Abstract
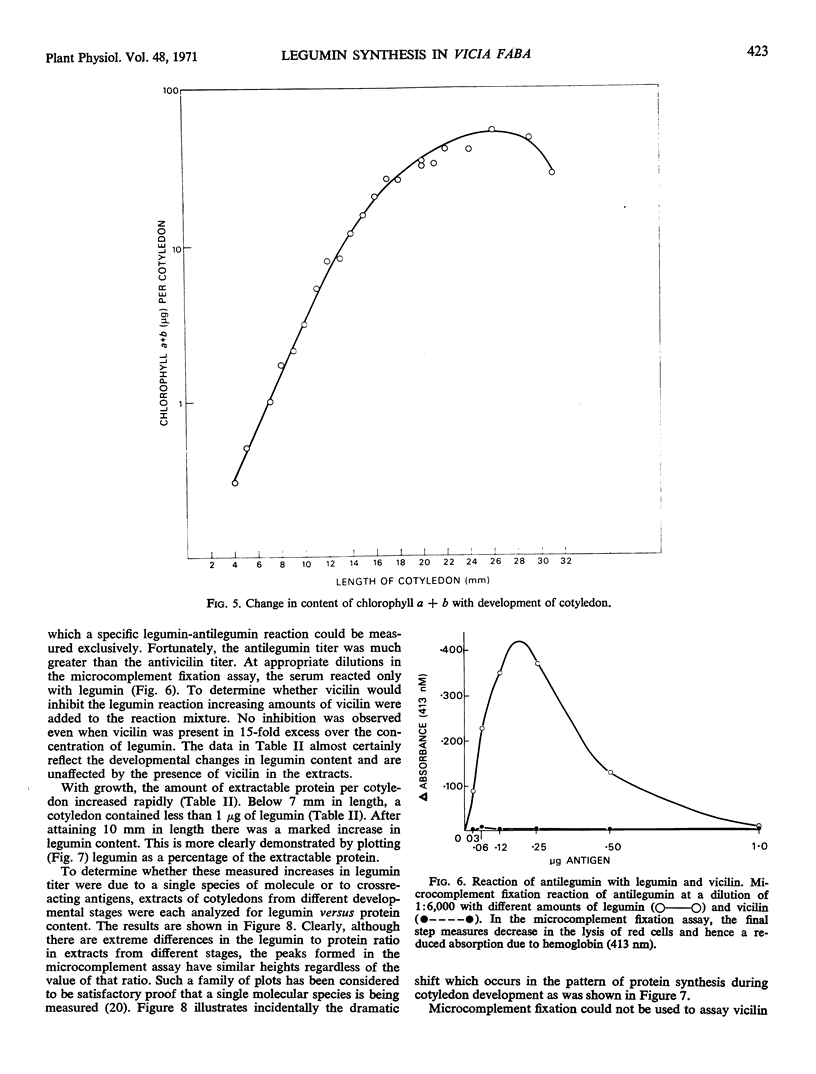
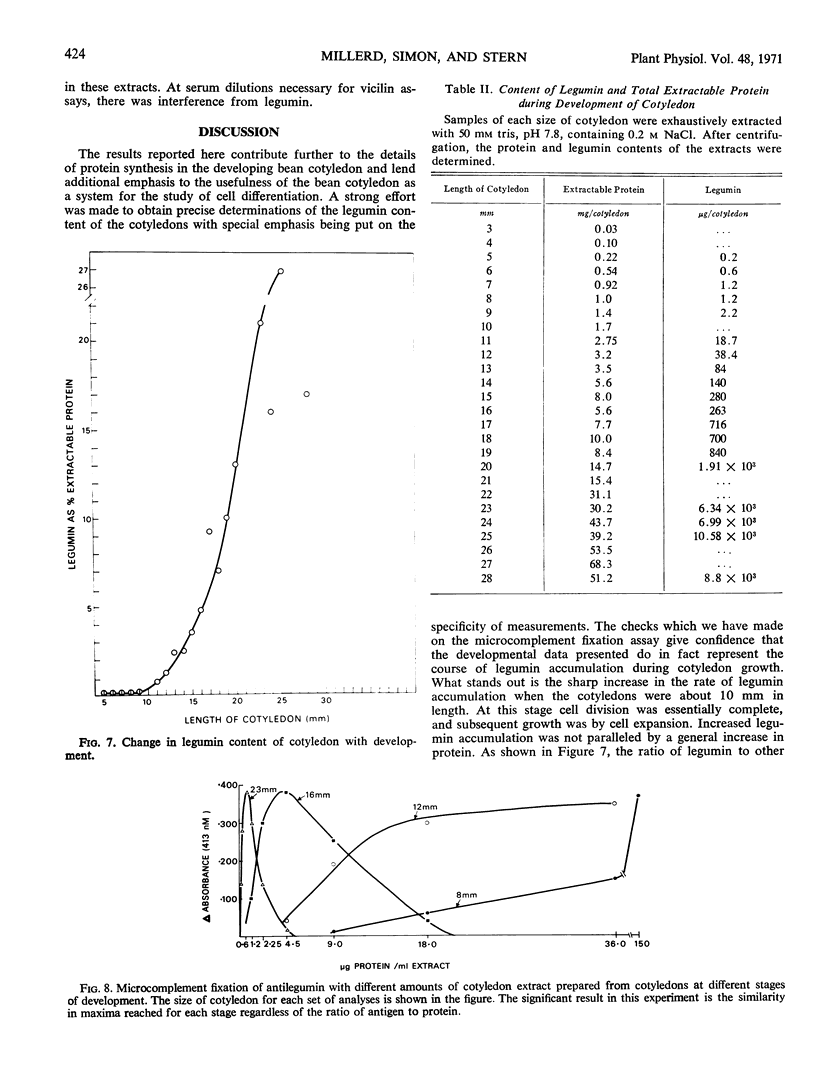
The synthesis of legumin in developing cotyledons of Vicia faba L. has been examined as a potential system for approaching the problem of differential gene expression. The pattern of legumin synthesis was determined during the growth of the cotyledon by microcomplement fixation which provided a sensitive and specific assay for legumin in the presence of vicilin. Legumin was detected even in young cotyledons. However, when the cotyledons were about 10 millimeters long, and cell division was essentially complete, there was a sharp increase in the rate of legumin accumulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNER J., HUANG R. C., GILDEN R. V. CHROMOSOMALLY DIRECTED PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Nov;50:893–900. doi: 10.1073/pnas.50.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. J., Boulter D. The structure of legumin, a storage protein of broad bean (Vicia faba) seed. Eur J Biochem. 1970 Dec;17(3):460–466. doi: 10.1111/j.1432-1033.1970.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Bonner J., Dahmus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- Bonner J., Dhamus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The biology of isolated chromatin. Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. [PubMed] [Google Scholar]
- GRANT D. R., LAWRENCE J. M. EFFECTS OF SODIUM DODECYL SULFATE AND OTHER DISSOCIATING REAGENTS ON THE GLOBULINS OF PEAS. Arch Biochem Biophys. 1964 Dec;108:552–561. doi: 10.1016/0003-9861(64)90441-2. [DOI] [PubMed] [Google Scholar]
- Graham T. A., Gunning B. E. Localization of legumin and vicilin in bean cotyledon cells using fluorescent antibodies. Nature. 1970 Oct 3;228(5266):81–82. doi: 10.1038/228081a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., RICHARDS E. G. The study of legumin by depolarization of fluorescence and other physicochemical methods. Arch Biochem Biophys. 1962 May;97:260–276. doi: 10.1016/0003-9861(62)90078-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rijven A. H., Wardlaw I. F. A method for the determination of cell number in plant tissues. Exp Cell Res. 1966 Feb;41(2):324–328. doi: 10.1016/s0014-4827(66)80140-4. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Schidlovsky G. Intracellular Distribution of Proteins in Pea Cotyledons. Plant Physiol. 1963 Mar;38(2):139–144. doi: 10.1104/pp.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]