Abstract
The current treatment regimen against drug susceptible tuberculosis (DS-TB) was defined by the 1980s. Since then the emergence of the global HIV pandemic and the escalation of drug resistant (DR−) forms of TB have presented new challenges for therapeutic research. Priority goals include shortening DS-TB treatment, improving DR-TB treatment and making combined TB-HIV therapy easier. To help achieve these goals, a range of new drugs and treatment strategies are currently being evaluated. Phase IIb and III clinical trials are ongoing to assess combinations involving the high-dose rifamycins, the 8-methoxyquinolones, a diarylquinoline (bedaquiline) and the nitroimidazoles. Other compounds (e.g. novel oxazolidinones and ethylenediamines) are at earlier stages of clinical development. Overall, there are grounds for optimism that recent advances will contribute towards achievement of new treatment regimens in the foreseeable future. However, long-term investment, political commitment and scientific endeavour are crucial to ensure that progress is sustained and the benefits of recent advances reach those in the greatest need.
Keywords: Bedaquiline (TMC-207), Delamanid (OPC-67683), Gatifloxacin, Moxifluxacin, PA-824, Rifampicin, Rifapentine, Sterilising activity
INTRODUCTION
After a series of groundbreaking advances between the 1940s and the 1980s, a prolonged hiatus in the development of new drugs against Mycobacterium tuberculosis was accompanied by a dramatic upsurge in morbidity and mortality from tuberculosis (TB) infections worldwide. In the last decade, a range of new treatment options have emerged. Evaluation of these is ongoing through an expanding portfolio of pre-clinical research and clinical trials.
In this review, contemporary challenges to successful treatment are placed in an historical context in order to outline priority goals for TB therapeutics. Recently completed and ongoing studies involving major drug classes against drug-susceptible (DS-) and drug-resistant (DR-) TB, including multidrug resistant (MDR) and extensively drug resistant (XDR) strains, are described in order to assess the likelihood that new treatment regimens will achieve these goals.
HISTORICAL PERSPECTIVE AND PRIORITY GOALS
Prior to the 1940s, TB chemotherapy was unavailable. Immunotherapy, environmental/nutritional measures and surgical intervention were potentially dangerous [1-3] and the case-fatality rate from smear positive pulmonary TB exceeded 50%. In 1944 streptomycin was isolated from the soil actinomycete Streptomyces griseus [4] and paraaminosalicylic acid (PAS) was discovered [5]. In 1952 isoniazid was discovered [6]. These drugs, with demonstrable efficacy against M. tuberculosis, were rigorously evaluated in the first randomised controlled trials to be performed in clinical medicine [7-11]. Whilst chemotherapy could be life-saving, it was established that single drug therapy was compromised by rapid emergence of drug-resistance [12, 13]. Combination regimens made durable cure of TB possible but until the 1970s, the shortest effective treatment duration was 18 months [14, 15].
The evidence supporting current regimens were derived from a series of international multi-centre trials mainly performed in the 1970s and 1980s by the Tuberculosis Research Unit at the British Medical Research Council (reviewed in detail by Fox [16]) and the US Public Health Service. Overall, these demonstrated 95% cure without relapse after short course chemotherapy comprising a two month “intensive phase” of 4 drugs (rifampicin, isoniazid, pyrazinamide and streptomycin or ethambutol) and a four month “continuation phase” of 2 drugs (rifampicin and isoniazid). No drug combination given for less than 6 months achieved a relapse rate below 10% and combinations which did not include rifampicin for the entire duration of therapy were associated with a relapse rate of 30% [17, 18].
Progress from no therapy to highly efficacious fully oral treatment within 40 years was impressive and for a brief time it seemed that the major obstacles to TB control had been overcome. However, no new first line drug has been developed since rifampicin in 1967. In the meantime, the global HIV pandemic has driven a rise in TB incidence and put considerable strain on healthcare resources. Much of the worldwide TB burden occurs in middle or low income countries of Africa and Asia where administration and monitoring of six month therapy is exceptionally difficult. Further reductions in treatment duration are necessary, especially as some mathematical models predict that “ultra-short course” therapy (less than 2 month duration) might prevent 20% of new TB cases and 25% of deaths [19].
Poor adherence to 6 month therapy and ineffective TB control has driven the emergence of MDR- TB with bacillary resistance to both rifampicin and isoniazid. Management of MDR-TB usually necessitates use of injectable agents (amikacin, kanamycin or capreomycin) for 4-6 months and toxic second-line oral drugs for several years. Although some success was recently reported with a shorter (9 month) regimen in Bangladesh this required use of 9 drugs [20]. XDR-TB with reduced susceptibility to second line drugs is also escalating and even more difficult to treat [21]. Shorter, simpler and preferentially oral regimens for MDR- and XDR-TB are urgently needed.
13% of worldwide TB cases are co-infected with HIV including 39% of cases in Africa [22]. Mortality in HIV-infected patients with pulmonary TB is lower when antiretroviral therapy (ART) is initiated during the first few weeks of TB treatment [23-25]. However, rifampicin potently induces the activity of multiple hepatic cytochrome P450 isoforms including CYP3A4 [26]. This accelerates the metabolism of some anti-retrovirals (particularly protease inhibitors and non-nucleoside reverse transcriptase inhibitors) and can result in sub-therapeutic drug concentrations. Overlapping toxicities and heavy pill burden also complicate dual HIV-TB treatment [27]. TB regimens which are easier to co-administer with ART are essential.
Finally, immunosuppression due to HIV infection or increased use of powerful immuno-modulatory drugs in modern medicine increases the possibility of early reactivation of latent TB infection, highlighting the need for better strategies to treat latent TB in high-risk populations.
The priority goals for therapeutics research are summarised in Table 1 [28]. The remainder of this review will analyse the potential for recent advances to contribute towards achievement of the first three goals concerning treatment of active TB.
Table 1. Priority Goals for Development of New TB Treatment Regimens.
| Patient Population | Current Therapy | Priority Goals | |
|---|---|---|---|
| 1. | Drug susceptible TB | 4 drugs; ≥ 6 month therapy (2 RHZE + 4RH) | Shorter, simpler therapy |
| 2. | Drug resistant (M[X]DR) TB | Few drugs (including injectable) | Totally oral, shorter, more efficacious and safer therapy |
| 3. | TB/HIV co-infection | Drug-drug interactions with ART | No or low drug-drug interactions, can be co-administered with ART |
| 4. | Latent TB infection | 6-9 months H | Shorter, safer therapy |
R=Rifampicin, H=Isoniazid, Z=Pyrazinamide, E=Ethambutol, ART=Anti-retroviral therapy.
METHODS IN THE DEVELOPMENT OF NEW REGIMENS AGAINST ACTIVE TB
It is believed that the pharmacodynamic response of TB infection to treatment is non-uniform, based on the efficacy of different drugs against sub-populations of M tuberculosis bacilli in varying metabolic states (Fig. 1A [29]). Quantitative sputum colony counting studies in DS-TB support a biphasic model of bacillary elimination comprising a 5-7 day “Early Bactericidal Phase” during which replicating organisms are killed by isoniazid [30, 31] and a prolonged “Sterilisation Phase” when metabolically quiescent “persisters” are eradicated more slowly (Fig. 1B [32-34]). Rifampicin and pyrazinamide are currently the best first-line sterilising drugs but it seems that faster Sterilisation Phase activity is essential for shorter regimens. For this reason, compounds with strong bactericidal activity against dormant/non-replicating M. tuberculosis cultures [35] and treatments which accelerate bacillary clearance in animal models are of particular interest.
Fig. (1).
Bacillary sub-populations theory of bacillary response to chemotherapy. A: Schematic diagram demonstrating probable response of distinct bacillary sub-populations during DS-TB therapy. During the Early Bactericidal Phase (first 5-7 days), isoniazid is key to killing metabolically active and replicating organisms. After day 7, the majority of viable bacilli are likely to be metabolically quiescent persisters, which are eradicated more slowly by sterilising drugs (especially rifampicin). The overall bacillary load falls in a biphasic manner, reflecting elimination of different bacillary populations at different times in therapy. B: Sputum colony counting data from 3 patient cohorts, demonstrating biphasic bacillary elimination in clinical practice, adapted from [39] and [95].
Drugs with these characteristics may proceed to efficacy trials in patients with pulmonary TB [36]. Phase IIa extended Early Bactericidal Activity (EBA) studies are often used to measure the fall in log10 colony forming units (CFU)/ ml of sputum achieved by new compounds compared to standard rifampicin-isoniazid-pyrazinamide-ethambutol (RHZE) therapy over 7-14 days. This provides a rapid assessment of potency but it is unclear whether studies of less than 2 weeks duration accurately reflect Sterilisation Phase activity. Phase IIb trials of 8 weeks duration are a better test of new drug combinations but the most suitable microbiological endpoints for these are unclear. Ultimately, new DS-TB regimens require evaluation in Phase III trials with a clinical end-point of post-treatment relapse. This means approximately 500 patients per arm on study for 18-30 months [37, 38]. MDR- and XDR-TB trials require multi-site recruitment and even longer treatment and follow-up. Improved standardisation and validation of the surrogate endpoints used in Phase IIb studies is needed to ensure that candidate regimens taken forward to Phase III trials have the highest chance of success [39].
TREATMENTS UNDER EVALUATION AND THE DRUG DEVELOPMENT PIPELINE
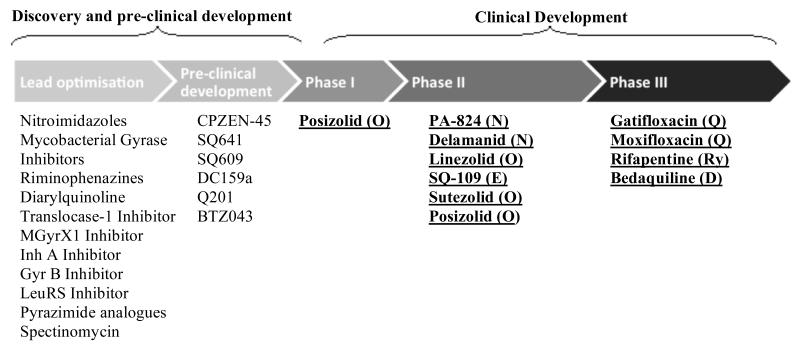
Fig. (2) shows compounds with anti-mycobacterial activity which are at different stages in the drug development pipeline (Fig. 2). Ongoing or planned Phase IIa trials of drugs in the major classes are summarised in Table 2 and Phase IIb/III trials of new treatment regimens against DS and DR-TB are summarised in Table 3.
Fig. (2). The Global Tuberculosis Drug Pipeline.
Schematic of the global drug development pipeline from the Working Group on New Drugs, Stop TB Partnership adapted from [36]. Drug names are followed by details of the relevant drug class in parenthesis. Drug classes are coded as follows: R=rifamycin, Q=fluoroquinolone, D=diarylquinoline, N=nitroimidazoles, Ed=ethylenediamine, O=oxazolidine. Compounds discussed in specific detail in this review are listed in bold and underlined.
Table 2. Ongoing and Completed But Unpublished Phase IIa Studies (Extended Early Bactericidal Activity 14 Day End-Point).
| Drug Classes | Study | Sites | Recruitment Groups | Regimens | Primary End-Point(s) | |
|---|---|---|---|---|---|---|
| Q | ACTG NCT01589497 |
S Africa | DS-TB HIV−/+(CD4>200/μl) |
2 days RHZE →12 days REZ 2 days RHZE → 12 days RMZE 14 days RHZE |
Rate of change in log10 CFU/ml in sputum from day 2-14 | |
| D | N | Global Alliance NCT01691534 |
S Africa | DS-TB HIV−/+ |
14 days JPaZC, JPaZ, JPaC, JZC, Z, C or RHZE | Rate of change in log10 CFU/ml in sputum from day 0-14 |
| N | Global Alliance NCT00944021 |
S Africa | DS-TB HIV−/+(CD4>300/μl) |
14 days Pa50, Pa100, Pa150, Pa200 or RHZE | Rate of change in log10 CFU/ml in sputum from day 0-14 | |
| N | Otsuka NCT00401271 |
S Africa | DS-TB HIV−/+(CD4>350/μl) |
14 days D100, D200, D300, D400 or RHZE | Rate of change in log10 CFU/ml in sputum from day 0-14 | |
| Ox | Pfizer NCT01225640 |
S Africa | DS-TB HIV −/+(CD4>350/μl) |
14 days Su60, Su1200 or RHZE | Rate of change in log10 CFU/ml in sputum from day 0-2 | |
| Ox | NIAID NCT01516203 |
S Africa | DS-TB HIV−/+(CD4>350/μl) |
14 days of Po500od, Po500bid, Po800bid, Po 1200bid or RHZE | Rate of change in log10 CFU/ml in sputum from day 0-14 | |
| Ed | Sequella NCT01218217 |
S Africa | DS-TB HIV−/+(CD4>250/μl) |
14 days of Sq75, Sq150, Sq300, Sq150R or R | Rate of change in log10 CFU/ml in sputum from day 0-14 | |
Drug classes are coded as in Table 1: Q=fluoroquinolones, D=Diarlyquinoline, N=nitroimidazopyran, Ox=Oxazolidinones, Ed=Ethylenediamines Numbers before drug regimens specify duration in months. Drugs are coded with the following initials: R=Rifampicin, H=Izoniazid, Z=Pyrazinamide, E=Ethambutol,M=Moxifloxacin, J=Bedaquiline, Pa=PA-824, C=Clofazimine, D=Delamanid, Su=Sutezolid,Sq=SQ109. Varying dosages of a drug in different arms of a trial, are indicated by a subscript next to the drug initial, bid=twice daily.
Table 3. Ongoing and Imminent Phase II/III Clinical Efficacy Trials Involving Major New Drug Classes Against Pulmonary TB.
| Drug Classes | Study | Sites | Recruitment Groups | Regimens | Primary End-Point(s) | |
|---|---|---|---|---|---|---|
| Phase III studies | ||||||
| Ry | Q | RIFAQUIN | S Africa, Zimbabwe, Zambia, Mozambique | DS-TB HIV−/+(CD4>200/μl) |
2RMZE→2Rp15/20mg/kgM[x2/wk] 2RHZE→4RH |
Evaluate at 18 months: Clinical failure/relapse |
| Q | OFLOTUB NCT00216385 |
Benin, Guinea, Kenya, Senegal, S Africa | DS-TB HIV−/+ |
2RHPG→2RHG 2RHZE→4RH |
Evaluate at 30 months: Clinical failure/relapse | |
| Q | REMoxTB NCT00864383 |
China, India, Kenya, Malaysia, Mexico, S Africa, Tanzania, Thailand, Zambia | DS-TB HIV−/+ |
2RHZM→2RHM 2RMZE→2 RM 2RHZE→4RH |
Evaluate at 18 months: Clinical failure/relapse | |
| D | TMC-C210 NCT01600963 |
Brazil, Cambodia, China, Colombia, Estonia, Korea, Latvia, Mexico, Peru, Phillipines, Russia, S Africa, Taiwan, Turkey, Ukraine, Vietnam | M (pre-X)DR-TB HIV −/+ |
9OB+J 9OB+placebo |
Evaluate at 15 months: Proportion of patients with SCC | |
| N | Otsuka NCT01424670 |
Estonia, India, Latvia, Lithuania, Moldova, Peru, Phillipines, S Africa | MDR-TB HIV− (HIV+ sub-study) |
4OB+D 4OB+placebo |
Evaluate at 2 and 6 months: Proportion of patients with SCC Time to SCC | |
| Phase IIb studies with 8-12 week microbiological end-points | ||||||
| Ry | Q | Uni of Munich NCT01785186 |
S Africa, Tanzanzia | DS-TB | 3R35mg/kgHZE, 3RHZQ 3R20mg/kgHZQ, 3R20mg/kgHZM or 3RHZE |
Proportion of patients with SCC Time to SCC |
| Ed | ||||||
| Ry | High RIF NCT00760149 |
Tanzania | DS-TB | 2R600/900/1200HZE | Bacillary elimination rate Time to SCC Proportion of patients with SCC | |
| Ry | HIRIF NCT01408914 |
Peru, Brazil | DS-TB HIV− /+ (CD4>350/μl) |
2R600/900/1200HZE | Bacillary elimination rate Time to SCC Proportion of patients with SCC | |
| Ry | JHU Study NCT00814671 |
S Africa | DS-TB HIV−/+(CD4>200/μl) |
2RHZE 2Rp450/600HZE |
Proportion of patients with SCC | |
| Ry | Q | JHU Study NCT00728507 |
Brazil | DS-TB HIV−/+(CD4>200/μl) |
2Rp300/450HZM 2RHZE |
Proportion of patients with SCC |
| Q | N | Global Alliance NCT01498419 |
Brazil, S Africa, Tanzania | DS and MDR-TB HIV−/+(CD4>200/μl) |
DS-TB: 2MPa100/200Z DS-TB: 2RHZE MDR-TB: 2MPa200Z |
Rate of change in log10 CFU/ml in sputum |
Drug classes coded as in Table 1: Ry=rifamycins, Q=fluoroquinolones, D=Diarlyquinoline, N=nitroimidazopyran, Ed=diethylamine. Numbers before drug regimens specify duration in months. Drugs: R=Rifampicin, Rp=Rifapentine, M=moxifloxacin, G=Gatifolxacin, O=Ofloxacin, Z=Pyrazinamide, E=Ethambutol, OB=Individualised Optimised Background for MDR-TB, J=Bedaquiline, D=Delamanid, Pa=PA-824. SCC=Sputum culture conversion. Varying dosages are indicated by subscript.
RE-EVALUATING THE RIFAMYCINS
The rifamycins work by inhibiting bacterial RNA synthesis. As rifampicin is the main sterilising drug in current DS-TB regimens further exploration of the treatment-shortening potential of this class is appropriate. The present rifampicin dose (10mg/kg once daily) was originally selected because it facilitated 6 month treatment whilst keeping drug acquisition costs low enough for global distribution [40]. Although the cost of rifampicin has fallen dramatically since the 1960s, toxicity concerns have previously discouraged dose escalation studies. However, recent use of up to 1200mg/day in non-mycobacterial infections, especially with meticillin resistant Staphylococcus aureus (MRSA), suggests that higher doses are safe [41]. Furthermore, animal and early clinical studies indicate that rifampicin’s activity against M. tuberculosis is concentration dependent and correlates well with the ratio of the area under the serum concentration-time curve (AUC)/minimum inhibitory concentration (MIC) [42]. A 10mg/kg human dose may be at the bottom end of a steep dose-response curve far below any observed maximum effect [43].
Two phase IIb dose-ranging studies of “high dose” rifampicin TB treatment with similar designs are now underway (Table 3). The High RIF study conducted in Tanzania (www.clinicaltrials.gov identifier NCT00760149) and the HIRIF study (NCT01408914) in Peru and Brazil will compare the effect of “intensive” phase treatment regimens containing rifampicin 600mg, 900mg or 1200mg on the rate of decline in sputum bacillary load, time to sputum culture conversion and proportion of patients who become culture negative during the first 8 weeks. An additional 12 week trial of 4 experimental regimens which include extended rifampicin dosing from 10-35mg/kg in combination with established (isoniazid/pyrazinamide) and new (moxifloxacin/SQ109) drugs is due to start soon (PanACAEA MAMS Study NCT01785186).
Rifapentine is an alternative semi-synthetic rifamycin with greater in vitro potency than rifampicin and a longer serum half-life. Mouse models suggest that high-dose rifapentine regimens may be given intermittently [44] while daily regimens may have treatment-shortening potential [45]. One multi-centre Phase clinical IIb study conducted in North America, South Africa, Uganda, Spain, Brazil, Peru and Vietnam (Tuberculosis Treatment Trial Consortium [TBTC] study 29) recently described equivalent 8 week sputum culture conversion rates between a 2 month intensive phase regimen of rifapentine (10mg/kg)-isoniazid,-pyrazinamideethambutol and standard RHZE [46]. Two further Phase IIb studies of alternative intensive phase rifapentine doses will report soon (Table 2, NCT00814671 and NCT00727507). A Phase III study (the RIFAQUIN trial) recently showed that a 2 month intensive phase of daily rifampicin (10mg/kg)-moxifloxacin-pyrazinamide-ethambutol followed by a 4 month continuation phase of once weekly rifapentine [20mg/kg]-moxifloxacin had similar efficacy to standard therapy. However, 2 months of rifampicin (10mg/kg)-moxifloxacin-pyrazinamide-ethambutol followed by 2 months of once weekly rifapentine (20mg/kg)-moxifloxacin was associated with a higher rate of post-treatment relapse. Therefore, whilst rifapentine may be useful, the appropriate drug combination for treatment shortening has yet to be found [40, 47].
Rifabutin is a less potent CYP3A inducer that the other rifamycins, a potential advantage for HIV-infected TB patients requiring ART. However, detailed dosing and long-term efficacy data on rifabutin are lacking [48] and some observational studies have reported high rates of acquired rifamycin resistance, particularly with intermittent regimens [49]. A Cochrane review in 2007 including meta-analysis of 924 patients from 5 trials suggested that rifabutin was as effective and safe as rifampicin but only 5% of recruited patients were HIV-infected [48]. A recently published case series from the UK suggests that daily rifabutin-based therapy achieves similar outcomes to rifampicin in TB-HIV co-infection [50] but further prospective studies are required. Some of the new drugs to be discussed in this review (e.g. bedaquiline) may be metabolised by CYP3A and rifabutin may be useful in allowing these agents to be combined with a rifamycin.
DEFINING THE ROLE OF 8-METHOXYFLUOROQUINOLONES
The fluoroquinolones are broad-spectrum antimicrobial drugs targeting DNA gyrase [51]. They have several attractive properties for TB treatment; high oral bio-availability, excellent EBA against M. tuberculosis, non-induction of CYP3A and a lack of cross-resistance with other anti-TB drugs. Despite a lack of randomised clinical trials, levofloxacin and the new 8-methoxyfluoroquinolones (moxifloxacin and gatifloxacin) are already regarded as a vital component of effective MDR-TB therapy [40].
Mouse models indicate that 8-methoxyfluoroquinolone-containing regimens may also be used to shorten DS-TB treatment [52, 53]. Four important Phase IIb studies have been conducted to assess the clinical efficacy of this approach (Table 4).
Table 4. Completed Phase II Studies Including Moxifloxacin for Treatment for DS-TB.
| Study | Site | Regimens | Duration | Endpoint(s) | Results |
|---|---|---|---|---|---|
| TBTC-27 [54] | North America Brazil South Africa Spain, Uganda | RHZM RHZE | 8 weeks | Sputum culture conversion in liquid broth | SCC in 99/139 (71.0%) RHZM patients vs 98/139 (71.0%) RMZE patients (p=0.97) Secondary analysis at 4 weeks showed SCC in 62/167 (37%) RHZM patients vs 43/165 (26%) RHZE patients (p=0.05) |
| TBTC-28 [55] | North America Brazil South Africa Spain, Uganda | RMZE RHZE |
8 weeks | Sputum culture conversion in liquid broth | SCC in 90/164 (54.9%) RHZE patients vs 99/164 (60.4%) RMZE patients (p=0.37); small but nonsignificant increase in culture conversion on RMZE |
| JHU [56] | Brazil | RHZM RHZE |
8 weeks | Sputum culture conversion on LJ slopes | SCC in in 59/74 (80%) RHZM patients vs 45/72 (63%) RHZE patients (p=0.03) Secondary analysis showed shorter time to culture conversion in RHZM patients (p=0.005) |
| OFLOTUB [33] | South Africa | RHZM, RHZG, RHZO RHZE |
8 weeks | Regression co-efficient of sterilisation phase bacillary elimination rate | Significantly greater regression co-efficients for RHZM and RHZG than RHZE (p=0.002 in both cases) No difference in regression co-efficients between RHZO and RHZE (p=0.14) |
R=Rifampicin, H=Isoniazid, Z=Pyrazinamide, E=Ethambutol, M=Moxifloxacin, G=Gatifloxacin, O=Ofloxacin, LJ=Lowenstein Jensen media, SCC=Sputum culture conversion.
Two multi-centre TBTC studies compared standard RHZE with regimens in which moxifloxacin replaced ethambutol (Study 27) [54] or isoniazid (Study 28) [55]. Both were based on an end-point of sputum culture conversion in liquid broth at 8 weeks and neither reported better outcomes with moxifloxacin. However, in Study 27 moxifloxacin was associated with a greater culture conversion at 4 weeks indicating that the choice of analysis time-point may influence study conclusions [54]. Investigators from John Hopkins University (JHU) also assessed moxifloxacin-ethambutol substitution. They described greater culture conversion with moxifloxacin at 8 weeks and shorter time to culture conversion with moxifloxacin during the first 2 months. The JHU study used Lowenstein Jensen slopes rather than liquid broth for sputum cultures, suggesting that the choice of media may affect outcome [56].
Finally, the OFLOTUB consortium performed a quantitative sputum colony counting study to evaluate the effect of ethambutol replacement by moxifloxacin, gatifloxacin or ofloxacin. Statistical modelling was used to generate regression co-efficients representing the rate of bacillary elimination during the sterilisation phase of treatment for each regimen. Co-efficients for moxifloxacin and gatifloxacin were significantly greater than for ethambutol or ofloxacin suggesting that the 8-methoxyfluoroquinolones accelerated eradication of persister organisms [33].
The choice of various methodologies and statistical analyses generated slightly different outcome data from these studies, illustrating the current difficulty in end-point selection for Phase II trials. It is uncertain which marker of treatment response during the first 8 weeks is most predictive of final clinical outcome and this reduces the ability of Phase II studies to select regimens for further evaluation. Nevertheless, the overall trend towards earlier culture conversion and greater sterilising activity with combinations including 8-methoxyfluoroquinolones has prompted commencement of several Phase III trials. The first of these to report was the RIFAQUIN trial, which has already been described and failed to show adequate sterilising activity from a four month regimen incorporating moxifloxacin [47]. Although this was disappointing, two other studies are ongoing. The OFLOTUB consortium is assessing a regimen based on 2 months of rifampicinisoniazid-pyrazinamide-gatifloxacin followed by 2 months of rifampicin-isoniazid-gatifloxacin (NCT00216385), whilst the REMoXTB trial is evaluating two separate combinations; 2 months of rifampicin-isoniazid-pyrazinamide-moxifloxacin followed by 2 months of rifampicin-isoniazid-moxifloxacin, and 2 months of rifampicin-moxifloxacin-pyrazinamideethambutol followed by 2 months of rifampicin-moxifloxacin (NCT00864383). Results of these trials will determine whether moxifloxacin becomes a first-line drug in the near future Further Phase IIa/b studies designed to understand how moxifloxacin could be used in new combinations with existing and novel drug classes are summarised in Tables 2 and 3.
Limitations to future fluoroquinolone-based combinations may include toxicity, drug interactions and resistance. Although QTc interval prolongation has previously been described with moxifloxacin, no Phase II studies showed increased incidence of cardiac arrthythmias in patients on fluoroquinolones. However, there may be a risk of additive toxicity if moxifloxacin is combined with other compounds which also prolong the QTc interval (e.g. bedaquiline [TMC-207]) [40]. The rifamycins induce metabolism of moxifloxacin, slightly reducing the AUC but the clinical significance of this is unknown [57, 58]. Finally, the propensity of fluoroquinolone monotherapy administered for presumed bacterial respiratory infections in the community to cause rapid emergence of resistance has led to a significant prevalence of fluoroquinolone resistance in some countries [59].
LICENSING AND ONGOING TRIALS OF BEDAQUILINE [TMC-207]
Diarylquinolines are selective inhibitors of mycobacterial ATP synthase with minimal activity against other bacteria or eukaryotic cells [60]. The first drug in the class, bedaquiline (previously known as R207910 and TMC-207), was discovered from high throughput screening of compounds against M smegmatis. It can be administered orally, is bactericidal against rapidly growing and metabolically quiescent organisms and is effective against DS and DR-TB [60-62]. Viewed together these characteristics give it considerable treatment-shortening potential. In view of the urgent need for new drugs, the US Food and Drug Administration granted accelerated approval and licensing of bedaquiline for use in MDR-TB regimens in 2012 [63].
Early animal studies indicated the potency of bedaquilinie as monotherapy [60] or in combination with pyrazinamide-rifapentine [64]. In humans, a Phase IIa EBA trial showed that a daily dose of 400-800mg had equivalent efficacy to isoniazid and rifampicin from Day 5 onwards [65]. The drug had no EBA for the first 4 days, perhaps reflecting the time required for depletion of intra-bacillary ATP stores or accumulation of therapeutic levels.
FDA provisional licensing for MDR-TB was based on emerging data from two Phase IIb studies; TMC207-C208 and C209 [66]. C208 was a placebo controlled trial performed in two stages. In the first stage, 47 South African patients with MDR-TB were recruited and treated with Optimised Background (OB) regimens supplemented by bedaquiline (400mg once daily) or placebo for the first 8 weeks. Time to sputum culture conversion in liquid media was the primary outcome measure and patients given bedaquiline performed significantly better [67, 68]. In the second stage, 161 patients were recruited at 15 centres worldwide and bedaquiline/placebo supplementation was extended to 24 weeks. Follow-up is ongoing but preliminary efficacy analysis at the end of the investigational period reported faster culture conversion in the bedaquiline arm [66]. C209 was a single arm open-label study which recruited 233 patients with M(X) DR-TB at 33 sites. All participants received OB therapy augmented by bedaquiline during the first 24 weeks. Once more, long-term follow-up is ongoing but 24 week interim analysis showed a culture conversion rate of 75.1-81.5% and median time to conversion of 57 days [66].
Although no Phase III studies of bedaquiline have been performed a current trial protocol proposes to compare 9 months of an OB plus bedaquiline or placebo in MDR-TB (Table 3, NCT1600963). Additionally, a Phase IIa study is currently underway to assess the extended EBA of 7combinations for DS-TB, 4 of which include bedaquiline (Table 2, Global Alliance NC-003, NCT01691534).
Possible limitations of using bedaquiline are toxicity, drug-drug interactions and development of resistance. QTc prolongation has been described in published trials and, although an increased incidence of cardiac arrhythmias has not been reported [67, 68] the drug does carry an FDA back box warning. Caution may be necessary during co-administration with other arrhythmogenic drugs (e.g. moxifloxacin). In study C208, higher all-cause mortality was reported on bedaquiline than placebo. This was multifactorial and has not been attributed to the study drug but will remain a cause of concern until Phase III trial data based on clinical end-points are available [69]. Bedaquiline is metabolised by hepatic CYP3A and the serum AUC falls by 50% when it is given alongside rifampicin. This may compromise its role in DS-TB. Interactions with ART have not been extensively studied but co-therapy with efavirenz does not appear to affect PK parameters [70]. In vitro resistance to bedaquiline is induced by several mechanisms including atpE gene mutations [71]. Cross-resistance with other first or second-line anti-TB drugs has not been described but careful monitoring will be necessary in settings of pre-existing complex drug resistance.
EMERGENCE OF THE NITROIMIDAZOLES
The nitroimidazoles are structurally related to metronidazole. The anti-TB effect of this class was identified in the 1990s [72] and is principally due to inhibition of mycolic acid synthesis in the mycobacterial cell wall. Although development of early compounds was thwarted by mutagenicity, two agents appear to be clinically useful; PA-824 [73] and delamanid (OPC-67683) [74]. Both are pro-drugs and several reactive intermediates created during their activation provide additional mechanisms of bactericidal activity [74, 75]. As with bedaquiline, both are potent against replicating and non-replicating organisms. Murine studies indicated treatment-shortening sterilizing activity and accelerated time to culture conversion when either drug is combined with rifampicin-pyrazinamide [74, 76] or PA-824 is combined with moxifloxacin-pyrazinamide [55].
In South Africa, a partially blinded Phase IIa clinical study demonstrated that oral PA-824 monotherapy at 200, 600, 1000 or 1200mg caused an average drop of 0.1 log10 CFU/ml sputum during the first 2 weeks of DS-TB treatment, only slightly below that of RHZE [77]. There was no dose dependent effect, suggesting that maximal extended EBA of PA-824 is achieved at a lower dose than 200mg/day or that the proportion of the dosing interval during which the active drug concentration exceed the MIC is more important than the maximum achievable serum concentration. A second study to assess doses ranging from 50-200mg (NCT00944021) has recently been completed but results are not yet available.
Delamanid has higher bactericidal activity than PA-824 in vitro. Doses up to 400mg are well tolerated and a Phase IIb trial including 481 pulmonary MDR-TB patients recently showed that supplementation of an OB regimen with oral delamanid 100mg twice daily increased the proportion of patients with sputum culture conversion at 2months when compared to an OB regimen supplemented by placebo [78]. A Phase III study of delamanid in MDR-TB is now underway with primary end-points based on time to sputum culture conversion in the first 6 months and secondary endpoints based on durability of culture conversion out to 30 months (Table 3, NCT01424670).
Both delamanid and PA-824 are well tolerated and neither cause significant CYP3A induction, suggesting that they may be co-administered with ART. Resistance to both drugs develops through mutations in the ddn gene responsible for nitroreductive activation of the pro-drug molecules. Other mutations affecting the F420 biochemical pathway (fbiA, fbiB, fbiC and fgd) may confer PA-824 resistance but it is unclear whether they cause cross-resistance with delamanid.
The success of recent 8-methoxyfluoroquinolone, diarylquinoline and nitroimidazopyran trials has raised the possibility of constructing new “universal” regimens composed predominantly or entirely of new drugs. Advantages of this approach may include avoidance of CYP3A induction by rifamycins and the creation of a single unifying regimen against DS- and DR-TB. Combinations of moxifloxacin-bedaquiline-PA-824 in the presence or absence of other experimental agents appear to have treatment shortening potential in mouse models [79, 80] and a Phase IIa clinical study has recently reported that moxifloxacin-PA-824-pyrazinamide has equivalent extended EBA to RHZE [81]. It remains to be seen whether these regimens will be robust in longer clinical trials using later outcome markers of sterilising activity.
OTHER NEW DRUG CLASSES AND TREATMENT STRATEGIES
At least one agent from each of the drug classes described so far is being evaluated in Phase III trials. Several compounds at earlier stages of development also deserve mention.
The oxazolidinones achieve broad spectrum antimicrobial activity by binding to the 70S initiation complex of bacterial ribosomes and interfering with protein synthesis. In vitro and clinical EBA studies of linezolid, the only currently licensed drug in this class, display activity against M. tuberculosis [82] and it has been used off-label in combination therapy for MDR-TB. A recent Phase II trial described accelerated sputum culture conversion when linezolid was added to an OB regimen in XDR-TB [83] but adverse events were reported in 82% of patients on the linezolid arm. Complications including myelosuppression and optic/peripheral neuropathy have fuelled concerns about long-term use of the drug. Remarkably, emergence of resistance was uncommon during this trial since in many cases the regimen amounted to linezolid monotherapy. Alternative oxazolidinones are under development including sutezolid (PNU-100480) and posizolid (AZD5847) [84]. It is hoped that these will show enhanced sterilising activity with lower toxicity.
The ethylenediamines, including the current first-line drug ethambutol are inhibitors of mycobacterial cell wall synthesis. SQ109, a new drug from this class, was identified by screening a library of ethambutol derivatives but it has several distinguishing features from the parent compound. Its precise mechanism of action is unknown but gene expression analyses suggest differences from ethambutol, and there is no evidence of cross-resistance between the drugs [85]. Studies in mice revealed extensive tissue distribution of SQ109 and bactericidal activity at serum concentrations below the MIC [85]. Monotherapy at doses of 1-25mg/kg showed equivalent efficacy to ethambutol but SQ109 performed better in a combination regimen with rifampicinisoniazid-pyrazinamide [86]. Considerable in vitro synergy has also been observed between SQ-109 and bedaquiline [87]. Phase I and IIa clinical studies are ongoing.
The β-lactam antibiotics (penicillins and cephalosporins) have traditionally been excluded from TB treatment because of β-lactamase mediated resistance. Co-amoxiclav, a combination of amoxicillin with clavulanate (an irreversible β-lactamase inhibitor), has higher anti-TB EBA than other antibiotics in the class [88] but it has poor sterilizing activity and is unlikely to be of value for treatment shortening [89]. However, the carbapenems (particularly imipenem and meropenem) also have activity against M. tuberculosis, particularly when combined with clavulanate, and a role has been proposed for them in the treatment of XDR-TB [90]. Their PK properties may limit their usefulness as they require multiple daily parenteral dosing.
Clofazimine is a riminophenzine dye developed in the 1950s. Its anti-mycobacterial activity has mainly been employed in the treatment of leprosy and it was initially overshadowed as a TB drug by the success of streptomycin, isoniazid and PAS [91]. Due to a shortage of second line chemotherapy options, it has been re-considered against MDR-TB and it was recently included in a successful 9 month 9 drug regimen [20]. Modern mouse studies have shown sterilising activity of clofazimine in combination with bedaquiline [80] and pyrazinamide. A Phase IIa clinical study is currently assessing the extended EBA study of clofazimine as monotherapy and in combination with bedaquiline, pyrazinamide and PA-824 (Table 2, NCT01691524)
Augmentation of host immune responses to M. tuberculosis has been proposed as an alternative means of shortening TB treatment. Although TB immunotherapy has historically been unsuccessful, calcitriol (the active component of Vitamin D) has several anti-mycobacterial properties and its role an adjunctive agent against DS-TB has recently been re-visited [92]. A double-blind randomised trial in the UK reported that administration of 2.5mg Vitamin D3 on days 0, 14, 28 and 42 of therapy did not significantly shorten the time to sputum culture conversion, except in a subpopulation of patients with the tt genotype of the TaqI Vitamin D receptor polymorphism [93]. Revised Vitamin D dosing strategies and detailed study of the pharmacogenetics of Vitamin D treatment may be considered, but it currently appears that novel anti-microbial combinations with enhanced sterilization phase activity will be the most likely route to shorter TB chemotherapy.
CONCLUSIONS
There are justifiable grounds for optimism that recent advances in TB drug development will result in achievement of some priority goals in the management of active TB. The recent licensing of bedaquiline and the encouraging performance of sterilising drugs such as the 8-methoxyfluoroquinolines and nitroimidazoles in Phase IIb clinical trials are concrete signs of progress towards shorter therapy.
However, this optimism should be tempered with a degree of caution. A regimen less than 6 months in duration with proven efficacy against DS-TB is still awaited, and may still require a CPY3A inducing rifamycin for the entire treatment course. Management of TB-HIV co-infection remains difficult, particularly in resource-poor settings where the disease burden is highest. The precise role of new drugs in the management of MDR-TB remains undefined and Phase III clinical trials will not report for several years. Extrapolating conclusions from Phase II studies is compromised by uncertainty surrounding the validation of surrogate end-points. Several additional questions are beyond the scope of this review; management of latent TB infection, appropriate dosing regimens for paediatric TB [36] and the best regimens for extra-pulmonary disease (e.g. TB meningitis) [94].
Overall, the last decade has been associated with the most encouraging advances in TB therapeutics since the 1960s but these breakthroughs have still to impact on the stubbornly high global burden of TB disease. Long-term investment, political commitment and scientific endeavour will be crucial to ensure that progress is sustained and the benefits of recent advances reach those in the greatest need.
ACKNOWLEDGEMENTS
Declared none.
Footnotes
CONFLICT OF INTEREST The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Koch R. Weitere mitteilungen uber ein heilmittel gegen tuberculose. Deutsche Medizinsche Wochenschrift. 1891;171:101–2. [Google Scholar]
- [2].Bodington G. An essay on the treatment and cure of pulmonary consumption. Sinopkin, Marshall, Hamilton and Kent; London: 1840. [Google Scholar]
- [3].Temple L. In: Surgery of pulmonary tuberculosis: a historical approach, in Clinical Tuberculosis. Davies P, editor. Chapman and Hall; London: 1998. pp. 21–33. [Google Scholar]
- [4].Schatz A, Bugie E, Waksman S. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proceedings of the Society for Experimental and Biological Medicine. 1944;55:66–9. [Google Scholar]
- [5].Lehmann J. Para-Aminosalicylic acid in the treatment of tuberculosis. Lancet. 1946;I:15–6. doi: 10.1016/s0140-6736(46)91185-3. [DOI] [PubMed] [Google Scholar]
- [6].Bernstein J, Lott WA, Steinberg BA, Yale HL. Chemotherapy of experimental tuberculosis V. Isonicotinic acid hydrazide (Nydrazid) and related compounds. Am Rev Tuberc. 1952;65:357–64. doi: 10.1164/art.1952.65.4.357. [DOI] [PubMed] [Google Scholar]
- [7].MRC Streptomycin treatment of tuberculous meningitis. Lancet. 1948;1:582–96. [PubMed] [Google Scholar]
- [8].MRC Streptomycin treatment of pulmonary tuberculosis. Br Med J. 1948;2:769–82. [PMC free article] [PubMed] [Google Scholar]
- [9].MRC The prevention of streptomycin resistance by combined chemotherapy. Br Med J. 1952;1:1157–62. [PMC free article] [PubMed] [Google Scholar]
- [10].MRC The treatment of pulmonary tuberculosis with isoniazid. Br Med J. 1952;2:735–46. [PMC free article] [PubMed] [Google Scholar]
- [11].MRC Isoniazid in the treatment of pulmonary tuberculosis. Second Report. Br Med J. 1953;1:521–36. [PMC free article] [PubMed] [Google Scholar]
- [12].Mitchison DA. Development of streptomycin resistant strains of tubercle bacilli in pulmonary tuberculosis. Results of simultaneous sensitivity tests in liquid and on solid media. Thorax. 1950;5:144–61. doi: 10.1136/thx.5.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Selkon JB, Devadatta S, Kulkarni KG, et al. The emergence of isoniazid-resistant cultures in patients with pulmonary tuberculosis during treatment with isoniazid alone or isoniazis plus PAS. Bull World Health Organ. 1964;31:273–94. [PMC free article] [PubMed] [Google Scholar]
- [14].MRC Long term chemotherapy in the treatment of chronic pulmonary tuberculosis with cavitation. Tubercle. 1962;43:201–67. [Google Scholar]
- [15].MRC Co-operative controlled trial of a standard regimen of streptomycin, PAS and isoniazid and three alternative regimens of chemotherapy in Britain. Tubercle. 1973;54:99–129. doi: 10.1016/0041-3879(73)90031-7. [DOI] [PubMed] [Google Scholar]
- [16].Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(10 Suppl 2):S231–79. [PubMed] [Google Scholar]
- [17].East African/British Medical Research Councils Controlled clinical trial of five short-course (4-month) chemotherapy regimens in pulmonary tuberculosis. Second report of the 4th study. Am Rev Respir Dis. 1981;123(2):165–70. doi: 10.1164/arrd.1981.123.2.165. [DOI] [PubMed] [Google Scholar]
- [18].East African and British Medical Research Councils Controlled clinical trial of five short-course (4-month) chemotherapy regimens in pulmonary tuberculosis. First report of 4th study. Lancet. 1978;2(8085):334–8. [PubMed] [Google Scholar]
- [19].Salomon JA, Lloyd-Smith JO, Getz WM, et al. Prospects for advancing tuberculosis control efforts through novel therapies. PLoS Med. 2006;3(8):e273. doi: 10.1371/journal.pmed.0030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Van Deun A, Maug AK, Salim MA, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–92. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- [21].Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- [22].World Health Organisation . Global Tuberculosis Report 2012. Geneva: 2012. [Google Scholar]
- [23].Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344(13):984–96. doi: 10.1056/NEJM200103293441307. [DOI] [PubMed] [Google Scholar]
- [27].Burman WJ. Issues in the management of HIV-related tuberculosis. Clin Chest Med. 2005;26(2):283–94. vi–vii. doi: 10.1016/j.ccm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [28].Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375(9731):2100–9. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]
- [29].Mitchison DA. Basic mechanisms of chemotherapy. Chest. 1979;76(6 Suppl):771–81. doi: 10.1378/chest.76.6_supplement.771. [DOI] [PubMed] [Google Scholar]
- [30].Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121(6):939–49. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- [31].Sirgel FA, Donald PR, Odhiambo J, et al. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J Antimicrob Chemother. 2000;45(6):859–70. doi: 10.1093/jac/45.6.859. [DOI] [PubMed] [Google Scholar]
- [32].Davies GR, Brindle R, Khoo SH, Aarons LJ. Use of nonlinear mixed-effects analysis for improved precision of early pharmacodynamic measures in tuberculosis treatment. Antimicrob Agents Chemother. 2006;50(9):3154–6. doi: 10.1128/AAC.00774-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rustomjee R, Lienhardt C, Kanyok T, et al. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12(2):128–38. [PubMed] [Google Scholar]
- [34].Sloan DJ, Corbett EL, Butterworth AE, et al. Optimizing outpatient serial sputum colony counting for studies of tuberculosis treatment in resource-poor settings. J Clin Microbiol. 2012;50(7):2315–20. doi: 10.1128/JCM.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Piccaro G, Giannoni F, Filippini P, Mustazzolu A, Fattorini L. Activity of drug combinations against Mycobacterium tuberculosis grown in aerobic and hypoxic acidic conditions. Antimicrob Agents Chemother. 2013;57(3):1428–33. doi: 10.1128/AAC.02154-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lienhardt C, Raviglione M, Spigelman M, et al. New drugs for the treatment of tuberculosis: needs, challenges, promise, and prospects for the future. J Infect Dis. 2012;205(Suppl 2):S241–9. doi: 10.1093/infdis/jis034. [DOI] [PubMed] [Google Scholar]
- [37].Burman WJ. Rip Van Winkle wakes up: development of tuberculosis treatment in the 21st century. Clin Infect Dis. 2010;50(Suppl 3):S165–72. doi: 10.1086/651487. [DOI] [PubMed] [Google Scholar]
- [38].van Niekerk C, Ginsberg A. Assessment of global capacity to conduct tuberculosis drug development trials: do we have what it takes? Int J Tuberc Lung Dis. 2009;13(11):1367–72. [PubMed] [Google Scholar]
- [39].Davies GR. Early clinical development of anti-tuberculosis drugs: science, statistics and sterilizing activity. Tuberculosis (Edinb) 2010;90(3):171–6. doi: 10.1016/j.tube.2010.03.007. [DOI] [PubMed] [Google Scholar]
- [40].Nuermberger EL, Spigelman MK, Yew WW. Current development and future prospects in chemotherapy of tuberculosis. Respirology. 2010;15(5):764–78. doi: 10.1111/j.1440-1843.2010.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Forrest GN, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev. 2010;23(1):14–34. doi: 10.1128/CMR.00034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47(7):2118–24. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Steingart KR, Jotblad S, Robsky K, et al. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2011;15(3):305–16. [PubMed] [Google Scholar]
- [44].Rosenthal IM, Williams K, Tyagi S, et al. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am J Respir Crit Care Med. 2006;174(1):94–101. doi: 10.1164/rccm.200602-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4(12):e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dorman SE, Goldberg S, Stout JE, et al. Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the tuberculosis trials consortium. J Infect Dis. 2012;206(7):1030–40. doi: 10.1093/infdis/jis461. [DOI] [PubMed] [Google Scholar]
- [47].Jindani A, Hatherill M, Charalambous S, et al. A Multicentre Randomised Clinical Trial to Evaluate High-dose Rifapentine with a Quinolone for Treatment of Pulmonary TB: The RIFAQUIN Trial. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, USA. 2013. [Google Scholar]
- [48].Davies G, Cerri S, Richeldi L. Rifabutin for treating pulmonary tuberculosis. Cochrane Database Syst Rev. 2007;(4):CD005159. doi: 10.1002/14651858.CD005159.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Burman W, Benator D, Vernon A, et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med. 2006;173(3):350–6. doi: 10.1164/rccm.200503-417OC. [DOI] [PubMed] [Google Scholar]
- [50].Singh R, Marshall N, Smith CJ, et al. No impact of rifamycin selection on tuberculosis treatment outcome in HIV coinfected patients. AIDS. 2013;27(3):481–4. doi: 10.1097/QAD.0b013e32835a67fb. [DOI] [PubMed] [Google Scholar]
- [51].Mitscher LA. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem Rev. 2005;105(2):559–92. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- [52].Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med. 2004;170(10):1131–4. doi: 10.1164/rccm.200407-885OC. [DOI] [PubMed] [Google Scholar]
- [53].Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004;169(3):421–6. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- [54].Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174(3):331–8. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- [55].Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180(3):273–80. doi: 10.1164/rccm.200901-0078OC. [DOI] [PubMed] [Google Scholar]
- [56].Conde MB, Efron A, Loredo C, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373(9670):1183–9. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Weiner M, Burman W, Luo CC, et al. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob Agents Chemother. 2007;51(8):2861–6. doi: 10.1128/AAC.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dooley K, Flexner C, Hackman J, et al. Repeated administration of high-dose intermittent rifapentine reduces rifapentine and moxifloxacin plasma concentrations. Antimicrob Agents Chemother. 2008;52(11):4037–42. doi: 10.1128/AAC.00554-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ginsburg AS, Hooper N, Parrish N, et al. Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin Infect Dis. 2003;37(11):1448–52. doi: 10.1086/379328. [DOI] [PubMed] [Google Scholar]
- [60].Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307(5707):223–7. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- [61].Koul A, Vranckx L, Dendouga N, et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem. 2008;283(37):25273–80. doi: 10.1074/jbc.M803899200. [DOI] [PubMed] [Google Scholar]
- [62].Rao SP, Alonso S, Rand L, Dick T, Pethe K, et al. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2008;105(33):11945–50. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cohen J. Infectious disease. Approval of novel TB drug celebrated--with restraint. Science. 2013;339(6116):130. doi: 10.1126/science.339.6116.130. [DOI] [PubMed] [Google Scholar]
- [64].Ibrahim M, Andries K, Lounis N, et al. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother. 2007;51(3):1011–5. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52(8):2831–5. doi: 10.1128/AAC.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].FDA Anti-Infective Drugs Advisory Committee Meeting Briefing Document: TMC-207 (bedaquiline) Treatment of Patients with MDR-TB DNA 204-384. 2012.
- [67].Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- [68].Diacon AH, Donald PR, Pym A, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother. 2012;56(6):3271–6. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Avorn J. Approval of a tuberculosis drug based on a paradoxical surrogate measure. JAMA. 2013;309(13):1349–50. doi: 10.1001/jama.2013.623. [DOI] [PubMed] [Google Scholar]
- [70].Dooley KE, Park JG, Swindells S, et al. Safety, tolerability, and pharmacokinetic interactions of the antituberculous agent TMC207 (bedaquiline) with efavirenz in healthy volunteers: AIDS Clinical Trials Group Study A5267. J Acquir Immune Defic Syndr. 2012;59(5):455–62. doi: 10.1097/QAI.0b013e3182410503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Huitric E, Verhasselt P, Koul A, Andries K, Hoffner S, Andersson DI. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2010;54(3):1022–8. doi: 10.1128/AAC.01611-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ashtekar DR, Costa-Perira R, Nagrajan K, Vishvanathan N, Bhatt AD, Rittel W. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1993;37(2):183–6. doi: 10.1128/aac.37.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stover CK, Warrener P, VanDevanter DR, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405(6789):962–6. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- [74].Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3(11):e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mukherjee T, Boshoff H. Nitroimidazoles for the treatment of TB: past, present and future. Future Med Chem. 2011;3(11):1427–54. doi: 10.4155/fmc.11.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tasneen R, Tyagi S, Williams K, Grosset J. Nuermberger E. Enhanced bactericidal activity of rifampin and/or pyrazinamide when combined with PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother. 2008;52(10):3664–8. doi: 10.1128/AAC.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54(8):3402–7. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–60. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- [79].Tasneen R, Li SY, Peloquin CA, et al. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55(12):5485–92. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Williams K, Minkowski A, Amoabeng O, et al. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother. 2012;56(6):3114–20. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Diacon AH, Dawson R, von Groote-Bidlingmaier F, et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet. 2012;380(9846):986–93. doi: 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- [82].Dietze R, Hadad DJ, McGee B, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008;178(11):1180–5. doi: 10.1164/rccm.200806-892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367(16):1508–18. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shaw KJ, Barbachyn MR. The oxazolidinones: past, present, and future. Ann N Y Acad Sci. 2011;1241:48–70. doi: 10.1111/j.1749-6632.2011.06330.x. [DOI] [PubMed] [Google Scholar]
- [85].Protopopova M, Hanrahan C, Nikonenko B, et al. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J Antimicrob Chemother. 2005;56(5):968–74. doi: 10.1093/jac/dki319. [DOI] [PubMed] [Google Scholar]
- [86].Nikonenko BV, Protopopova M, Samala R, Einck L, Nacy CA. Drug therapy of experimental tuberculosis (TB): improved outcome by combining SQ109, a new diamine antibiotic, with existing TB drugs. Antimicrob Agents Chemother. 2007;51(4):1563–5. doi: 10.1128/AAC.01326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Reddy VM, Einck L, Andries K, Nacy CA. In vitro interactions between new antitubercular drug candidates SQ109 and TMC207. Antimicrob Agents Chemother. 2010;54(7):2840–6. doi: 10.1128/AAC.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chambers HF, Kocagöz T, Sipit T, Turner J, Hopewell PC. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis. 1998;26(4):874–7. doi: 10.1086/513945. [DOI] [PubMed] [Google Scholar]
- [89].Donald PR, Sirgel FA, Venter A, et al. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand J Infect Dis. 2001;33(6):466–9. doi: 10.1080/00365540152029954. [DOI] [PubMed] [Google Scholar]
- [90].Payen MC, De Wit S, Martin C, et al. Clinical use of the meropenem-clavulanate combination for extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16(4):558–60. doi: 10.5588/ijtld.11.0414. [DOI] [PubMed] [Google Scholar]
- [91].Grosset JH, Singer TG, Bishai WR. New drugs for the treatment of tuberculosis: hope and reality. Int J Tuberc Lung Dis. 2012;16(8):1005–14. doi: 10.5588/ijtld.12.0277. [DOI] [PubMed] [Google Scholar]
- [92].Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103(3-5):793–8. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- [93].Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–50. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- [95].Sloan DJ, Davies GR. Anti-tuberculous chemotherapy: Serial Sputum Colony Counting in development, in Progress in Respiratory Research. Karger; Basel: 2011. [Google Scholar]