Abstract
Background
Heart failure (HF) and obesity are associated with cognitive impairment. However, few studies have investigated the relationship between adiposity and cognitive functioning in HF for each sex, despite observed sex differences in HF prognosis. We tested the hypothesis that greater body mass index (BMI) would be associated with poorer cognitive functioning, especially in men, in sex-stratified analyses.
Methods and Results
Participants were 231 HF patients (34% female, 24% nonwhite, overall age 68.7 ± 7.3 years). Height and weight were used to compute BMI. A neuropsychology battery tested global cognitive function, memory, attention, and executive function. Composites were created using averages of age-adjusted scaled scores. Regressions adjusting for demographic and medical factors were conducted. The sample was predominantly overweight/obese (76.2%). For men, greater BMI predicted poorer attention (ΔR2 = 0.03; β = −0.18; P = .01) and executive function (ΔR2 = 0.02; β = −0.13; P = .04); these effects were largely driven by men with severe obesity (BMI ≥40 kg/m2). BMI did not predict memory (P = .69) or global cognitive functioning (P = .08). In women, greater BMI was not associated with any cognitive variable (all P ≥ .09).
Discussion
Higher BMI was associated with poorer attention and executive function in male HF patients, especially those with severe obesity. These patients may therefore have more difficulties with the HF treatment regimen and may have poorer outcomes.
Keywords: Attention, executive function, gender differences, obesity
Heart failure (HF) affects an estimated 5.1 million individuals in the United States.1 Approximately 50% of these HF patients die within = years of diagnosis,1 with men having poorer prognosis than women.2,3 Most HF patients can expect to have decreased quality of life4 and reduced functioning in their activities of daily living.5 In addition to these medical consequences, patients with HF are at increased risk for poor neurocognitive outcomes, including development of Alzheimer disease and other dementias.6 Less severe impairments in cognitive function are also found in up to 80% of HF patients with deficits observed across multiple cognitive domains, including attention, executive function, and memory.7,8
Another widespread disabling disease,9,10 obesity is prevalent in HF, with >40% of HF patients defined as obese (body mass index [BMI] ≥30 kg/m2).11 Similarly, obese individuals are 2 times more likely than their normal-weight peers to have HF.12 These high rates of comorbidity–combined with evidence that obesity predicts cognitive impairment in individuals without HF13–16—have encouraged researchers to explore how obesity and HF may act together to affect cognitive function.
One recent review17 posits that obesity likely contributes to the cognitive impairments observed in HF via several potential mechanisms, including increased vascular dysfunction, elevated adipokines, and inflammation. Unfortunately, few empirical studies have directly investigated the relationship between obesity indicators and cognitive functioning in HF populations. Of the available evidence, higher BMI has been found to predict poorer processing speed in patients with HF,18 as well as reduced attention/executive functioning and language.19 These findings may appear to contradict those in which overweight/obese persons with HF have been found to have lower risk for adverse outcomes than normal-weight individuals (ie, the “obesity paradox”).3 However, some authors speculate that these paradoxic findings are a statistical artifact resulting from the inadequacy of BMI as a measure of adiposity in older adults and/or HF patients as well as the failure of studies to include severely obese individuals owing to their elevated rate of premature mortality.20,21 Together, these findings suggest that the relationship between cognition and BMI is complex, especially with increasing age. However, the possibilities that obesity may confer unique risk for cognitive impairment in patients with HF and that obese patients may have even greater cognitive deficits than normal-weight patients should be fully explored.
A limitation of the extant obesity-HF-cognition literature is that sex-stratified analyses have not been conducted. Such analyses are important because male HF patients have poorer prognosis2,3 and differ from female HF patients in a number of prognostic factors that are potential confounders of the BMI—cognitive functioning relationship, such as age, ischemic vs nonischemic HF etiology, left ventricular function, and rate of atrial fibrillation.22 Therefore, we conducted sex-stratified analyses to determine whether BMI predicts cognitive functioning in HF patients by examining multiple cognitive domains, including attention, executive functioning, memory, and global cognitive functioning. Based on the literature, we hypothesized that greater BMI would be associated with poorer cognitive functioning and that men may have greater cognitive deficits than women, owing to higher rates of poor prognostic indicators in men.
Methods
Participants
The sample was 235 persons with HF enrolled in the larger ongoing Heart Failure Adherence, Behavior, and Cognition Study (Heart ABC).23 Study eligibility requirements were as follows: 1) age 50–85 years at enrollment; 2) documented systolic HF diagnosis within 36 months of study enrollment; 3) New York Heart Association (NYHA) functional class II or III of ≥3 months duration; 4) no cardiac surgery within past 3 months; 5) no history of neurologic disorder or injury (eg, Alzheimer disease, dementia, stroke, seizures); 6) no history of moderate or severe head injury; 7) no history of psychotic disorders, bipolar disorder, learning disorder, developmental disability, renal failure requiring dialysis, or untreated sleep apnea; 8) no substance abuse within the past 5 years; and 9) no current use of home tele-health monitoring program for HF. For the present study, 4 underweight participants (BMI < 18.5 kg/m2) were also excluded, owing to the link between malnutrition and cognitive impairment.24 Thus, the final sample was 231 participants (see Table 1 for characteristics).
Table 1.
Characteristics of Participants
| Total Sample (n = 231) | Men (n = 153) | Women (n = 78) | |
|---|---|---|---|
| Demographic factors | |||
| Age | 68.7 ± 9.4 | 69.3 ± 9.4 | 67.5 ± 9.3 |
| Female gender | 78 (33.8) | — | — |
| Nonwhite race/ethnicity | 121 (23.8) | 23 (15.0) | 32 (41.0)* |
| Education Level | |||
| 8th Grade or Less | 2 (.9) | 2 (1.3) | 0 (0.0) |
| 9th–11th Grade | 17 (7.4) | 6 (3.9) | 11 (14.1)* |
| High school | 64 (27.7) | 40 (26.1) | 24 (30.8) |
| Technical or trade school | 25 (10.8) | 15 (9.8) | 10 (12.8) |
| Some college | 66 (28.6) | 39 (25.5) | 27 (34.6) |
| Bachelor’s degree | 32 (13.9) | 27 (17.6) | 5 (6.4)* |
| Master’s degree | 25 (10.8) | 24 (15.7) | 1 (1.3)* |
| SES z-score | 0.32 ± 4.4 | .91 ± 4.4 | −.81 ± 4.1* |
| Medical and psychologic factors | |||
| Charlson comorbidity scorea | 3.32 ± 1.9 | 3.4 ± 1.9 | 3.1 ± 1.4 |
| NYHA functional class | |||
| I | 28 (12.1) | 23 (15.0) | 5 (6.4) |
| II | 53 (22.9) | 36 (23.5) | 17 (21.8) |
| III | 140 (60.6) | 87 (56.9) | 53 (67.9) |
| IV | 10 (4.3) | 7 (4.6) | 3 (3.8) |
| Patient Health Questionnaire–9 | 4.6 ± 5.1 | 4.1 ± 4.9 | 5.7 ± 5.4* |
| BMI categoryb | |||
| Normal weight (BMI 18.5–24.9 kg/m2) | 56 (24.2) | 35 (22.9) | 21 (26.9) |
| Overweight (BMI 25.0–29.9 kg/m2) | 66 (28.6) | 47 (30.7) | 19 (24.4) |
| Obese class I (BMI 30–34.9 kg/m2) | 61 (26.4) | 44 (28.8) | 17 (21.8) |
| Obese class II (BMI 35–39.9 kg/m2) | 30 (13.0) | 17 (11.1) | 13 (16.7) |
| Obese class III (BMI ≥40.0 kg/m2) | 18 (7.8) | 10 (6.5) | 8 (10.3) |
| BMI (kg/m2) | 30.3 ± 6.7 | 30.2 ± 6.4 | 30.6 ± 7.0 |
| Cognitive factors | |||
| Global cognitive function | 92.3 ± 6.4 | 92.1 ± 6.5 | 92.6 ± 6.4 |
| Estimated IQ from NAART | 110.9 ± 10.5 | 111.8 ± 10.0 | 109.3 ± 11.2 |
| Attention Composite Score | 8.5 ± 2.3 | 8.5 ± 2.2 | 8.6 ± 2.4 |
| Trails A scaled score | 8.0 ± 3.1 | 8.0 ± 3.0 | 7.9 ± 3.2 |
| Stroop Color scaled score | 8.1 ± 2.9 | 8.5 ± 2.8 | 9.0 ± 2.9 |
| Stroop Word scaled score | 8.7 ± 2.8 | 8.0 ± 2.8 | 8.3 ± 3.0 |
| Letter-Number Sequencing scaled score | 9.5 ± 3.1 | 9.6 ± 3.0 | 9.2 ± 3.1 |
| Executive function composite score | 8.4 ± 3.0 | 8.5 ± 2.9 | 8.1 ± 3.1 |
| Trails B scaled score | 7.9 ± 3.6 | 8.0 ± 3.5 | 7.7 ± 3.8 |
| Stroop Color-Word scaled score | 8.8 ± 3.1 | 9.0 ± 3.0 | 8.6 ± 3.3 |
| Memory composite score | 8.4 ± 2.0 | 8.3 ± 2.1 | 8.5 ± 2.0 |
| RAVLT Long Delay scaled score | 9.4 ± 2.7 | 8.9 ± 2.6 | 10.3 ± 2.8* |
| Complex Figure Long Delay scaled score | 7.4 ± 2.5 | 7.8 ± 2.5 | 6.7 ± 2.2* |
Continuous variables represented as mean ± SD, categoric variables as n (%). SES, socioeconomic status; NYHA, New York Heart Association; BMI, body mass index; NAART, North American Adult Reading Test; RAVLT, Rey Auditory Verbal Learning Test.
Percentage of participants who reported having diabetes (45%), myocardial infarction (52%), peripheral vascular disease (13%).
Underweight participants (n = 4) were excluded from analyses.
P < .05 for independent t test or chi-square test comparing men and women.
Measures
Body Mass Index
Participants’ BMI was calculated as kg/m2 using weight and height. For the Heart ABC study, all patients were given an electronic scale to use for home weighing. For the purpose of the present study, baseline weights were obtained from the electronic scale at visit 3. Patients’ most recent heights were self-reported at visit 3 or obtained from the medical record. For the present study, we used both continuous BMI as well as the BMI categories endorsed by the World Health Organization,25 including normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obese class I (30.0–34.9 kg/m2), obese class II (35.0–39.9 kg/m2), and obese class III (≥40 kg/m2).
Cognitive Functioning
Cognitive functioning across multiple domains was measured employing commonly used neuropsychologic tests with strong psychometric properties. The 4 cognitive domains were as follows:
1. Attention
The ability to attend to and process information was measured with the Trail Making Test A,26 Stoop Word and Color subtests,27 and Letter-Number Sequencing (LNS).28
2. Executive Function
The ability to reason, plan, problem solve, and inhibit was assessed with the Trail Making Test B26 and Stroop Color Word subtest.27
3. Memory
The ability to retain and recall verbal and visuospatial information was measured with the use of the Rey Auditory Verbal Learning Test Long Delay score29 and Rey Complex Figure Long Delay score.30
4. Global Cognitive Function
A broad assessment of general cognitive function was assessed with the use of the Modified Mini Mental State Examination.31
Age-adjusted scaled scores were calculated for each neuropsychology test in each domain. Scaled scores are standardized scores ranging from 1 to 19 with a mean of 10 and SD of 3. Scores of 7–13 represent average performance, whereas lower scores represent borderline (5–6), mild (4), moderate (3), and severe (≤2) cognitive impairment. The relevant tests were then averaged to create a composite score for the domains of attention, executive function, and memory. Global cognitive function was measured by a single test, so no composite was created. The composite scores and the global cognitive function score were used as the outcome variables in separate regressions.
Covariates
The following variables were included as covariates and potential confounders of any observed relationship between BMI and cognitive impairment: race (0 = white; 1 = nonwhite), education (1 = no schooling; 2 = ≤8th grade; 3 = 9th–11th grade; 4 = high school; 5 = technical or trade school; 6 = some college; 7 = bachelor’s degree; 8 = master’s degree), estimated IQ,32 socioeconomic status (SES), Charlson comorbidity index score,33 heart failure severity (as estimated by the 4 functional class levels of the NYHA,34 and depressive symptoms as measured by the Patient Health Questionnaire–9 (PHQ-9).35 The North American Adult Reading Test (NAART)32 was used to estimate participants’ IQs, with higher scores indicating higher IQ. SES was estimated with the use of subjects’ zip codes via a method similar to that described by Roux et al.36 Z-scores were calculated for the SES scores with the use of indicators of income and education for each zip code. Higher scores indicate higher SES. The Charlson comorbidity index is a summary score of several medical conditions, including diabetes, peripheral vascular disease, myocardial infarction, etc.33 Medical diagnoses are assigned points, with more severe conditions receiving higher points; thus, higher Charlson scores indicate a higher number and greater severity of medical comorbidities. The NYHA HF severity levels34 include the following: class I (mild), class II (mild), class III (moderate), and class IV (severe). The PHQ-9 assesses depressive symptom severity, with higher scores indicating greater severity.
Procedure
The Heart ABC study is an ongoing observational study conducted at 2 separate health care systems in northeastern Ohio. Patients with documented diagnoses of systolic HF were recruited from the cardiac inpatient units and outpatient practices. Each of the patients gave written informed consent to participate in the study. The Institutional Review Boards of Kent State University, Summa Health Systems, and Case Western Reserve University approved all study procedures. After recruitment and consent, a research assistant conducted a series of self-report questionnaires and neuropsychologic testing (visit 1) either at the medical center or at the patient’s home. The research assistant also arranged visits 2 and 3 within the next 2 weeks to drop off materials at the patient’s home for the larger study. Visits 1–3 are considered to be the baseline period for this study and occurred within 2–4 weeks of study enrollment.
Statistical Analyses
Independent t tests and chi-square analyses were used to assess differences between men and women and between obese and non-obese patients in the study variables. To examine the associations of BMI and cognitive function, 4 sets of multiple linear regression analyses were performed for men and women separately. Each primary analysis was conducted with the age-adjusted global cognitive function score or the attention, executive function, or memory composite score as the criterion variable. The effects of BMI on cognitive function were examined by entering estimated IQ, education, SES, race, medical comorbidities, and HF severity level in step 1 and BMI in step 2. Given the potential influence of depression on cognition among patients with HF,37 we entered PHQ-9 scores in step 3 to determine whether depressive symptoms eliminated or reduced the relationship between BMI and cognitive function. Of note, age was not included as a covariate, given that the cognitive domain variables were created with the use of test scores that already corrected for age using normative data. If continuous BMI was related to a cognitive variable in the regression model, an analysis of covariance (ANCOVA) was run to compare the variable across the BMI categories, adjusting for the same covariates as the regression models. All analyses were conducted with the use of IBM SPSS version 20.0 statistical software.
Results
Demographic and Medical Differences Between HF Patients Across Sex and/or Obesity Status
As presented in Table 1, the majority of the sample was overweight (28.6%) or obese (47.6%), with no sex differences across the BMI categories: χ2 (4; n = 231) = 4.16; P = .383. Obese male HF patients did not differ from nonobese men in age (t(151) = 0.34; P = .735), SES (t(147) = −0.27; P = .789), estimated IQ (t(151) = 0.38; P = .703), Charlson score (t(151) = 0.12; P = .892), NYHA functional classification (χ2 (3; n = 153) = 5.15; P = .161), or PHQ-9 scores (t(151) = 0.67; P = .51). Obese female patients were younger than their nonobese peers (t(76) = 4.28; P < .001), but did not differ in SES (t(75) = 1.31; P = .194), estimated IQ (t(76) = 0.87; P = .387), Charlson score (t(76) = −.42; P = .676), NYHA functional classification (χ2 (3; n = 78) = 3.23; P = .358), or PHQ-9 scores (t(76) = −.49; P = .63. Of note, obese females were also younger than obese males, t(107) = 3.44; P = .001.
Compared with the total sample of men, women had significantly lower SES (t(224) = 2.86; P = .005) and education (χ2 (6; n = 231) = 25.25; P < .001) and were more likely to be nonwhite (χ2 (1; n = 231) = 22.38; P < .001). Women had higher PHQ-9 scores: t(229) = 2.31; P = .02. They also had higher verbal memory scores (t(229) = −3.89; P < .001) and lower visuospatial memory scores (t(229) = 3.10; P < .001) than men.
BMI and Cognitive Functioning in Men
In the total sample of men, cognitive performance across the domains was in the average range (Table 1). Regression results in this group revealed that higher BMI predicted poorer attention (β = −0.18; P = .009) and executive function (β = −0.13; P = .043), but not memory (β = −0.03; P = .687) or global cognitive functioning (β = 0.12; P = .080; Table 2). In men, BMI accounted for 3% of the variance in attention beyond estimated IQ, education, SES, race, medical comorbidities, and HF severity level. The addition of PHQ-9 scores to the model did not eliminate the effect of BMI on attention, as the association remained significant and of similar magnitude (β = −0.17; P = .016; Table 2, Step 3). Similarly, BMI accounted for 2% of the variance in executive functioning after adjusting for the covariates. Adding the PHQ-9 to the model reduced the significance of the effect to a trend but the magnitude of the effect remained relatively unchanged (β = −0.12; P = .067; Table 2, Step 3).
Table 2.
Regressions of BMI Predicting Domains of Cognitive Function in Men (n = 149)
| Attention |
Executive Function |
Memory |
Global Cognitive
Function |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 | ΔR2 | P Value | β | R2 | ΔR2 | P Value | β | R2 | ΔR2 | P Value | β | R2 | ΔR2 | P Value | |
| Step 1 | — | 0.33 | — | .00* | — | 0.40 | — | .00* | — | 0.21 | — | .00* | — | 0.38 | — | .00* |
| Race | −0.02 | .84 | −0.03 | .73 | 0.03 | .72 | 0.09 | .25 | ||||||||
| Education | −0.07 | .44 | −0.07 | .40 | 0.05 | .61 | −0.08 | .37 | ||||||||
| SES | 0.24 | .00* | 0.19 | .01* | 0.12 | .16 | 0.03 | .67 | ||||||||
| CCI | 0.06 | .41 | 0.03 | .66 | −0.04 | .56 | −0.06 | .41 | ||||||||
| NYHA | −0.09 | .20 | −0.10 | .14 | −0.07 | .36 | −0.00 | .98 | ||||||||
| Estimated IQ | 0.53 | .00* | 0.62 | .00* | 0.35 | .00* | 0.61 | .00* | ||||||||
| Step 2 | — | 0.37 | 0.03 | .01* | — | 0.42 | 0.02 | .04* | — | 0.21 | 0.00 | .69 | — | 0.40 | 0.01 | .08 |
| BMI | −0.18 | .01* | −0.13 | .04* | −0.03 | .69 | 0.12 | .08 | ||||||||
| Step 3Aa | 0.38 | 0.01 | .15 | 0.43 | 0.01 | .17 | 0.21 | 0.00 | .75 | 0.41 | 0.01 | .08 | ||||
| BMI | −0.17 | .02* | −0.12 | .07† | −0.03 | .72 | 0.10 | .13 | ||||||||
| PHQ-9 | 0.04 | .15 | −0.10 | .17 | −0.03 | .75 | 0.13 | .08 | ||||||||
CCI, Charlson comorbity index; PHQ-9, Patient Health Questionnaire–9; other abbreviations as in Table 1.
The PHQ-9 was entered separately on step 3. BMI on step 3 is presented to indicate how the addition of PHQ-9 to step 3 affected the effect of BMI.
Significant (P < .05).
Significant (P < .07).
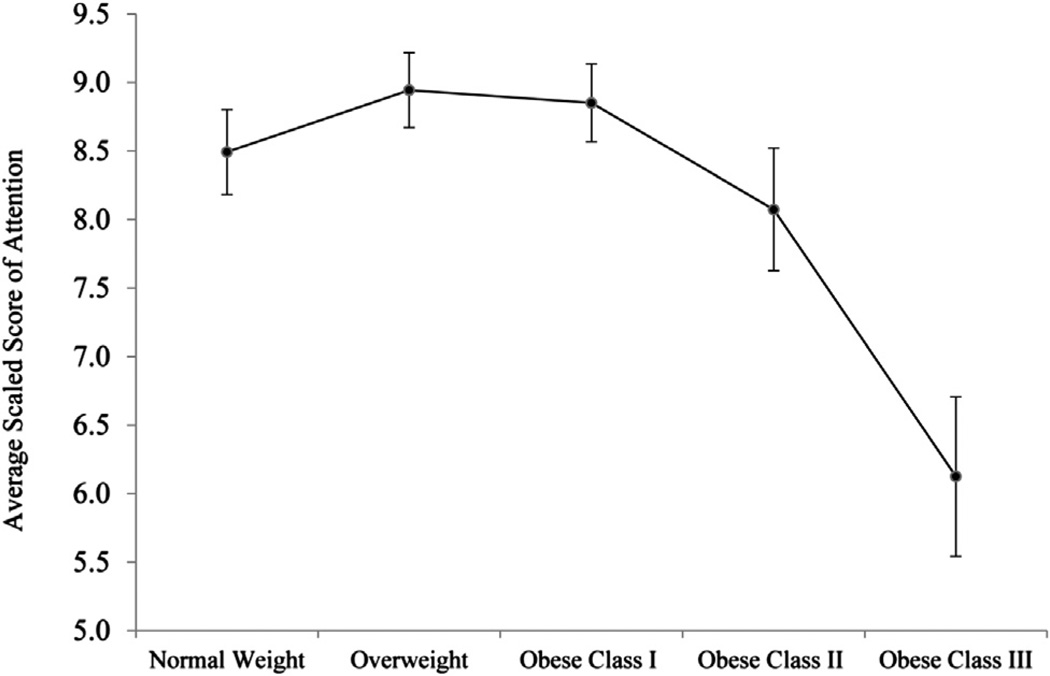
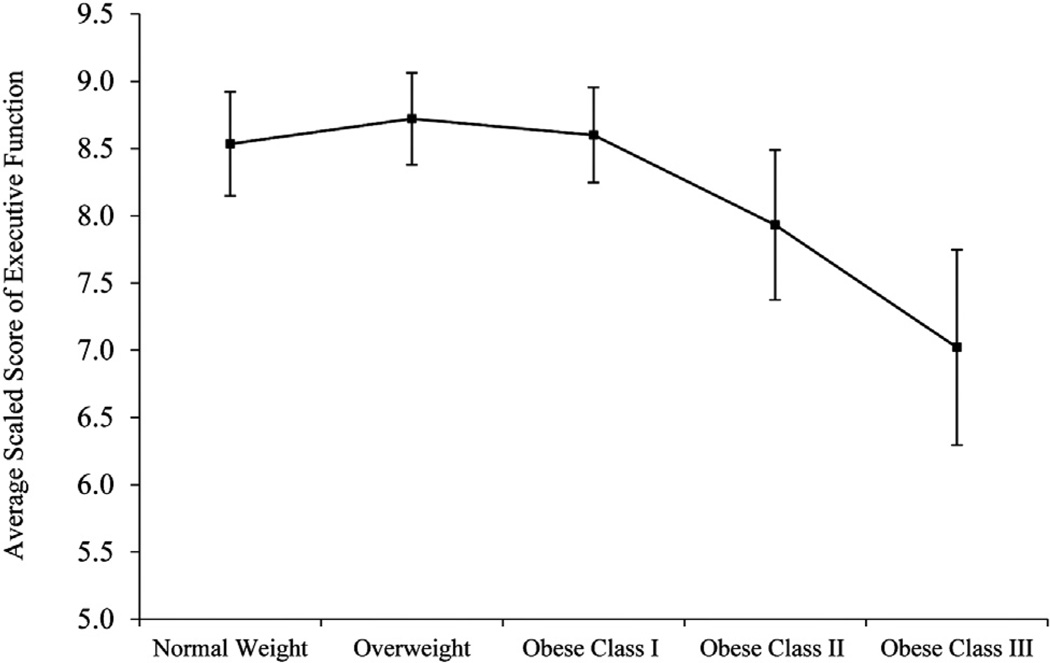
Given that BMI was negatively associated with attention and executive function in men, an ANCOVA was conducted to determine whether attention (Fig. 1) and executive function (Fig. 2) differed across BMI categories. The ANCOVA omnibus test adjusted for the same covariates as the regression models and was significant for attention (F(4,138) = 3.37; P = .012; η2 = 0.09) but not for executive function (F(4,138) = 1.39; P = .240; η2 = 0.04). Pairwise comparisons indicated that for men with BMI ≥40 kg/m2 (obese class III), average attention scores (mean 6.13, SD 1.65) were significantly lower than for all other BMI groups (means 8.07–8.94, SDs 1.93–2.48; P ≤ .027) and were in the borderline impaired range. On average, these patients’ attention scores were nearly 1 SD below their normal-weight peers’ scores. The attention scores of the other BMI categories did not differ from one another (P ≥ .23). For executive function, the only significant difference was between overweight (mean 9.00, SD 2.52) and obese class III patients (mean 6.40, SD 2.38), such that obese class III patient had lower scores (P = .036); the other BMI categories did not differ from one another in executive function (means 7.82–8.72, SDs 2.52–3.25); however, male patients with BMI ≥40 kg/m2 had executive function scores that were w20% lower than those with normal weight and fell into the borderline impaired range.
Fig. 1.
Fig. 2.
BMI and Cognitive Function in Women
In women, greater BMI was not associated with any cognitive variables (all P ≥.09) (See Table 3). Specifically, BMI was not associated with attention (β = −0.05; P = .604), executive functioning (β = 0.15; P = .092), memory (β = 0.09; P = .365), or global cognitive functioning (β = −0.08; P = .358). Addition of the PHQ-9 to the models did not change the pattern of results (Table 3).
Table 3.
Regressions of Body Mass Index (BMI) Predicting Domains of Cognitive Function in Women (n = 77)
| Attention |
Executive Function |
Memory |
Global Cognitive
Function |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 | ΔR2 | P Value | β | R2 | ΔR2 | P Value | β | R2 | ΔR2 | P Value | β | R2 | ΔR2 | P Value | |
| Step 1 | — | 0.47 | — | .00* | — | 0.49 | — | .00* | — | 0.29 | — | .00* | — | 0.45 | — | .00 |
| Race | −0.01 | .95 | 0.09 | .47 | 0.12 | .40 | −0.26 | .04† | ||||||||
| Education | 0.19 | .12 | 0.10 | .36 | −0.03 | .80 | −0.08 | .48 | ||||||||
| SES | −0.03 | .73 | 0.03 | .77 | −0.07 | .53 | 0.12 | .23 | ||||||||
| CCI | −0.20 | .04† | 0.06 | .56 | −0.17 | .13 | −0.05 | .61 | ||||||||
| NYHA | −0.03 | .79 | −0.06 | .50 | −0.19 | .08 | −0.11 | .24 | ||||||||
| Estimated IQ | 0.54 | .00* | 0.56 | .00* | 0.39 | .02† | 0.77 | .00* | ||||||||
| Step 2 | — | 0.47 | 0.00 | .60 | — | 0.51 | 0.02 | .09 | — | 0.30 | 0.01 | .37 | — | 0.46 | 0.01 | .36 |
| BMI | −0.05 | .60 | 0.15 | .09 | 0.09 | .37 | 0.08 | .36 | ||||||||
| Step 3a | 0.47 | 0.00 | .68 | 0.51 | 0.01 | .31 | 0.30 | 0.00 | .62 | 0.46 | 0.00 | .85 | ||||
| BMI | −0.05 | .57 | 0.16 | .07 | 0.10 | .34 | 0.09 | .35 | ||||||||
| PHQ-9 | 0.04 | .68 | −0.10 | .31 | −0.06 | .62 | −0.02 | .85 | ||||||||
Discussion
The objective of the present study was to examine whether BMI predicts cognitive functioning in HF patients.
To better define the relationship between adiposity and cognitive function and to explore the potential influence of gender on this relationship, we conducted sex-stratified analyses. Higher BMI was associated with poorer attention and executive function in men, even after accounting for demographic and medical variables and depressive symptoms. This effect was largely driven by men with severe obesity (BMI ≥40 kg/m2). Although the curves of Figures 1 and 2 may visually suggest slightly better cognitive performance in overweight versus normal-weight men, the scores in these groups were not statistically different and consequently do not support an “obesity paradox” pattern. BMI was not associated with any cognitive variables in women. Several aspects of these findings warrant discussion.
Our results are consistent with recent investigations that have implicated excess adiposity as a contributor to cognitive impairment in HF populations.18,19 Similarly to our findings, Alosco et al19 found that elevated BMI was associated with poorer performance on tests of attention/executive function, but not of memory. Riegel et al18 also demonstrated that higher BMI predicted poor cognitive function (ie, below-average processing speeds) in a group of HF patients. Because those studies did not report sex-stratified analyses, it is unknown whether sex differences in the BMI—cognitive function relationship existed. Addressing this omission in the literature is important, given earlier evidence that male HF patients have poorer prognosis 2,3 and differ from female HF patients in a number of demographic and medical variables.22 For example, male HF patients typically are older, are more likely to have ischemic HF etiology, have poorer left ventricular function, and have higher rates of atrial fibrillation than women with HF,22 all factors which have been associated with greater adiposity38–41 and poorer cognition.42–45 Sex differences in these prognostic factors may not only explain why males have poorer HF survival outcomes,22 they may also explain why BMI was unrelated to cognitive function in our sample female HF patients. Future studies are needed to clarify whether gender differences also exist in long-term neurologic outcomes in this population, including Alzheimer disease and stroke.
Although we could not examine ischemic versus nonischemic HF etiology, left ventricular function, or rates of atrial fibrillation, because these variables were not assessed in Heart ABC, we did explore potential age differences across obesity status and sex. We found that, although nonobese men and women in our sample did not significantly differ in age, obese women were, on average, 5.7 years younger than obese males and 8.2 years younger than nonobese females. The significantly younger age of obese females may have mitigated, to some degree, any cognitive deficits associated with a higher BMI in this group. Future studies should conduct sex-stratified analyses to assess this possibility as well as to explore the role that ischemic heart disease and atrial fibrillation may play in the relationship between BMI and cognitive function in male and female HF patients.
Several mechanisms might explain why greater BMI predicts poorer attention and executive function in male HF patients. One potential reason is that greater BMI is associated with elevated risk for vascular diseases (eg, diabetes and hypertension)46,47 and vascular dysfunction (eg, hypoperfusion and endothelial dysfunction),19,48 which in turn confer greater risk for cognitive impairment.49–51 Reduced vascular functioning ultimately results in cognitive deficits through structural and functional damage to the brain, including tissue atrophy, white matter hyperintensities, and reduced neuroactivation.52–55 Of note, obesity’s effect on cognitive function in the present sample was detected after adjusting for medical comorbidities, suggesting that BMI confers unique risk for cognitive impairment beyond that of other diseases such as diabetes and peripheral vascular disease. The effect of BMI on tests of attention and executive function (but not memory) is also consistent with the vascular cognitive impairment literature which indicates that these cognitive domains are often more impaired by vascular dysfunction and disease than are memory or language domains.56,57
Other physiologic mechanisms of the BMI—cognitive function relationship include elevated circulating adipokines (eg, leptin)58 and systemic inflammation,59 both of which are linked to poorer neurocognitive outcomes.60,61 Behavioral mechanisms may also be implicated, because individuals with elevated BMI are typically more sedentary than their normal-weight peers.62 Given the strong association between physical activity and cognitive function,63,64 higher BMI may reflect patients with reduced physical fitness. Prospective studies are needed which assess these potential mechanisms of the BMI—cognitive function relationship in patients with HF.
Study Limitations
The present findings are limited in several ways. First, the cross-sectional nature of the study precludes the opportunity to determine the directionality of the BMI—cognitive function relationship. Therefore, we do not know whether excess adiposity was a predictor or consequence of cognitive deficits, as studies have shown that poorer cognitive abilities earlier in life also predict higher risk of becoming obese.65 Second, the use of BMI as a measure of adiposity is not optimal, because BMI can not distinguish between changes in fat mass and other factors causing weight changes in an HF population (eg, water retention, sarcopenia). Future studies with the use of more precise measures of adiposity would clarify the possible mechanisms linking obesity and cognitive impairment in persons with HF. Third, although we speculate that ischemic versus nonischemic HF etiology, left ventricular function, and/or rates of atrial fibrillation are factors that could explain the potential sex differences in our study, these variables were not collected in the Heart ABC study. Future studies should assess these and other potential variables (eg, medication dosing and duration, documented hypertension diagnosis) that may be associated with BMI and/or cognitive function. Finally, although earlier work indicates that deficits in attention and executive function are related to functional impairment,66,67 future studies should directly examine whether obesity-related cognitive deficits are associated with impaired decision making or self-care abilities.
Conclusion
The current findings reveal that elevated BMI is associated with poorer attention and executive functioning in male HF patients, especially those with BMI ≥40 kg/m2. BMI was not associated with cognitive functioning among women. These findings highlight the importance of conducting sex-stratified analyses and exploring demographic and medical moderators of the BMI—cognitive function relationship. Regarding clinical implications, it is possible that obese male HF patients have more difficulty adhering to the complex HF treatment regimen and ultimately experience poorer clinical outcomes, given the association between mental abilities and daily function.66,67 Future studies are needed to clarify this possibility and to determine whether tailored interventions are needed to promote optimal adherence in this group.
Acknowledgments
Funding: 1 R01 HL096710-01A1 to Drs Hughes and Dolansky
The authors thank all members of the Heart ABC team for their efforts in data collection and dataset preparation for this project, particularly Julie T. Shaefer, MS, RD, and Michael J. Fulcher, BA.
Footnotes
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. AHA statistical update. Heart disease and stroke statistics—2013 update. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon T, Mary-Krause M, Funck-Brentano C, Jaillon P. Investigators. Sex Differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II) Circulation. 2001;103:375–380. doi: 10.1161/01.cir.103.3.375. [DOI] [PubMed] [Google Scholar]
- 3.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Bennett SJ, Oldridge NB, Eckert GJ, Embree JL, Browning S, Hou N, et al. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Seo Y, Roberts BL, Piña I, Dolansky M. Predictors of motor tasks essential for daily activities among persons with heart failure. J Card Fail. 2008;14:296–302. doi: 10.1016/j.cardfail.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 7.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauvé MJ, et al. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraro KF, Su YP, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. Am J Public Health. 2002;92:834–840. doi: 10.2105/ajph.92.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J. 2010;159:75–80. doi: 10.1016/j.ahj.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 13.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Cournot M, Marquie J, Ansiau D, Martinaud C, Fonds H, Ferrieres J, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 15.Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology. 2010;34:222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beydoun M, Beydoun H, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alosco ML, Spitznagel MB, Gunstad J. Obesity as a risk factor for poor neurocognitive outcomes in older adults with heart failure. Heart Fail Rev. 2013:1–9. doi: 10.1007/s10741-013-9399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riegel B, Lee CS, Glaser D, Moelter ST. Patterns of change in cognitive function over six months in adults with chronic heart failure. Cardiol Res Pract. 2012;2012 doi: 10.1155/2012/631075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Colbert LH, et al. Obesity interacts with cerebral hypoperfusion to exacerbate cognitive impairment in older adults with heart failure. Cerebrovasc Dis Extra. 2012;2:88–98. doi: 10.1159/000343222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth W, Jr, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghali JK, Krause-Steinrauf HJ, Adams KF, Khan SS, Rosenberg YD, Yancy CW, et al. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–2134. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Clinicaltrials.gov. Self-Management and Cognitive Function in Adults With Heart Failure (Heart ABC) Bethesda, Maryland: National Library of Medicine; 2011. [Accessed August 22, 2013]. Available at: http://clinicaltrials.gov/ct2/show/NCT01461629. [Google Scholar]
- 24.González-Gross M, Marcos A, Pietrzik K. Nutrition and cognitive impairment in the elderly. Br J Nutr. 2001;86:313–321. doi: 10.1079/bjn2001388. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Obesity: preventing and managing the the global epidemic. Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- 26.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 27.Golden JC. Stroop Color and Word Test. Chicago, Illinois: Stoelting; 1978. [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale. Third Edition. San Antonio, Texas: The Psychological Corporation; 1997. [Google Scholar]
- 29.Lezak MD. Neuropsychological assessment. New York, New York: Oxford University Press; 1995. [Google Scholar]
- 30.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. Odessa, Florida: Psychological Assessment Resources; 1995. [Google Scholar]
- 31.Teng E, Chui H. The Modified Mini-Mental State Examination (3MS) Can J Psychiatry. 1987;41:114–121. [PubMed] [Google Scholar]
- 32.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Committee NYHAC, New York Heart Association (NYHA) Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Little: Brown Medical Division; 1979. [Google Scholar]
- 35.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:1–7. [Google Scholar]
- 36.Roux AVD, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 37.Pullicino PM, Wadley VG, McClure LA, Safford MM, Lazar RM, Klapholz M, et al. Factors contributing to global cognitive impairment in heart failure: results from a population-based cohort. J Card Fail. 2008;14:290–295. doi: 10.1016/j.cardfail.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DS, Williamson DF, Croft JB, Ballew C, Byers T. Relation of body fat distribution to ischemic heart disease: the National Health and Nutrition Examination Survey I (NHANES I) epidemiologic follow-up study. Am J Epidemiol. 1995;142:53–63. doi: 10.1093/oxfordjournals.aje.a117545. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno T, Shu I-W, Makimura H, Mobbs C. Obesity over the life course. Science’s SAGE KE. 2004;2004:re4. doi: 10.1126/sageke.2004.24.re4. [DOI] [PubMed] [Google Scholar]
- 41.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 42.Thacker EL, McKnight B, Psaty BM, Longstreth W, Sitlani CM, Dublin S, et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013 doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham Study. Stroke. 2009;40:1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craik FM, Byrd M. Aging and cognitive deficits. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. Springer US; 1982. pp. 191–211. [Google Scholar]
- 45.Zuccalà G, Cattel C, Manes-Gravina E, di Niro MG, Cocchi A, Bernabei R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. J Neurol Neurosurg Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hossain P, Kawar B, el Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 47.Sims EA, Berchtold P. Obesity and hypertension. JAMA. 1982;247:49–52. [PubMed] [Google Scholar]
- 48.al Suwaidi J, Higano ST, Holmes DR, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- 49.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paglieri C, Bisbocci D, Caserta M, Rabbia F, Bertello C, Canadè A, et al. Hypertension and cognitive function. Clin Exp Hypertens. 2008;30:701–710. doi: 10.1080/10641960802563584. [DOI] [PubMed] [Google Scholar]
- 51.Umemura T, Kawamura T, Umegaki H, Mashita S, Kanai A, Sakakibara T, et al. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry. 2011;82:1186–1194. doi: 10.1136/jnnp.2010.217380. [DOI] [PubMed] [Google Scholar]
- 52.Menon U, Kelley RE. Subcortical ischemic cerebrovascular dementia. Int Rev Neurobiol. 2009;84:21–33. doi: 10.1016/S0074-7742(09)00402-4. [DOI] [PubMed] [Google Scholar]
- 53.Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people 1. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- 54.Vogels RL, Oosterman JM, van Harten B, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;24:418–423. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- 55.Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, et al. Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail. 2005;11:437–446. doi: 10.1016/j.cardfail.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 58.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactiveleptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 59.Ferrante AW. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 60.Pannacciulli N, Le DSN, Chen K, Reiman EM, Krakoff J. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci Lett. 2007;412:248–253. doi: 10.1016/j.neulet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40(3 Suppl 1):S20–S23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jebb SA, Moore MS. Contribution of a sedentary lifestyle and inactivity to the etiology of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31(11 Suppl):S534–S541. doi: 10.1097/00005768-199911001-00008. [DOI] [PubMed] [Google Scholar]
- 63.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 64.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 65.Chandola T, Deary I, Blane D, Batty G. Childhood IQ in relation to obesity and weight gain in adult life: the National Child Development (1958) study. Int J Obes. 2006;30:1422–1432. doi: 10.1038/sj.ijo.0803279. [DOI] [PubMed] [Google Scholar]
- 66.Bell-McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- 67.Pelton G, Devanand D. The impact of mild cognitive impairment on functional abilities in the elderly. Curr Psychiatry Rep. 2002;4:64–68. doi: 10.1007/s11920-002-0015-8. [DOI] [PubMed] [Google Scholar]