in 35 (18%); sulfonylurea + metformin in 57 (29%); sulfonylurea + metformin + DPP-IV inhibitors/exenatide in 53 (27%); sulfonylurea + metformin + basal insulin ± DPP-IV inhibitors in 36 (18.4%); sulfonylurea + metformin + premix insulin in 8 (4%); and sulfonylurea + metformin + basal-bolus insulin regimen in 7 (3.6%). Twenty-one patients discontinued Liraglutide before completion of 3 months for a variety of reasons. The reasons for discontinuation were adverse events (n = 7), financial constraints (n = 2), self-discontinued due to lack of follow-up (n = 5), pain at injection site (n = 3), nephropathy (n = 3), and bariatric surgery (n = 3).
Table 1.
Baseline parameters
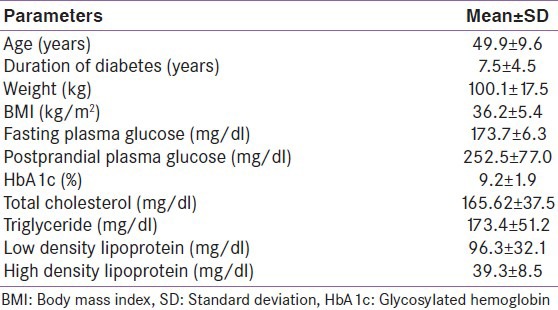
Therefore, 3 months data were available for 175 patients. Significant decline in mean HbA1c, FPG, PPG, and weight was seen at 3 months [Table 2]. Patients were divided into 6 groups according to the treatment modalities at the end of 3 months; only Liraglutide (group 1, n = 1), Liraglutide + metformin (group 2, n = 30), Liraglutide + metformin + sulfonylurea (group 3, n = 97), Liraglutide + metformin + basal insulin ± sulfonylurea (group 4, n = 35), Liraglutide + metformin + sulfonylurea + premixed insulin (group 5, n = 10), Liraglutide + metformin + sulfonylurea + basal-bolus insulin combination (group 6, n = 2). HbA1c reduction, PPG reduction and weight reduction was seen in all groups; however, it was not statistically significant in sulfonylurea + metformin + premix insulin group [Table 3 and Figures 1-3]. FPG reduction was statistically significant in all the groups [Figure 4].
Table 2.
Various parameters at 3 months (mean±SD)
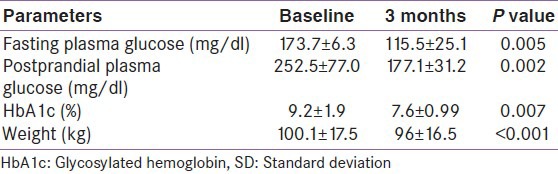
Table 3.
Change in various parameters from baseline in different groups
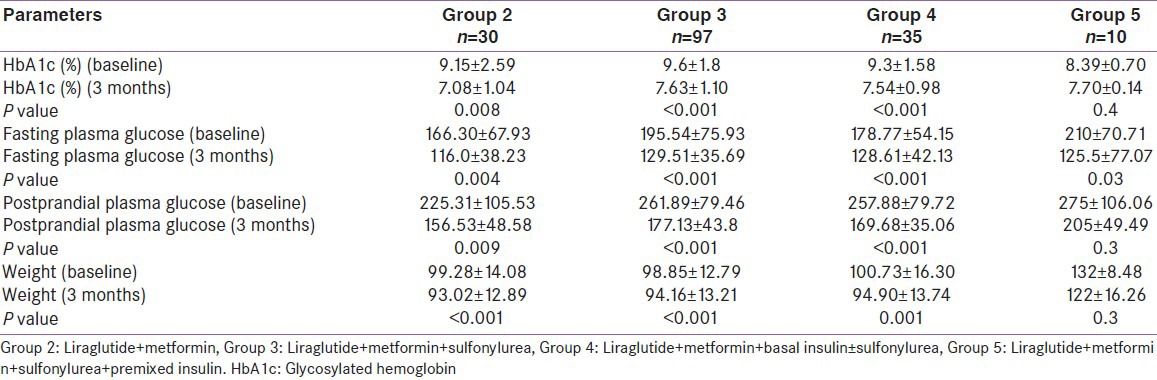
Figure 1.
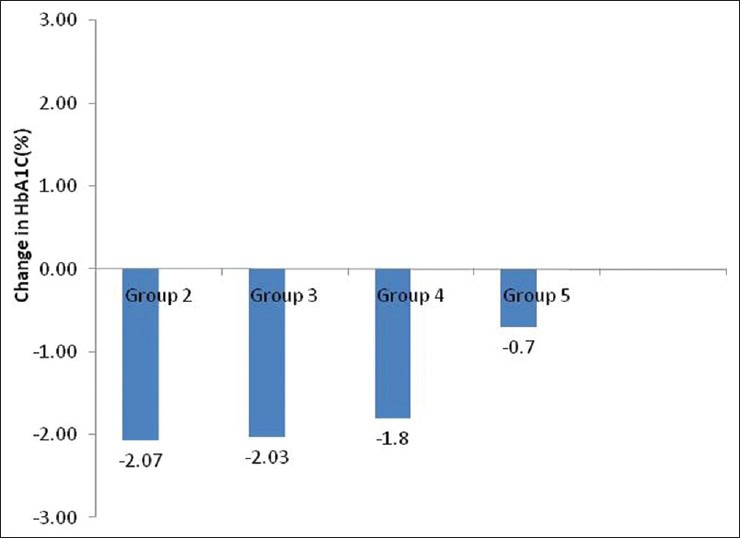
Change in mean glycosylated hemoglobin level from baseline in different groups
Figure 3.
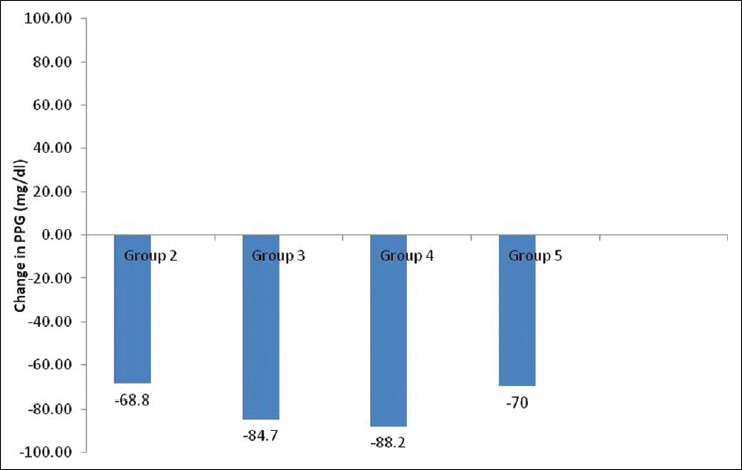
Change in mean postprandial plasma glucose (PPG) level from baseline in different groups
Figure 4.
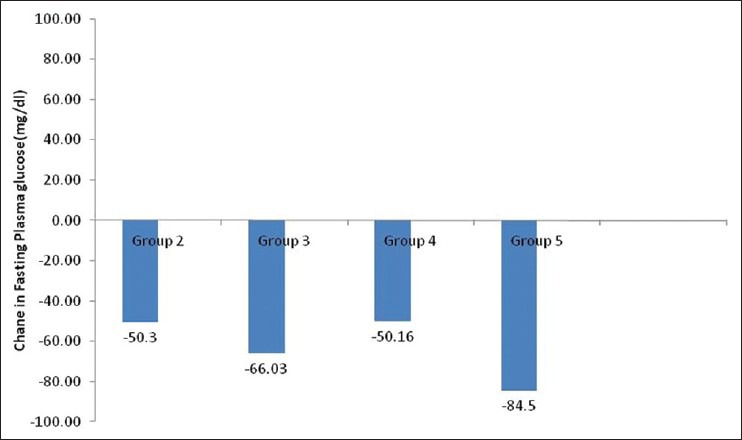
Change in mean fasting plasma glucose level from baseline in different groups
Figure 2.
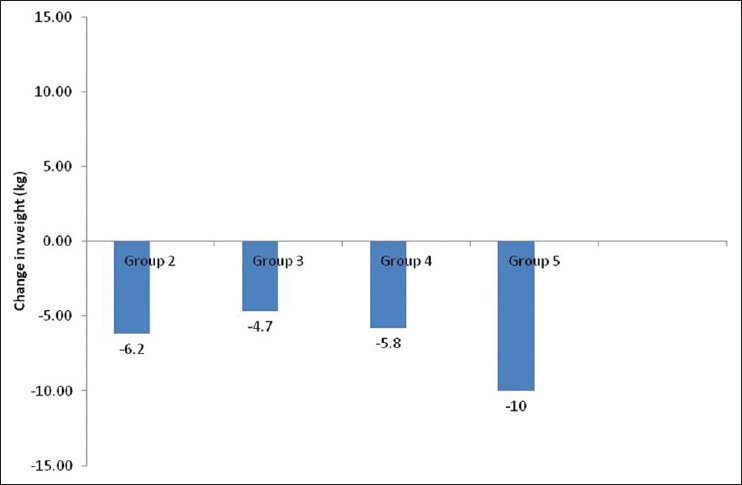
Change in mean weight from baseline in different groups
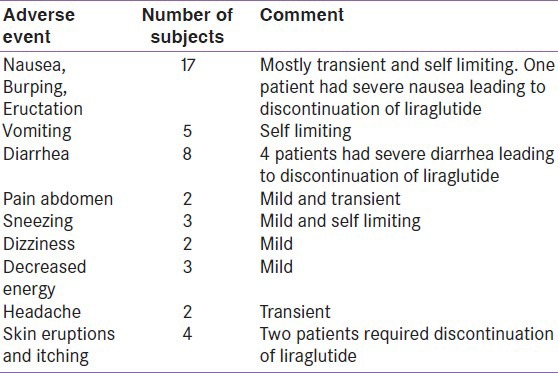
Adverse events are listed in Table 4. None of the patients had major hypoglycemia. Maximal daily dose of Liraglutide, i.e., 1.8 mg was reached in 15% patients, and, in the rest, the dose was maintained at 1.2 mg daily.
Table 4.
Adverse events
DISCUSSION
Type 2 diabetes is the most common disorder to present in our endocrinology OPD services with annual visits crossing over 25,000. We intend to investigate the effect of promising drug, Liraglutide, on glycemic control and weight in obese Indian patients with type 2 diabetes. We present observational follow-up data on Liraglutide use in 196 obese patients with type 2 diabetes who had suboptimal glycemic control on oral medications ± insulin. At 3 months, HbA1c showed a significant decline of 1.55% from baseline. Similarly, there was significant decline of 58.2 mg/dl in FPG level and decline of 75.4 mg/dl in PPG level at 3-months follow-up. HbA1c reduction was seen in all groups, but it was not statistically significant in sulfonylureas + metformin + premix insulin group. Our glycemic control results are in accordance with the efficacy of Liraglutide reported in phase 3 LEAD trials, where Liraglutide was shown to significantly reduce HbA1c. As the primary endpoint in the LEAD trials, HbA1c reductions ranged from 0.84% to 1.50% with Liraglutide 1.2 and 1.8 mg.[11]
The proposed advantages of Liraglutide, i.e. weight loss and minimal hypoglycemia also fared well in our patients. Most of the patients experienced weight loss after starting Liraglutide. Weight loss was noted as early as 4 weeks, this trend further continued and maximum weight loss was seen at 3 months. No change in weight was seen in 3 patients. Mean body weight loss of approximately 4 kg was noted at 3-months follow-up. All of the LEAD studies, except LEAD-1,[12,13,14,15,16,17] showed weight loss of up to a maximum of 3.2 kg with Liraglutide use, and maximum weight loss was seen in the first 16 weeks. In our experience, weight loss was one of the most important factors besides good glycemic control, which kept the patients motivated to continue Liraglutide use.
Out of 175 patients, 44 (25%) experienced adverse drug reactions (ADR). Most common adverse events were nausea, burping, and eructation (10%), followed by diarrhea (4.7%) and skin reactions (2.3%). However, nausea was transient, decreasing after 4 weeks of Liraglutide therapy. Nausea was managed with proton pump inhibitors and prokinetics with mixed response. Diarrhea was managed with antidiarrheal agent-loperamide. However, Liraglutide had to be discontinued in 7 patients due to ADR not responding to symptomatic treatment (1 had intractable nausea and vomiting, 4 had severe diarrhea, and 2 developed skin eruptions and itching). Five patients (3%) had mild hypoglycemia. These patients were those on metformin, sulfonylurea, and/or insulin + Liraglutide combination and did not require hospitalization. Hypoglycemia improved after modification of oral antidiabetic agents. The Liraglutide clinical trials have shown that Liraglutide is generally well tolerated. Nausea was the most frequently reported adverse event in the LEAD trials (7-40%).[12,13,14,15,16] Other adverse events noted in these trials were diarrhea, vomiting, dyspepsia, and constipation. The gastrointestinal events reported in these trials were often dose-dependent, mild, and noted to diminish within few days or weeks on continued therapy. Therefore, it is recommended that Liraglutide be titrated from 0.6 mg at weekly increments.[18]
Initial dose of Liraglutide used in our patients was 0.6 mg once daily, which was up-titrated to 1.2 mg once daily after 1 week, maximal daily dose of Liraglutide, i.e., 1.8 mg was reached in 15% patients. As the blood glucose values started decreasing after adding Liraglutide, dose adjustment of the concurrent oral antidiabetic drugs was done. Majority of the patients required reduction in sulfonylurea and insulin dosage with no change in metformin dosage after adding Liraglutide.
Education regarding Liraglutide use and its adverse events was provided to all patients at the initiation of therapy and at regular intervals thereafter, which helped improving patients’ adherence to treatment. Acceptance for Liraglutide treatment was optimal in most of our patients. Majority of our patients reported no difficulty in injecting Liraglutide with easy-to-use pre-filled pen. In our observation, patients found injecting Liraglutide as more convenient compared to insulin as Liraglutide could be administered once daily independent of meals.
Much of the available information on Liraglutide use in type 2 diabetes is from clinical trials conducted in European and Japanese population. Limited data is available in Asian population. The results of a study in patients with type 2 diabetes in China, Korea, and India were consistent with efficacy and safety of Liraglutide reported in previous LEAD studies.[19] Interestingly, a greater HbA1c lowering effects of Liraglutide are seen on Japanese type 2 diabetes[9,20,21] than those in the above mentioned study.
The results of our earlier study on the use of exenatide in obese north Indian patients with type 2 diabetes[22] showed a decline in mean HbA1c value by 1.1% from baseline at 3 months, which is lower than the decline in mean HbA1c value noted in the present study (1.5%). Mean weight loss of around 3.8 kg was noted at 3 months with the use of exenatide.
Prevalence of diabetes is rising globally, particularly in developing countries.[23] Prevalence of obesity is also rising rapidly in developing countries, including India. Our previous data analysis showed that, out of total 269 patients with type 2 diabetes, 45.3% were overweight and 36% were obese (unpublished data). Obesity and type 2 diabetes go hand in hand. Achieving ADA set HbA1c target of <7% in obese patients with type 2 diabetes is a big challenge. Our initial results indicate that Liraglutide helps in achieving good glycemic control in obese patients with type 2 diabetes. Furthermore, patients who were already on insulin also responded well to Liraglutide in terms of glycemic control. This suggests that Liraglutide works well in combination with insulin and can be used as an add-on treatment for patients having suboptimal glycemic control with insulin. In Indian scenario, due to cultural, social, and sedentary life style factors, pharmacotherapy with significant weight loss as secondary benefit carries an immense potential of the use of Liraglutide in patients with type 2 diabetes and obesity. In our experience, the initial weight loss accompanying improved glycemic control gives stronger motivation for patient to strictly adhere to diabetic program. In LEAD-3 trial, weight loss occurred mostly in the initial 16 weeks; however, it was subsequently continued throughout the 52 weeks of the study, which signifies sustained weight-loss effect of Liraglutide.
CONCLUSIONS
Liraglutide significantly improves glycemic control with low risk of hypoglycemia and is associated with significant weight loss in obese Indian patients with type 2 diabetes Most patients on prior insulin/oral antidiabetic combinations also responded well to Liraglutide. Liraglutide use was associated with gastrointestinal adverse events; these adverse events were usually mild and transient and led to very few withdrawals.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. ICMR-INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31:81–6. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 3.Harris SB, Ekoe JM, Zdanowicz Y, Webster-Bogaert S. Glycemic control and morbidity in the Canadian primary care setting (results of the diabetes in Canada evaluation study) Diabetes Res Clin Pract. 2005;70:90–7. doi: 10.1016/j.diabres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Uwaifo GI, Ratner RE. Novel pharmacologic agents for type 2 diabetes. Endocrinol Metab Clin North Am. 2005;34:155–97. doi: 10.1016/j.ecl.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez C, Beruto V, Keller G, Santoro S, Di Girolamo G. Investigational treatments for Type 2 diabetes mellitus: Exenatide and liraglutide. Expert Opin Investig Drugs. 2006;15:887–95. doi: 10.1517/13543784.15.8.887. [DOI] [PubMed] [Google Scholar]
- 6.Wajcberg E, Amarah A. Liraglutide in the management of type 2 diabetes. Drug Des Devel Ther. 2010;4:279–90. doi: 10.2147/DDDT.S10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35:S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponzani P, Corsi A. Incretin-based therapies in clinical practice: From efficacy to effectiveness. Focus on liraglutide. Minerva Endocrinol. 2012;37:1–8. [PubMed] [Google Scholar]
- 9.Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26:1013–22. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 10.Zenari L. Efficacy and tolerability of liraglutide in combination with other antidiabetic drugs in type 2 diabetes. Acta Biomed. 2011;82:251–3. [PubMed] [Google Scholar]
- 11.Raskin P, Mora PF. Glycaemic control with liraglutide: The phase 3 trial programme. Int J Clin Pract Suppl. 2010:21–7. doi: 10.1111/j.1742-1241.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 12.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 13.Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, et al. LEAD-1 SU study group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): A randomised controlled trial. Diabetologia. 2009;52:2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 18.Princeton, NJ: Novo Nordisk Inc; 2010. Jan, Victoza®. (Package insert) [Google Scholar]
- 19.Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: A 16-week, randomized, double-blind, active control trial (*) Diabetes Obes Metab. 2011;13:81–8. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaku K, Rasmussen MF, Clauson P, Seino Y. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human glucagon-like peptide-1 analogue liraglutide as add-on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:341–7. doi: 10.1111/j.1463-1326.2009.01194.x. [DOI] [PubMed] [Google Scholar]
- 21.Seino Y, Rasmussen MF, Nishida T, Kaku K. Glucagon-like peptide-1 analog liraglutide in combination with sulfonylurea safely improves blood glucose measures vs. sulfonylurea monotherapy in Japanese patients with type 2 diabetes: Results of a 52-week, randomized, multicenter trial. J Diabetes Invest. 2011;2:280–6. doi: 10.1111/j.2040-1124.2011.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bawa T, Dhingra V, Malhotra N, Wasir JS, Mithal A. Clinical experience with exenatide in obese North Indian patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2013;17:91–4. doi: 10.4103/2230-8210.107804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]


