Abstract
Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) activates the fibroblast growth factor-inducible-14 (Fn14) receptor. TWEAK has actions on intrinsic kidney cells and on inflammatory cells of potential pathophysiological relevance. The effects of TWEAK in tubular cells have been explored in most detail. In cultured murine tubular cells TWEAK induces the expression of inflammatory cytokines, downregulates the expression of Klotho, is mitogenic, and in the presence of sensitizing agents promotes apoptosis. Similar actions were observed on glomerular mesangial cells. In vivo TWEAK actions on healthy kidneys mimic cell culture observations.
Increased expression of TWEAK and Fn14 was reported in human and experimental acute and chronic kidney injury. The role of TWEAK/Fn14 in kidney injury has been demonstrated in non-inflammatory compensatory renal growth, acute kidney injury and chronic kidney disease of immune and non-immune origin, including hyperlipidaemic nephropathy, lupus nephritis (LN) and anti-GBM nephritis. The nephroprotective effect of TWEAK or Fn14 targeting in immune-mediated kidney injury is the result of protection from TWEAK-induced injury of renal intrinsic cells, not from interference with the immune response.
A phase I dose-ranging clinical trial demonstrated the safety of anti-TWEAK antibodies in humans. A phase II randomized placebo-controlled clinical trial exploring the efficacy, safety and tolerability of neutralizing anti-TWEAK antibodies as a tissue protection strategy in LN is ongoing. The eventual success of this trial may expand the range of kidney diseases in which TWEAK targeting should be explored.
Keywords: apoptosis, clinical trials, fibrosis, inflammation, necroptosis, proteinuria
INTRODUCTION
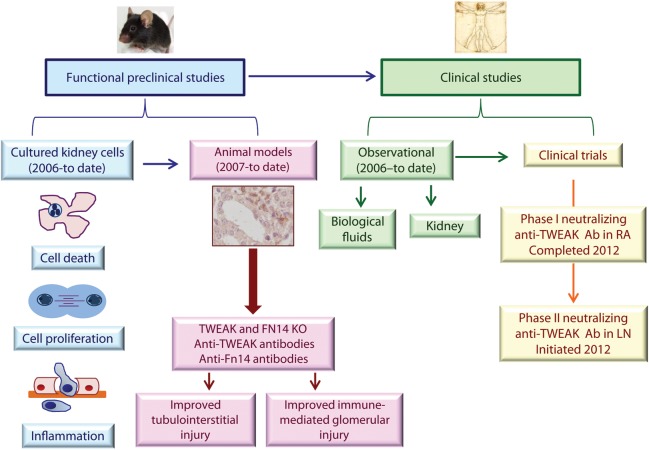
Tumour necrosis factor-like weak inducer of apoptosis [TWEAK, Apo3L, tumour necrosis factor superfamily (TNFSF)12] is a cytokine that belongs to the TNFSF that activates Fibroblast growth factor-inducible-14 (Fn14, TWEAK receptor, TNFRSF12A, CD266), a TNF receptor superfamily (TNFRSF) protein [1–4]. In recent years, evidence has accumulated supporting a role for TWEAK activation of intrinsic renal cell Fn14 receptors in the pathogenesis of acute and chronic kidney injury, glomerular and tubulointerstitial damage and non-immune and immune-mediated kidney disease. This has led to the launch of a clinical trial of neutralizing anti-TWEAK antibodies in human lupus nephritis (LN). We now review the pathophysiology of TWEAK/Fn14 signalling and the preclinical and clinical data on the involvement of this system in kidney disease (Figure 1).
FIGURE 1:
Progress in the study of TWEAK as a therapeutic target in kidney injury. Understanding the role and regulation of TWEAK and Fn14 in kidney injury has progressed very rapidly since the early functional studies published in 2006 to clinical trials in kidney disease launched in 2012. RA, rheumatoid arthritis; LN, lupus nephritis; Ab, antibody.
TWEAK AND ITS RECEPTOR
The human TWEAK gene encodes a type II transmembrane glycoprotein of 249 amino acids. The C-terminal extracellular TNF homology domain participates in self-trimerization and receptor binding [5], and contains two furin cleavage sites. Proteolytical processing of membrane TWEAK generates soluble TWEAK (156-amino acids) [5, 6]. The N-terminal cytoplasmatic domain has a potential protein kinase C phosphorylation site, and a furin recognition site, suggesting that full-length TWEAK can be processed intracellularly [5, 7, 8]. Moreover, TWEAK has nuclear localization sequences and may localize to nuclei, but the biological significance is unknown [8]. TWEAK is broadly expressed. In the kidneys, both resident kidney cells and infiltrating leucocytes express TWEAK [1, 9–11].
Fn14 is the only characterized signal-transducing TWEAK receptor. Both soluble and membrane TWEAK activate Fn14 [6, 12]. Fn14 was originally described as an immediate-early response gene regulated by growth factors in fibroblasts [13]. The human Fn14 gene encodes a type I transmembrane protein (129 amino acids) that is processed into a mature protein (102 amino acids). The extracellular domain (53 amino acids) contains a cystein-rich domain necessary for TWEAK binding [14, 15]. In contrast to many TNFRSF members, the intracellular domain (29 amino acids) of Fn14 lacks a death domain. However, it contains TNFR-associated factor (TRAF)-binding sites [16]. TWEAK can also bind the monocyte/macrophage scavenger receptor CD163. TWEAK binding to CD163 results in TWEAK internalization, but not in signalling. This suggests that CD163 behaves as a TWEAK scavenger [17, 18].
In healthy tissues Fn14 expression is low [13]. However, cellular Fn14 levels are increased in response to stress or injury [19]. In the kidney tubular cells, mesangial cells and podocytes express Fn14 [20, 21]. Moreover, renal infiltrating cells, such as macrophages, may express Fn14 and CD163 [22, 23].
TWEAK regulates processes with potential physiopathological relevance for kidney injury such as cell death, proliferation, differentiation, migration, inflammation, neoangiogenesis and tissue regeneration. Fn14 binding to TWEAK leads to TRAF2 and TRAF5 recruitment and activation of intracellular signalling pathways [24–26]. The specific signalling pathways activated by TWEAK/Fn14 system and the outcome of TWEAK binding depend on cell type, cell state and the microenvironment [19].
TWEAK has a potential role in injury of different organs, including the central nervous system, liver, gut, the vasculature, skeletal muscle, heart and kidneys [26–28].
TWEAK HAS MITOGENIC, LETHAL AND PROINFLAMMATORY ACTIONS ON CULTURED RENAL CELLS
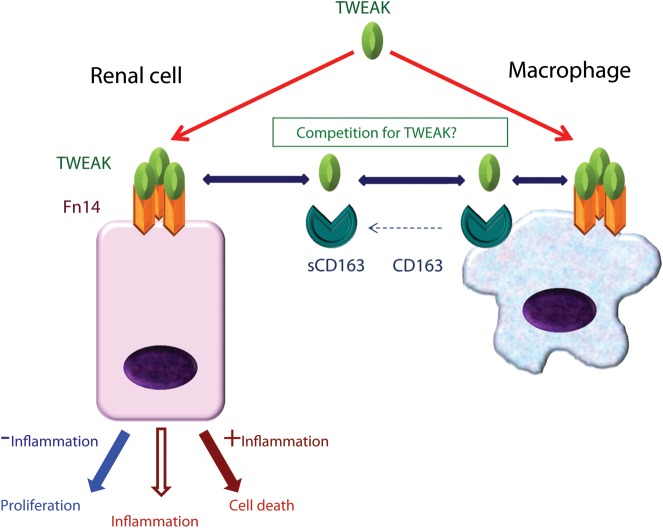
TWEAK has actions on intrinsic kidney cells and on inflammatory cells of potential pathophysiological relevance (Figure 2).
FIGURE 2:
TWEAK and TWEAK receptors. TWEAK binds Fn14 and transduces proinflammatory signals in kidney cells. In the absence of inflammation TWEAK may also induce cell proliferation, while in the presence of inflammation it promotes cell death. Chimeric mouse studies have established a key role of renal cell Fn14 in kidney injury. Macrophages also express Fn14 and may respond to TWEAK. In addition, they express CD163, which may be released in a soluble form and scavenges TWEAK. The precise role of CD163 in TWEAK dynamics is not fully understood. There is not enough information on the site of Fn14 expression in polarized epithelia.
Several intrinsic renal cells respond to TWEAK through Fn14 receptor signalling. The effects of TWEAK in tubular cells have been explored in most detail. In contrast, there is very little information on TWEAK and podocytes or kidney fibroblasts.
In cultured murine tubular cells TWEAK activates several signalling pathways. Thus, TWEAK activates mitogen-activated protein kinases (MAPK) such as extracellular signal-regulated kinase1/2 (ERK1/2) and p38 MAPK, phosphoinositide 3-kinase/Akt, JAK2 kinase and canonical or non-canonical NFκB activation [29–32]. As a result of canonical NFκB pathway activation and p65/RelA nuclear translocation, TWEAK induces the expression of inflammatory cytokines, such as MCP-1, IL-6, RANTES and CXCL16 and downregulates the expression of Klotho [30, 33, 34]. TWEAK also activates the non-canonical NFκB pathway, inducing the expression of CCL21 and CCL19 [32]. The proinflammatory effect of TWEAK on cultured tubular cells is enhanced by the simultaneous presence in the cell environment of additional inflammatory mediators, such as CXCL16, TNFα and interferon-γ [29, 33].
TWEAK has a proliferative effect in quiescent renal tubular cells [31]. This effect is modulated by the cell microenvironment and is increased by factors present in serum that enhance Fn14 expression [31]. TWEAK-induced tubular cell proliferation requires ERK1/2, p38 MAPK, phosphoinositide 3-kinase/Akt and NFκB in a manner that disruption of one of them prevents proliferation [31].
TWEAK alone does not promote renal tubular cell apoptosis. However, co-stimulation with sensitizing agents present in injured kidneys, such as TNFα and interferon-γ, leads to tubular cell apoptosis [20]. Apoptosis induced by TWEAK/TNFα/interferon-γ recruits the mitochondrial apoptosis pathway [20]. Both the time course and the response to caspase inhibitors differ from TNFα-induced apoptosis in the same cells [20, 35]. The combination of cytokines accelerates cell death. Caspase inhibition prevents features of TWEAK/TNFα/interferon-γ-induced apoptosis but increases overall cell death through a reactive oxygen species-dependent necrotic pathway [20]. Studies are underway to further assign this form of cell death to one of the known regulated necrosis forms of death [36].
TWEAK also induced the NFκB-dependent expression of inflammatory mediators, such as MCP-1, RANTES, CXCL10 and CXCL1 in human glomerular mesangial cells [21, 37]. In human glomerular mesangial cells, TWEAK-induced more prolonged NFκB p65/RelA phosphorylation than TNFα [21]. TWEAK promoted proliferation in humans but not in murine mesangial cells [37]. TWEAK alone did not induce human mesangial cell death, but the combination of TWEAK and interferon-γ increased apoptosis [21, 37].
Podocytes are also TWEAK responsive. TWEAK stimulation of human and murine podocytes induces a proinflammatory response via the activation of the NFκB signalling pathway [21] (Sanchez-Niño et al., JASN Abstr Suppl 2011; 656).
TWEAK AND INFLAMMATORY CELLS
TWEAK and Fn14 have been reported to be expressed by NK cells, macrophages, dendritic cells and Th17 cells [38, 39], but not by T and B cells [38, 40, 41]. Fn14 was not detected in peripheral blood mononuclear cells [42]. In addition, monocytes and macrophages express CD163 that can scavenge TWEAK [17]. A variety of actions of TWEAK on leucocytes have been reported. In human NK cells and macrophages, TWEAK induced prolonged p65 phosphorylation, inhibited STAT-1 and repressed interferon-γ and IL-12 through HDAC-1 activation [38]. In human macrophage-like THP-1 cells, TWEAK induced the expression of inflammatory mediators, such as IL-6, MCP-1, IL-8 and MMP-9 [11]. In naïve murine T cells, TWEAK promoted Th17 differentiation [39]. In addition, TWEAK has been reported to contribute to autologous human monocyte death induced by lupus T cells [10] and to human H9 CD4+ T cell line death induced by monocyte-derived dendritic cells [43]. Fn14 KO mice had milder chronic colitis induced by a hapten. Reduced epithelial cell synthesis of thymic stromal lymphopoietin appeared to contribute to reduced inflammation, fibrosis and T helper 2-type immunity [44]. Adaptive immunity defects described in a TWEAK KO mouse strain [38] were not observed in TWEAK KO mice generated by other authors [19]. Thus, the overall contribution of leucocytes to TWEAK availability and the in vivo significance of TWEAK effects on leucocytes are unclear.
IN VIVO TWEAK ACTIONS ON HEALTHY KIDNEYS MIMIC CELL CULTURE OBSERVATIONS
TWEAK actions in vivo in healthy kidneys have been explored in mice receiving a single intraperitoneal or intravenous recombinant TWEAK dose [21, 30–32, 37]. Systemic TWEAK-induced tubulointerstitial inflammation decreases kidney Klotho expression and promotes tubular cell proliferation within 4–24 h [31, 34]. TWEAK activated canonical (RelA) and non-canonical (RelB/p52) NFκB pathways in healthy kidney, resulting in early (4 h) RelA and delayed (24 h) RelB and p52 nuclear translocation in tubular cells [30, 32], and it increased total kidney MCP-1, IL-6, RANTES, CXCL16, CCL21, IP-10 and VCAM-1 expression [21, 30, 32, 33, 37]. Immunohistochemistry identified tubular cells as expressing inflammatory mediators and disclosed increased interstitial macrophages and CD3+ cells [21, 30, 32, 33, 37].
Systemic injection of TWEAK also increased the number of proliferating kidney cells assessed as tubular PCNA+ or Ki-67+ cells or Ki-67+ glomerular cells [21, 31]. However, at the times and doses tested TWEAK did not induce cell death in healthy mouse kidneys. This is an expected result since TWEAK-induced renal cell death requires a concomitant inflammatory microenvironment [20, 21, 37].
TWEAK PROMOTES ACUTE AND CHRONIC KIDNEY DISEASE
The potential role of TWEAK and Fn14 in kidney injury has been explored in non-inflammatory compensatory renal growth, non-immune tubulointerstitial kidney injury and immune-mediated kidney injury (Table 1). However, the role of TWEAK in non-immune glomerular injury remains insufficiently studied.
Table 1.
TWEAK targeting and kidney injury: animal models
| Experimental model | Target | Results | References |
|---|---|---|---|
| Non-immune kidney injury | |||
| Acute kidney injury (Folic acid) | TWEAK | ↑Renal function | [30] |
| ↓Interstitial inflammation | [31] | ||
| ↓Tubular cell death | [32] | ||
| ↓Tubular cell proliferation | [33] | ||
| ↑Klotho | [34] | ||
| AKI-to-chronic injury (I/R) | Fn14 | ↑Renal function | [49] |
| ↓Interstitial inflammation | |||
| ↓Tubular cell ell death | |||
| ↓Residual interstitial fibrosis | |||
| Chronic kidney injury(hyperlipidaemia-induced kidney injury in ApoE −/− mice) | TWEAK | ↓Inflammation | [50] |
| ↓Histological injury | |||
| CKD: unilateral ureteral obstruction | TWEAK | decreased inflammation/fibrosis | [51] |
| Immune injury | |||
| SLE (cGVH model) | TWEAK or Fn14 | = Titres autoantibodies | [60] |
| ↓Proteinuria | |||
| ↓Inflammation | |||
| ↓Glomerular IgG deposition | |||
| Fn14 in haematopoietic cells | = Initial nephritis | [22] | |
| Fn14 in kidney resident cells | Delayed nephritis | [22] | |
| Nephrotoxic serum nephritis (NTN) | Fn14 | ↓Proteinuria | [61] |
| ↓Histological renal injury | |||
| TWEAK | = Titres autoantibodies | [61] | |
| ↓IgG deposition | |||
| ↓Histological renal injury | |||
| ↓Proteinuria | |||
| ↓Tubulointerstitial fibrosis | |||
| ↓Inflammation | |||
| Non-inflammatory kidney regeneration | |||
| Uninephrectomy-induced renal hyperplasia | TWEAK | ↓Tubular cell proliferation | [31] |
SLE, systemic lupus erythematosus; cGVH model, chronic graft-versus-host model.
Non-inflammatory compensatory renal growth
Compensatory remnant kidney hypertrophy and hyperplasia follow unilateral nephrectomy. Clinical examples include nephrectomies for malignant tumour or living kidney donation and kidney transplant recipients [45]. Compensatory renal growth is thought to be a response to poorly characterized growth factors, including growth hormone [46]. TWEAK promotes remnant kidney growth following unilateral nephrectomy in mice [31]. Indeed, systemically administered TWEAK increased tubular cell proliferation in the remnant kidney, while TWEAK knockout mice had decreased remnant kidney tubular cell proliferation and kidney size. Tubular cell Fn14 expression was upregulated in remnant kidneys and may sensitize tubular cells to TWEAK-induced proliferation. In this regard, the remnant kidney expression of inflammatory cytokines, such as TNFα and interferon-γ, was low [31]. Thus, compensatory kidney growth is a non-inflammatory model where the proliferative action of TWEAK on tubular cells can be fully appreciated in vivo, confirming the relevance of cell culture data. A role for TWEAK/Fn14 in tissue regeneration has been observed in other organs, including the liver [47] and skeletal muscle [48]. This effect of TWEAK/Fn14, observed in precursor cells in some organs [47, 48], may eventually be modulated to develop regenerative therapies in clinical situations characterized by an acute non-inflammatory loss of kidney mass or in tissue engineering.
Non-immune kidney injury
Increased expression of TWEAK and Fn14 has been observed in human and experimental non-immune tubulointerstitial kidney injury [33, 49]. In addition, there is functional in vivo evidence for a role of TWEAK/Fn14 in acute kidney injury (AKI) and chronic kidney disease (CKD) of non-immune origin [30, 32–34, 50, 51].
Tubular cell Fn14 upregulation was observed within 24 h in experimental models of AKI induced by a folic acid overdose or by renal ischaemia–reperfusion injury (IRI). In folic acid-induced AKI, increased Fn14 expression persisted longer (up to 3 days) than in IRI (up to 1 day) [20, 49]. Kidney TWEAK was also increased in experimental renal injury, but to a lesser extent than Fn14 [20, 49]. Increased tubular Fn14 has also been observed in human ischaemic AKI and in acute or chronic human tubulointerstitial inflammation associated with glomerular injury or caused by acute tubulointerstitial nephritis [33, 49].
Functional studies in experimental models have characterized TWEAK/Fn14 as a therapeutic target in AKI. Targeting TWEAK with neutralizing anti-TWEAK antibodies, the use of TWEAK knock-out mice or targeting Fn14 by blocking anti-Fn14 antibodies improved histological and functional features of AKI [1, 30–34, 49].
Better preserved renal function, histological tubular injury and kidney Klotho expression, as well as reduced chemokine expression, tubular cell apoptosis, compensatory tubular cell proliferation and interstitial leucocyte infiltration were observed in mice with folic acid-induced AKI treated with neutralizing anti-TWEAK antibodies or in TWEAK knock-out mice (TWEAK KO) [1, 30–34, 49].
Seven days after induction of AKI, renal function had recovered in control and TWEAK-targeted mice, suggesting that the decreased tubular cell proliferation was secondary to the lower initial injury and did not compromise recovery of kidney function [1].
Fn14 blockade in renal IRI decreased peak serum creatinine and urea (at 24 h), tubular injury, inflammation, apoptosis (at 24 h) and residual fibrosis (at 30 days) [49]). This study addressed the transition from AKI to CKD and indicated that protection from a transient insult by TWEAK targeting resulted in longer term benefits over renal fibrosis. However, it did not explore potential specific anti-fibrotic functions of TWEAK/Fn14 targeting, or the consequences of TWEAK/Fn14 targeting when the kidney insult is persistent. A recent report revealed that TWEAK KO mice were protected from kidney fibrosis resulting from persistent unilateral ureteral obstruction [51].
In hyperlipidaemic ApoE−/− mice with non-immune chronic kidney injury repeated TWEAK administration activated kidney cell NFκB and increased the severity of tubulointerstitial and glomerular injury and inflammation [50]. In contrast, neutralizing anti-TWEAK antibodies improved these parameters [50].
Immune-mediated glomerular injury
Lu et al. [52] examined the expression of TWEAK and Fn14 in kidney biopsies obtained from LN patients. As compared with controls, lupus patients had significantly higher glomerular and tubulointerstitial mRNA expression of both TWEAK and Fn14. Moreover, TWEAK expression was significantly higher in class V than in class IV nephritis, and was inversely correlated with the activity index but not SLEDAI score, anti-DNA antibody titres, or complement levels. In a follow-up study, Lu et al. [53] looked at changes in TWEAK and Fn14 expression between an initial and a repeat kidney biopsy, and found that tubulointerstitial TWEAK, glomerular TWEAK and glomerular Fn14 increased when the histology evolved from membranous to proliferative nephritis. In contrast, glomerular Fn14 decreased when the pattern changed from proliferative to membranous histopathology. Glomerular Fn14 and tubulointerstitial TWEAK correlated with the renal histology index. Although the findings in these particular studies are not entirely concordant, the significant changes found in the expression of this pathway, and between histological classes, do support a role for TWEAK and Fn14 in LN pathogenesis. Finally, Zhi-Chun et al. [54] reported that patients with LN also display significantly higher levels of TWEAK in PBMC, although this finding was not replicated [55].
We [56] and others [55] found that serum TWEAK levels cannot distinguish between lupus patients with and without nephritis. Since inflammatory mediators produced locally in the kidney are important in pathogenesis and urinary levels can closely reflect renal production, we investigated the use of urinary TWEAK (uTWEAK) as a possible LN biomarker. In a cross-sectional study, we found that uTWEAK levels were significantly higher in patients with renal involvement and correlated with the renal SLEDAI score (rSLEDAI). Moreover, renal flares were associated with significantly higher uTWEAK levels. Significant positive correlations were found between uTWEAK and serum anti-DNA antibody titres and urinary MCP-1, while a negative correlation was found with serum C3 and C4 [57]. We confirmed the potential of uTWEAK as an LN biomarker in a subsequent study as well [56]. uTWEAK levels distinguished between lupus patients with nephritis and lupus patients without nephritis, healthy individuals and RA controls, and correlated with the rSLEDAI. In lupus patients followed longitudinally, a significant association was found between uTWEAK levels and renal disease activity over time, with uTWEAK levels peaking at the time of flare. El-shehaby et al. [58] similarly found significantly higher uTWEAK in LN when compared with lupus patients without nephritis or healthy controls. While uTWEAK levels did not differentiate between the various histopathological classes in this study, they did show a good correlation with rSLEDAI. Finally, Xuejing et al. [59] found a similar association between uTWEAK and active LN. Furthermore, significant correlations were found between uTWEAK with glomerular and tubulointerstitial activity indices, but not with chronicity.
Based upon the proinflammatory effects of TWEAK on murine and resident kidney cells, the preliminary studies in humans, and an observation that kidney (but not splenic) expression of TWEAK and Fn14 increases with disease progression in a spontaneous murine lupus model (the MRL-lpr/lpr strain) [Xia et al., Arthritis Rheum 63(10) (Suppl.): S212–S213 (abstract)], we hypothesized that TWEAK/Fn14 interactions are instrumental in LN pathogenesis. Furthermore, this pathway may be a novel target for therapeutic intervention. To examine this question, we used two models of antibody mediated nephritis that share many similarities with spontaneous disease. In the cGVH model [60], injection of MHC-mismatched bm12 splenocytes into non-autoimmune C57Bl6 mice induces an alloreactive reaction. This immune response promotes the activation of recipient B cells, with production of autoantibodies and the development of nephritis. In the second animal model, nephrotoxic serum nephritis, passive transfer of preformed rabbit anti-mouse glomerular antibodies induces a necrotizing proliferative glomerulonephritis that is very similar histologically to LN [61]. Via a genetic approach, we found that both Fn14 deficient C57Bl/6 recipients of bm12 splenocytes, as well as Fn14 knockout 129/SvJ mice injected with nephrotoxic serum, were significantly less susceptible to the development of nephritis than wild-type, Fn14 sufficient littermates. Furthermore, an identical result was achieved pharmacologically, where nephritis was attenuated in those Fn14 wild-type mice treated with an anti-TWEAK mAb. The adaptive immune response was not affected by anti-TWEAK treatment; therefore, the most likely mechanism of protection was through the observed reduction in downstream targets of TWEAK/Fn14 signalling, particularly chemokines and other inflammatory mediators.
TWEAK, INFLAMMATION AND AGEING
CKD is associated with accelerated ageing and premature death [62]. The reason for this association is probably multifactorial, but inflammation may play a prominent role as suggested by observational studies linking inflammatory biomarkers to adverse patient outcomes [63, 64]. In this regard, higher than expected circulating TWEAK levels were associated with accelerated mortality, especially in the context of systemic inflammation [65]. A recent hypothesis suggests that CKD is a state of Klotho deficiency and that Klotho deficiency might contribute to accelerated ageing in uraemia [62]. Klotho is an anti-ageing hormone with anti-inflammatory properties, which is highly expressed in tubular renal cells [34, 66, 67]. TWEAK reduces renal Klotho expression through canonical NFκB RelA activation and histone H3 and H4 deacetylation at the Klotho promoter [34]. Indeed, TWEAK was a key contributor to kidney Klotho downregulation in experimental AKI [34]. Thus, TWEAK/Fn14 system promotes both NFκB-mediated activation of inflammation pathways and suppression of anti-inflammatory/anti-ageing pathways and is a potential player in inflammation-associated ageing [68].
CLINICAL TRIALS EXPLORING TWEAK/FN14 TARGETING
Clinical trials exploring TWEAK/Fn14 targeting are underway in inflammation and cancer (Table 2). A phase I dose ranging clinical trial was recently completed in rheumatoid arthritis patients [69]. A single intravenous dose of neutralizing anti-TWEAK antibodies resulted in undetectable serum TWEAK levels for up to one month as well in a trend towards decreased levels of several circulating biomarkers of systemic inflammation. No serious adverse effects were recorded.
Table 2.
TWEAK/Fn14 targeting in clinical trials
| ClinicalTrials.gov Identifier | Agent | Target | Phase | Disease | Status | Company |
|---|---|---|---|---|---|---|
| NCT00771329 | BIIB023 | Neutralizing anti-TWEAK | 1 | Rheumatoid arthritis | Completed | Biogen Idec |
| NCT01499355 | BIIB023 | Neutralizing anti-TWEAK | 2 | Lupus nephritis | Recruiting | Biogen Idec |
| NCT01383733 | RO5458640 | Anti-TWEAK | 1 | Advanced solid tumours | Ongoing, not recruiting | Hoffmann-La Roche |
| NCT00738764 | PDL192 | Anti-Fn14 | 1 | Advanced solid tumours | Completed | Abbott |
http://www.clinicaltrials.gov/ct2/results?term=tweak&Search=Search (5 May 2013, date last accessed).
A phase II randomized placebo-controlled clinical trial (ATLAS, Anti-Tweak in Lupus Nephritis Patients) is exploring the efficacy, safety and tolerability of the BIIB023 anti-TWEAK antibody as add-on therapy in class III/IV LN patients that fail to undergo a complete remission within 3 months of initial therapy with steroids and mycophenolate mofetil [70]. The primary outcome measure is the proportion of subjects with complete and partial renal response from baseline to Week 52. ATLAS was launched in 2012 and is expected to recruit 300 patients. Results are expected no earlier than end of 2014.
In addition, several tumours express Fn14 and TWEAK may induce apoptosis, depending on cell type and the environment [71]. Both agonistic anti-Fn14 that recruit antibody-dependent cellular cytotoxicity and neutralizing anti-TWEAK antibodies are being studied in human clinical trials to treat cancer [72–75]. A potential side effect of Fn14 activation would be nephrotoxicity in those patients with prior kidney injury and upregulated kidney cell Fn14.
THE FUTURE OF TWEAK TARGETING IN KIDNEY DISEASE
An increasing body of evidence suggests that TWEAK has a deleterious role in kidney injury associated with inflammation through actions on intrinsic renal cells that include further promotion of inflammation and cell death. Based on evidence of beneficial effects of TWEAK targeting in preclinical models of kidney injury, a clinical trial is exploring the contribution of TWEAK to LN. The eventual success of this clinical trial may expand the range of kidney disease in which TWEAK should be explored as a therapeutic target. In addition, a potential role of TWEAK in kidney regeneration should be explored. Finally, a better understanding of TWEAK signalling and its role in cytokine networks may identify therapeutic strategies beyond direct TWEAK/Fn14 targeting. Thus, a nanoconjugate molecule prevented TWEAK-induced cell death and inflammatory signalling in diverse cell types, including tubular cells [29, 76].
ACKNOWLEDGEMENTS
Grant support: FIS PS09/00447, FIS PI10/00072, ISCIII-RETIC, REDinREN/RD06/0016, FEDER funds, RD12/0021, Comunidad de Madrid/CIFRA S2010/BMD-2378, CIBERDEM, Fundacion Lilly and FRIAT. Salary support: FIS to MDSN (Sara Borrell), ABS (Miguel Servet). Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM) to A.O. These studies were supported by National Institute of Health (USA), AR048692 and DK090319, to C.P.
REFERENCES
- 1.Sanz AB, Sanchez-Niño MD, Ortiz A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011;80:708–718. doi: 10.1038/ki.2011.180. [DOI] [PubMed] [Google Scholar]
- 2.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohi T, Burkly LC. The TWEAK/Fn14 pathway as an aggravating and perpetuating factor in inflammatory diseases: focus on inflammatory bowel diseases. J Leukoc Biol. 2012;92:265–279. doi: 10.1189/jlb.0112042. [DOI] [PubMed] [Google Scholar]
- 4.Burkly LC, Michaelson JS, Zheng TS. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol Rev. 2011;244:99–114. doi: 10.1111/j.1600-065X.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 5.Chicheportiche Y, Bourdon PR, Xu H, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 6.Brown SA, Ghosh A, Winkles JA. Full-length, membrane-anchored TWEAK can function as a juxtacrine signaling molecule and activate the NF-kappaB pathway. J Biol Chem. 2010;285:17432–17441. doi: 10.1074/jbc.M110.131979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsters SA, Sheridan JP, Pitti RM, et al. Identification of a ligand for the death-domain-containing receptor Apo3. Curr Biol. 1998;8:525–528. doi: 10.1016/s0960-9822(98)70204-0. [DOI] [PubMed] [Google Scholar]
- 8.Baxter FO, Came PJ, Abell K, et al. IKKbeta/2 induces TWEAK and apoptosis in mammary epithelial cells. Development. 2006;133:3485–3494. doi: 10.1242/dev.02502. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama M, Kayagaki N, Yamaguchi N, et al. Involvement of TWEAK in interferon gamma-stimulated monocyte cytotoxicity. J Exp Med. 2000;192:1373–1380. doi: 10.1084/jem.192.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan MJ, Lewis EE, Shelden EA, et al. The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169:6020–6029. doi: 10.4049/jimmunol.169.10.6020. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Kang YJ, Kim WJ, et al. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J. 2004;68:396–399. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- 12.Roos C, Wicovsky A, Müller N, et al. Soluble and transmembrane TNF-like weak inducer of apoptosis differentially activate the classical and noncanonical NF-kappa B pathway. J Immunol. 2010;185:1593–1605. doi: 10.4049/jimmunol.0903555. [DOI] [PubMed] [Google Scholar]
- 13.Meighan-Mantha RL, Hsu DK, Guo Y, et al. The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem. 1999;274:33166–33176. doi: 10.1074/jbc.274.46.33166. [DOI] [PubMed] [Google Scholar]
- 14.Brown SA, Hanscom HN, Vu H, et al. TWEAK Binding to the Fn14 cysteine-rich domain depends on charged residues located in both the A1 and D2 modules. Biochem J. 2006;397:297–304. doi: 10.1042/BJ20051362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He F, Dang W, Saito K, et al. Solution structure of the cysteine-rich domain in Fn14, a member of the tumor necrosis factor receptor superfamily. Protein Sci. 2009;18:650–656. doi: 10.1002/pro.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown SA, Richards CM, Hanscom HN, et al. The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J. 2003;371(Pt 2):395–403. doi: 10.1042/BJ20021730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bover LC, Cardó-Vila M, Kuniyasu A, et al. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J Immunol. 2007;178:8183–8194. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- 18.Moreno JA, Muñoz-García B, Martín-Ventura JL, et al. The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis. Atherosclerosis. 2009;207:103–110. doi: 10.1016/j.atherosclerosis.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Burkly LC, Michaelson JS, Hahm K, et al. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16. doi: 10.1016/j.cyto.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Justo P, Sanz AB, Sanchez-Niño MD, et al. Cytokine cooperation in renal tubular cell injury: the role of TWEAK. Kidney Int. 2006;70:1750–1758. doi: 10.1038/sj.ki.5001866. [DOI] [PubMed] [Google Scholar]
- 21.Gao HX, Campbell SR, Burkly LC, et al. TNF-like weak inducer of apoptosis (TWEAK) induces inflammatory and proliferative effects in human kidney cells. Cytokine. 2009;46:24–35. doi: 10.1016/j.cyto.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Molano A, Lakhani P, Aran A, et al. TWEAK Stimulation of kidney resident cells in the pathogenesis of graft versus host induced lupus nephritis. Immunol Lett. 2009;125:119–128. doi: 10.1016/j.imlet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Martín Cleary C, Moreno JA, Fernández B, et al. Glomerular haematuria, renal interstitial haemorrhage and acute kidney injury. Nephrol Dial Transplant. 2010;25:4103–4106. doi: 10.1093/ndt/gfq493. [DOI] [PubMed] [Google Scholar]
- 24.Saitoh T, Nakayama M, Nakano H, et al. TWEAK Induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem. 2003;278:36005–36012. doi: 10.1074/jbc.M304266200. [DOI] [PubMed] [Google Scholar]
- 25.Tran NL, McDonough WS, Savitch BA, et al. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J Biol Chem. 2005;280:3483–3492. doi: 10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- 26.Mittal A, Bhatnagar S, Kumar A, et al. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol. 2010;188:833–849. doi: 10.1083/jcb.200909117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirnitz-Parker JE, Viebahn CS, Jakubowski A, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52:291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 28.Haile WB, Echeverry R, Wu J, et al. The interaction between tumor necrosis factor-like weak inducer of apoptosis and its receptor fibroblast growth factor-inducible 14 promotes the recruitment of neutrophils into the ischemic brain. J Cereb Blood Flow Metab. 2010;30:1147–1156. doi: 10.1038/jcbfm.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ucero AC, Berzal S, Ocaña-Salceda C, et al. A polymeric nanomedicine diminishes inflammatory events in renal tubular cells. PLoS One. 2013;8:e51992. doi: 10.1371/journal.pone.0051992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz AB, Justo P, Sanchez-Niño MD, et al. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz AB, Sanchez-Niño MD, Izquierdo MC, et al. Tweak induces proliferation in renal tubular epithelium: a role in uninephrectomy induced renal hyperplasia. J Cell Mol Med. 2009;13(9B):3329–3342. doi: 10.1111/j.1582-4934.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanz AB, Sanchez-Niño MD, Izquierdo MC, et al. TWEAK Activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One. 2010;5:e8955. doi: 10.1371/journal.pone.0008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izquierdo MC, Sanz AB, Mezzano S, et al. TWEAK (tumor necrosis factor-like weak inducer of apoptosis) activates CXCL16 expression during renal tubulointerstitial inflammation. Kidney Int. 2012;81:1098–1107. doi: 10.1038/ki.2011.475. [DOI] [PubMed] [Google Scholar]
- 34.Moreno JA, Izquierdo MC, Sanchez-Niño MD, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz A, Lorz C, Catalán MP, et al. Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int. 2000;57:969–981. doi: 10.1046/j.1523-1755.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 36.Linkermann A, De Zen F, Weinberg J, et al. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant. 2012;27:3412–3419. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 37.Campbell S, Burkly LC, Gao HX, et al. Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J Immunol. 2006;176:1889–1898. doi: 10.4049/jimmunol.176.3.1889. [DOI] [PubMed] [Google Scholar]
- 38.Maecker H, Varfolomeev E, Kischkel F, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–944. doi: 10.1016/j.cell.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 39.Park JS, Park MK, Lee SY, et al. TWEAK Promotes the production of interleukin-17 in rheumatoid arthritis. Cytokine. 2012;60:143–149. doi: 10.1016/j.cyto.2012.06.285. [DOI] [PubMed] [Google Scholar]
- 40.van Kuijk AW, Wijbrandts CA, Vinkenoog M, et al. TWEAK and its receptor Fn14 in the synovium of patients with rheumatoid arthritis compared to psoriatic arthritis and its response to tumour necrosis factor blockade. Ann Rheum Dis. 2010;69:301–304. doi: 10.1136/ard.2008.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñoz-García B, Martín-Ventura JL, Martínez E, et al. Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: modulation by atorvastatin. Stroke. 2006;37:2044–2053. doi: 10.1161/01.STR.0000230648.00027.00. [DOI] [PubMed] [Google Scholar]
- 42.Desplat-Jégo S, Feuillet L, Creidy R, et al. TWEAK Is expressed at the cell surface of monocytes during multiple sclerosis. J Leukoc Biol. 2009;85:132–135. doi: 10.1189/jlb.0608347. [DOI] [PubMed] [Google Scholar]
- 43.Lichtner M, Marañón C, Vidalain PO, et al. HIV Type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res Hum Retroviruses. 2004;20:175–182. doi: 10.1089/088922204773004897. [DOI] [PubMed] [Google Scholar]
- 44.Son A, Oshio T, Kawamura YI, et al. TWEAK/Fn14 pathway promotes a T helper 2-type chronic colitis with fibrosis in mice. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.10. March 6. doi: 10.1038/mi.2013.10. [Epub ahead of print] PMID:23462911. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Langer WJ, Devish K, et al. Compensatory kidney growth in estrogen receptor-alpha null mice. Am J Physiol Renal Physiol. 2006;290:F319–F323. doi: 10.1152/ajprenal.00271.2005. [DOI] [PubMed] [Google Scholar]
- 46.Flyvbjerg A, Bennett WF, Rasch R, et al. Compensatory renal growth in uninephrectomized adult mice is growth hormone dependent. Kidney Int. 1999;56:2048–2054. doi: 10.1046/j.1523-1755.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 47.Jakubowski A, Ambrose C, Parr M, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115:2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girgenrath M, Weng S, Kostek CA, et al. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 2006;25:5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hotta K, Sho M, Yamato I, et al. Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int. 2011;79:179–188. doi: 10.1038/ki.2010.379. [DOI] [PubMed] [Google Scholar]
- 50.Muñoz-García B, Moreno JA, López-Franco O, et al. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:2061–2068. doi: 10.1161/ATVBAHA.109.194852. [DOI] [PubMed] [Google Scholar]
- 51.Ucero AC, Benito-Martin A, Fuentes-Calvo I, et al. TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta. 2013;1832:1744–1755. doi: 10.1016/j.bbadis.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 52.Lu J, Kwan BC, Lai FM, et al. Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrology (Carlton) 2011;16:426–432. doi: 10.1111/j.1440-1797.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- 53.Lu J, Szeto CC, Tam LS, et al. Relationship of intrarenal gene expression and the histological class of lupus nephritis—a study on repeat renal biopsy. J Rheumatol. 2012;39:1942–1947. doi: 10.3899/jrheum.120177. [DOI] [PubMed] [Google Scholar]
- 54.Zhi-Chun L, Qiao-Ling Z, Zhi-Qin L, et al. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) mediates p38 mitogen-activated protein kinase activation and signal transduction in peripheral blood mononuclear cells from patients with lupus nephritis. Inflammation. 2012;35:935–943. doi: 10.1007/s10753-011-9396-3. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Chen LL, Pan HF, et al. Expression of human tumor necrosis factor-like weak inducer of apoptosis in patients with systemic lupus erythematosus. Clin Rheumatol. 2012;31:335–339. doi: 10.1007/s10067-011-1865-4. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz N, Rubinstein T, Burkly LC, et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther. 2009;11:R143. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz N, Su L, Burkly LC, et al. Urinary TWEAK and the activity of lupus nephritis. J Autoimmun. 2006;27:242–250. doi: 10.1016/j.jaut.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 58.El-Shehaby A, Darweesh H, El-Khatib M, et al. Correlations of urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and IL-8 with lupus nephritis. J Clin Immunol. 2011;31:848–856. doi: 10.1007/s10875-011-9555-1. [DOI] [PubMed] [Google Scholar]
- 59.Xuejing Z, Jiazhen T, Jun L, et al. Urinary TWEAK level as a marker of lupus nephritis activity in 46 cases. J Biomed Biotechnol. 2012;2012:359647. doi: 10.1155/2012/359647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Z, Burkly LC, Campbell S, et al. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179:7949–7958. doi: 10.4049/jimmunol.179.11.7949. [DOI] [PubMed] [Google Scholar]
- 61.Xia Y, Campbell SR, Broder A, et al. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin Immunol. 2012;145:108–121. doi: 10.1016/j.clim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izquierdo MC, Perez-Gomez MV, Sanchez-Niño MD, et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant. 2012;27(Suppl. 4):iv6–i10. doi: 10.1093/ndt/gfs426. [DOI] [PubMed] [Google Scholar]
- 63.Elewa U, Sanchez-Niño MD, Martin-Cleary C, et al. Cardiovascular risk biomarkers in CKD: the inflammation link and the road less traveled. Int Urol Nephrol. 2012;44:1731–1744. doi: 10.1007/s11255-012-0271-4. [DOI] [PubMed] [Google Scholar]
- 64.Ortiz A, Massy ZA, Fliser D, et al. Clinical usefulness of novel prognostic biomarkers in patients on hemodialysis. Nat Rev Nephrol. 2012;8:141–150. doi: 10.1038/nrneph.2011.170. [DOI] [PubMed] [Google Scholar]
- 65.Carrero JJ, Ortiz A, Qureshi AR, et al. Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:110–118. doi: 10.2215/CJN.02790608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 67.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Izquierdo MC, Sanz AB, Sánchez-Niño MD, et al. Acute kidney injury transcriptomics unveils a relationship between inflammation and ageing. Nefrologia. 2012;32:715–723. doi: 10.3265/Nefrologia.pre2012.Aug.11667. [DOI] [PubMed] [Google Scholar]
- 69. http://clinicaltrials.gov/ct2/show/NCT00771329. 5 May 2013, date last accessed.
- 70. http://clinicaltrials.gov/ct2/show/NCT01499355. 14 April 2013, date last accessed.
- 71.Sanz AB, Sanchez-Niño MD, Carrasco S, et al. Inflammatory cytokines and survival factors from serum modulate tweak-induced apoptosis in PC-3 prostate cancer cells. PLoS One. 2012;7:e47440. doi: 10.1371/journal.pone.0047440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michaelson JS, Amatucci A, Kelly R, et al. Development of an Fn14 agonistic antibody as an anti-tumor agent. MAbs. 2011;3:362–375. doi: 10.4161/mabs.3.4.16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Culp PA, Choi D, Zhang Y, et al. Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms. Clin Cancer Res. 2010;16:497–508. doi: 10.1158/1078-0432.CCR-09-1929. [DOI] [PubMed] [Google Scholar]
- 74. http://clinicaltrials.gov/ct2/show/NCT00738764. 5 May 2013, date last accessed.
- 75. http://clinicaltrials.gov/ct2/show/NCT01383733. 5 May 2013, date last accessed.
- 76.Santamaría B, Benito-Martin A, Ucero AC, et al. A nanoconjugate Apaf-1 inhibitor protects mesothelial cells from cytokine-induced injury. PLoS One. 2009;4:e6634. doi: 10.1371/journal.pone.0006634. [DOI] [PMC free article] [PubMed] [Google Scholar]