Abstract
Reduced signaling through the IGF type 1 (IGF-1) receptor increases life span in multiple invertebrate organisms. Studies on mammalian longevity suggest that reducing levels of IGF-1 may also increase life span. However, the data are conflicting and complicated by the physiology of the mammalian neuroendocrine system. We have performed life-span analysis on mice homozygous for an insertion in the Igf1 gene. These mice produce reduced levels of IGF-1 and display a phenotype consistent with a significant decrease in IGF-1. Life-span analysis was carried out at three independent locations. Although the life-span data varied between sites, the maximum life span of the IGF-1-deficient mice was significantly increased and age-specific mortality rates were reduced in the IGF-1-deficient mice; however, mean life span did not differ except at one site, where mean life span was increased in female IGF-1-deficient animals. Early life mortality was noted in one cohort of IGF-1-deficient mice. The results are consistent with a significant role for IGF-1 in the modulation of life span but contrast with the published life-span data for the hypopituitary Ames and Snell dwarf mice and growth hormone receptor null mice, indicating that a reduction in IGF-1 alone is insufficient to increase both mean and maximal life span in mice.
Key Words: IGF-1, Insulin, Metabolism, Obesity, Gluconeogenesis.
Introduction
A reduction in signaling through the insulin/IGF receptor increases life span in multiple organisms. Seminal studies identifying this intracellular pathway as a modulator of longevity were performed using Caenorhabditis elegans as a model organism, and the results were later confirmed in Drosophila (1–3). Epistasis approaches have defined intracellular signaling pathways, which are required for enhanced longevity in these invertebrates, including the PI-3 kinase/Akt signaling pathway and the Daf16/Foxo transcription factors (1,4,5). Subsequent studies using mouse models of reduced neuroendocrine signaling have generally supported the concept that the IGF type 1 (IGF-1) pathway is a primary modulator of longevity. Landmark studies on neuroendocrine signaling and longevity involved the Ames dwarf, which carries a mutation in the Prop-1 gene required for pituitary development (6). Subsequent studies on a similar mouse known as the Snell Dwarf, which carries a mutation in the Pit-1 gene that is also required for pituitary development, have confirmed these findings (7). Although both dwarf mutations result in reduced IGF-1, several other hormones (including growth hormone and thyroid hormone) that could affect life span are also altered in Snell and Ames dwarf mice. A specific link between the growth hormone/IGF-1 axis and longevity is supported by studies on a mouse model of the Laron syndrome mutation in humans and the lit/lit mouse model (8,9). The Laron mouse line harbors a point mutation in the growth hormone receptor. This mutation disrupts both the soluble and membrane-bound forms of the growth hormone receptor, whereas the lit/lit mice harbor a mutation in the growth hormone–releasing hormone receptor leading to a specific reduction in growth hormone levels (10,11). In both cases, the resulting mice exhibit a dwarf phenotype and life-span extension relative to genetically matched controls. Due to the fact that growth hormone is the primary regulator of IGF-1 production in the liver, the life-span extension provided by the pituitary and growth hormone receptor mutations has been interpreted as consistent with the invertebrate studies linking IGF-1 signaling with longevity. However, a direct assessment of the importance of IGF-1 signaling in mammalian longevity has been difficult due to the essential role that IGF-1 plays in growth and development. Null mutations of the IGF-1 gene or the IGF-1 receptor gene induce perinatal lethality in a large percentage of homozygotes depending on the genetic background (12–15). Further, the growth and development of the survivors is severely affected (16,17). Mice heterozygous for a null mutation in the IGF-1 receptor gene have been tested for longevity (18). These animals displayed an increased life span relative to controls in females but not in males in an initial study; however, subsequent studies suggest that this increase in life span may be dependent upon housing conditions (19). In order to gain a better understanding of the relative role of IGF-1 and growth hormone in the modulation of mammalian longevity, we have examined a mouse line that harbors a hypomorphic Igf1 gene due to the insertion of a gene-targeting vector (20). This mouse line, known as the IGF-1-deficient mouse due to its intermediate size relative to wild-type and IGF-1 null mice, was the first demonstration that gene loci harboring a gene-targeting vector may be expressed through alternative splicing. Importantly, the relative messenger RNA transcript levels are significantly reduced relative to mice with a wild-type allele in all tissues tested. This approach provides the opportunity to examine the impact of reduced expression of genes that are critical to survival. We have established a cohort of these IGF-1-deficient mice and have performed longevity studies in three independent locations, the Lankenau Institute for Medical Research in Philadelphia, PA; The University of Texas Health Sciences Center at San Antonio, and at the Pennsylvania State University in University Park, PA.
Materials and Methods
Animals
Mice used in this study were derived from mice homozygous for the Igf1 hypomorphic allele (11) obtained from The Jackson Laboratory (Bar Harbor, ME) on a 129sv/C57/CD-1 genetic background. These mice were mated to C57BL/6J mice from The Jackson Laboratory to generate mice heterozygous for the Igf1 hypomorphic allele. Heterozygous mice were mated to produce litters containing control mice, mice heterozygous for the Igf1 hypomorphic allele, and mice homozygous for the Igf1 hypomorphic allele. Mice were housed as sibling groups of three to four mice in a high-efficiency particulate air–filtered positive-flow isolation rack. Food and water were provided ad libitum throughout the study. All animal care and procedures were in compliance with the U.S. National Institutes of Health and the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, The Pennsylvania State University, the Lankenau Institute for Medical Research, or the Drexel University College of Medicine. Chow-fed animals were fed standard vegetable-derived chow (Harlan–Teklad 7912; Harlan Laboratories, Madison, WI); composition energy content (kcal/g) was as follows: protein 24%, carbohydrates 62%, and fat 14%. End-of-life pathology was performed on mice in the Lankenau cohort following established parameters (21).
Tissue Extraction and IGF-1 Measurements
Tissue levels of IGF-I were measured as previously described (22). Briefly, approximately 100mg of frozen tissue was powdered under liquid nitrogen and suspended in 5 mL of acetic acid/g of tissue. Tissue suspensions were incubated on ice for 30 minutes, and insoluble material was removed by centrifugation (10 min at 5,000g, 4°C). Extracts were lyophilized and resuspended in 0.1M Tris (pH 8.0) at 2mL/g of tissue. Samples were cleared by centrifugation (10 min at 10,000g, 4°C). Protein concentrations of cleared lysates were determined by the method described by Bradford (23). An equal amount of protein was used to determine the IGF-I content using an IGF-I ELISA kit (Immuno Diagnostic Systems; Fountain Hills, AZ). Input volumes of the samples were increased to 25 μL, and the volume of diluent was decreased to 0.25mL. The remainder of the assay was performed according to the manufacturer’s instructions. Female mice were used for tissue and serum IGF-1 measurements (n = 4 per group).
Body Composition and Bone Mineral Density
Analysis of body composition was obtained using dual-energy x-ray absorptiometry and quantitative MRI technologies. An EchoMRI100 (Echo Medical Systems LLC., Houston, TX) Mouse quantitative MRI machine was used to perform whole-body composition analysis without anesthesia as per manufacturer’s recommendations. Bone mineral density (BMD) measurements were performed using a Lunar Pixmus dual-energy x-ray absorptiometry instrument (n = 40 per group).
Tumorigenesis Assay
Tumor assays were performed using MC17 cells, a cell line that is syngeneic to C57/BL6 mice (24). Cells were tested for the presence of known viruses and rodent pathogens prior to tumor studies. Cells were plated at 1 × 104/cm2 24 hours prior to injection. Cells were rinsed three times with sterile saline and removed for the tissue culture plate by the addition of 1 mL 0.25% trypsin EDTA. Cells were collected by centrifugation, washed three times with sterile saline and counted using a guava easycyte flow cytometer. A total of 3 × 106 cells were injected in a volume of 100 µL sterile saline into the flank of control or IGF-1-deficient female mice (n = 4 per group). Tumor growth was monitored by caliper assessment for 21 days. At this time, tumors were excised and measured for volume and mass and subjected to histochemical analysis.
Statistical Analysis
Either SPSS or SAS v.9.1.3 was used to perform all statistical analysis. Survival analysis was used to assess longevity. Descriptive statistics were used to give an overview of data obtained, whereas t tests and multifactorial analysis of variance were performed to analyze the remaining data as appropriate. Power analysis performed using SAS v. 9.1.3 PROC POWER indicated n = 9 per group (by sex and genotype) would give the longevity study 0.80 power with 50% survival expected at 24 months of age. Our total N was larger to increase the power over 0.90. Similarly, the cross-sectional studies using younger mice required an n = 9 per group to give 0.80 power. In our studies, we had 9–12 mice per group (by sex and genotype). Median life span of a group was defined as the number of days at which 50% of a group had died. Maximum life span was defined as the age in days at which 90% of mice of a particular group had died. Each group was defined by sex (male or female) and genotype (IGF-1 deficient or control). Life tables were produced using SPSS and were modified to include Lx, Tx, and ex (25). Age-specific mortality rates were plotted using 30-day intervals throughout life span based upon the life tables by calculating the proportion of animals entering each interval and the number which terminated during that interval.
Results
We verified the phenotype of the IGF-1-deficient mouse by examining IGF-1 levels in serum and tissue, as well as several physiologic characteristics known to be influenced by IGF-1. IGF-1 levels were examined through the use of a tissue extraction method that releases IGF-1 from the IGF-binding proteins allowing total IGF-1 to be assessed by ELISA (26). We have successfully used this approach to examine the response of the IGF-1 to injury in aged animals (27) and in the measurement of IGF-1 levels in the brain as it relates to proteasome activity and oxidative damage (28). Relative IGF-1 levels in several tissues in the IGF-1 deficient mice have been reported elsewhere (Salmon et al. submitted) and were further examined to provide additional verification that IGF-1 production was reduced in the IGF-1-deficient mice (Table 1). IGF-1 levels were reduced in IGF-1 deficient mice serum levels and tissues of the IGF-1 deficient mice relative to wild-type animals. In order to verify that the reduction in IGF-1 levels were physiologically relevant, several physiological parameters known to be sensitive to IGF-1 were examined in the IGF-1-deficient mice. For example, gene-targeting studies have established that a reduced IGF-1 expression results in lower body size (29) and thus adult body weight can serve as a measure of the relative phenotypic impact of manipulations involving IGF-1 expression. Adult body weight of the IGF-1-deficient mice was significantly reduced in the IGF-1-deficient mice relative to controls (males 39 ± 4 g vs 30 ± 6 g; females 28 ± 4 g vs 17 ± 4 g wild type vs IGF-1 deficient p < .02 in all cases). Food intake was slightly increased in the IGF-1-deficient mice relative to wild-type mice when corrected for body weight (0.095 vs 0.155; 0.125 vs 0.145; control vs IGF-1-deficient for females and males, respectively). Organ size was reduced in all cases commensurate with the reduction in IGF-1 levels; however, the relative organ size expressed as a percent of body weight increased. For example, liver, kidney, and brain were slightly increased relative to total body weight in the IGF-1-deficient mice (7.3% vs 6.3%; 1.1% vs 0.9%; 2.4% vs 2.1%, respectively), whereas the relative heart size was slightly smaller (0.8% vs 1.0%). Body fat is reduced in the IGF-1-deficient mice making it unlikely that the differences in organ weight reflect differences in body composition and normalization to tibia length provided similar results in terms of relative differences in relative organ size (see Table 1).
Table 1.
Physiologic Parameters of IGF-1 Deficient and Wild-Type Mice Related to IGF-1 Function
| IGF-1 Levels | ||||
|---|---|---|---|---|
| Genotype | Liver | Kidney | Muscle | Serum |
| IGF-1 deficient | 1980±555ng/g | 1620±208ng/g | 678±181ng/g | 480±58ng/mL |
| Wild type | 2808±245ng/g | 2396±434ng/g | 1541±706ng/g | 909±123ng/m |
| Organ Weight (g) | ||||
| Genotype | Liver | Kidney | Heart | Brain |
| IGF-1 deficient | 1.37±0.001 | 0.21±0.007 | 0.155±0.0015 | 0.44±0.028 |
| Wild type | 1.39±0.002 | 0.21±0.022 | 0.22±0.0033 | 0.47±0.019 |
| Organ Weight (% of body weight) | ||||
| Genotype | Liver | Kidney | Heart | Brain |
| IGF-1 deficient | 7.3% | 1.1% | 0.8% | 2.4% |
| Wild type | 6.3% | 0.9% | 1.0% | 2.1% |
| Organ Weight/Tibia Length (g/cm) | ||||
| Genotype | Liver | Kidney | Heart | Brain |
| IGF-1 deficient | 1.41 | 0.175 | 0.129 | 0.367 |
| Wild type | 1.06 | 0.161 | 0.169 | 0.361 |
| Litter Size | ||||
| IGF-1 deficient | 6±2 | |||
| Wild type | 8±2 | |||
| Bone Mineral Density | ||||
| 100 d | 495 d | |||
| Male | Female | Male | Female | |
| IGF-1 deficient | 0.0425g/cm2 | 0.0475g/cm2 | 0.0435g/cm2 | 0.0440g/cm2 |
| ±0.002 | ±0.002 | ±0.002 | ±0.002 | |
| Wild type | 0.0535g/cm2 | 0.0565g/cm2 | 0.0525g/cm2 | 0.0535g/cm2 |
| ±0.002 | ±0.002 | ±0.002 | ±0.002 | |
| End-of-Life Pathology | ||||
| Male | Female | |||
| Wild Type (N = 26) | IGF-1 Deficient (N = 6) | Wild Type (N = 26) | IGF-1 Deficient (N = 12) | |
| Cause of death | ||||
| Neoplastic | 15 (57.7%) | 2 (33.3%) | 15 (57.7%) | 6 (50.0%) |
| Lymphoma | 1 | 1 | 1 | 4 |
| Hemangioma | 9 | 0 | 9 | 1 |
| Hepatocellular carcinoma | 2 | 1 | 2 | 1 |
| Others | 3 | 0 | 3 | 0 |
| Non-neoplasm | 5 | 2 | 5 | 3 |
| Glomerurosclerosis | 4 (26.7%) | 1 (16.7%) | 4 (26.7%) | 3 (25%) |
| Thrombus, heart | 1 | 1 | 1 | 0 |
| Other | 6 | 2 | 6 | 3 |
| Total | 26 | 6 | 26 | 12 |
Note: IGF-1 = IGF type 1.
Physiological parameters predicted to be influenced by changes in IGF-1 levels were examined in wild-type and IGF-1-deficient mice.
Total IGF-1 levels were assessed by ELISA using extracts from the tissues indicated or using serum in female mice. Tissue IGF-1 levels were examined in two separate assays with similar results, whereas serum IGF-1 levels presented are representative of at least three similar assays; animal numbers were n = 4 for kidney, n = 5 for liver and muscle, n = 15 for IGF-1-deficient serum, and n = 4 for wild-type serum. Absolute values of IGF-1 levels varied between assays, whereas relative differences between wild-type and IGF-1-deficient animals remained consistent. All differences between wild-type and IGF-1-deficient animals were significant at p < .05.
Average body weights were 18.62 g for IGF-1-deficient females and 22 g for control females at 7 months of age. Tibia length at this age was 1.2cm for IGF-1 deficient and 1.3 for controls.
Litter size was determined during breeding for longevity cohorts. Organ weight was examined at 3 months of age during necropsy. Organs were washed in phosfate-buffered saline and briefly dried prior to weighing, n = 4 for each genotype.
Bone mineral density was assessed by dual-energy x-ray absorptiometry; bone mineral density was lower (p < .0001) in the IGF-1-deficient mice compared with controls. Bone mineral density was reduced at 495 days relative to 100 days, and sex also influenced bone mineral density (p < .01); n = 10 in each age group and for each sex.
Pathological examination was performed at the University of Texas Health Sciences Center on a subset of animals from the Lankenau Cohort.
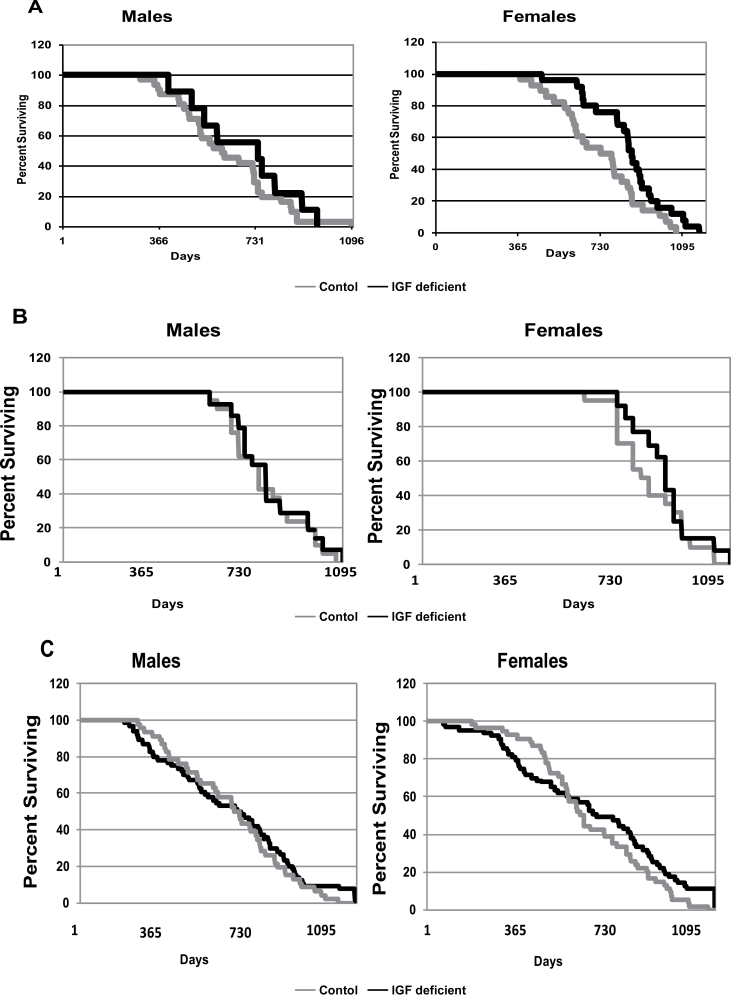
The mean and maximum life span achieved for the IGF-1-deficient and control mice at each institution is presented. The data labeled “All Cohorts” contains life-span data from all mice at all three institutions. Maximum life span represents the average life span of the final 10% of each cohort. The maximum life-span difference between IGF-1-deficient and control animals at all sites is significant at p = .0001 for males and p = .0016 for females.
Because IGF-1 is known to be a critical factor for the differentiation of multiple tissues (16), we examined several additional parameters of the IGF-1-deficient mice. For example, IGF-1 is known to play an important role in oocyte maturation (30–33), and a reduction in IGF-1 levels would be predicted to affect fertility. Consistent with this prediction, litter size was decreased in the IGF-1-deficient mice relative to wild-type animals (6 ± 2 pups vs 8 ± 2 pups, p < .05). IGF-1 has also been reported to be important for proper bone development (34–36), so BMD was examined in the IGF-1-deficient mice. In order to assess potential age-related changes, BMD was compared at 100 days and at 495 days of age. At 100 days of age, BMD was lower (p < .0001) in IGF-1-deficient mice compared with controls, with no differences between sexes of either genotype (p = .321). At 495 ± 5 days, BMD was lower in IGF-1-deficient mice (p < .0001) compared with controls. In addition, BMD exhibited a sexual dimorphism in that males of both genotypes exhibiting lower BMD in older animals (p = .010).
IGF-1 is known to be a positive factor for cellular transformation (37) and tumor formation in multiple tissues (38–43), and the reduced IGF-1 environment in the IGF-1-deficient mice would be expected to be inhibitory for tumor formation. We focused on female mice for these studies due to the fact that the female IGF-1-deficient mice displayed an increased mean life span in the Lankenau cohort and thus would be most likely to demonstrate phenotypic changes associated with increased life span. When female IGF-1-deficient mice were challenged, tumor formation by MC17 mouse fibrosarcoma cells was significantly reduced relative to controls (34% reduction in tumor mass; control 0.8 ± 0.15 g vs IGF-1 deficient 0.5 ± 0.18 g, p < .05). Additionally, end-of-life pathology on a subset of animals indicates a reduced incidence of neoplastic disease (Table 1).
Given that in all parameters we examined the IGF-1-deficient mouse line demonstrated significant differences from controls we anticipated that the reduced IGF-1 environment in the IGF-1-deficient mouse would provide a benefit in terms of life span. Life-span analysis was carried out at three independent locations, the Lankenau Institute for Medical Research in Wynnewood, Pennsylvania, The University of Texas Health Sciences Center at San Antonio, and at the Pennsylvania State University in State Park, Pennsylvania (Table 2). In all cases, mouse colonies were maintained in AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care)-approved SPF (specific pathogen free) barrier facilities. At the Lankenau Institute for Medical Research, a cohort of animals was bred within a 6-week period through the breeding of mice heterozygous for the IGF-1-deficient allele. Heterozygous animals showed no difference from wild type in terms of body size, IGF-1 levels or litter size and were classified as wild type. For this cohort of animals, the mean life span of the female animals was 729 ± 174 days for wild type (n = 43) and 863 ± 184 days for homozygous IGF-1-deficient females (n = 17). Maximum life span was 1093 days for female wild type and 1170 days for female IGF-1-deficient animals. The difference between wild-type and IGF-1-deficient females in terms of the mean and maximum life span were significant. Additional animals bred outside this time window were included in additional life-span analyses that increased the number of animals in the wild type group to 53 and the IGF-1-deficient cohort to 25. Homozygous IGF-1-deficient mice were under-represented in litters of heterozygous pairs. Male IGF-1-deficient animals were present at less than 10% of all offspring. For this reason, the male cohort at Lankenau contained only nine animals, whereas the wild-type group contained 53 animals. Nonetheless, there was no statistical difference between IGF-1-deficient and wild-type males in terms of mean or maximum survival.
Table 2.
Mean and Maximum Life Span for IGF-1 Deficient and Control Mice
| Mean Life Span | Maximum Life Span | |||
|---|---|---|---|---|
| Wild Type | IGF-1 Deficient | Wild Type | IGF-1 Deficient | |
| Lankenau cohort | ||||
| Female | 729±174 (n = 43) | 863±184 (n = 17) | 1093±87 | 1170±38 |
| Male | 624±297 (n = 53) | 688±246 (n = 9) | 935±62 | 893±41 |
| UTHSC cohort | ||||
| Female | 732±256 (n = 46) | 692±292 (n = 64) | 1079±95 | 1209±130 |
| Male | 698±227(n = 54) | 714±247(n = 63) | 1075±62 | 1204±63 |
| Penn state cohort | ||||
| Female | 822±161 (n = 21) | 867±169 (n = 14) | 1075±42 | 1143±50 |
| Male | 800±136 (n = 20) | 849±130 (n = 14) | 1002±64 | 1120±127 |
| All cohorts | ||||
| Female | 756±218 (n = 110) | 779±289 (n = 95) | 1118±58 | 1233±92 |
| Male | 704±213 (n = 127) | 738±296 (n = 86) | 1067±54 | 1172±61 |
Note: IGF-1 = IGF type 1; UTHSC = University of Texas Health Sciences Center.
Additional life-span analyses were carried out at the Pennsylvania State University and at the University of Texas Health Sciences Center in San Antonio. Due to the difficulties encountered when breeding the heterozygous mice, IGF-1-deficient cohorts were generated from homozygous pairs for these studies. Litter size in the IGF-1-deficient mice was significantly reduced (see Table 1) compared with wild-type animals consistent with the low-IGF-1 phenotype in these animals. At the Pennsylvania State University, the life-span cohort consisted of 75 mice, 21 wild-type females, 14 IGF-1-deficient females, 20 wild-type males, and 14 IGF-1-deficient males. In this case, the mean life span of the males was 849 ± 130 days versus 800 ± 136 days, IGF-1-deficient versus controls, whereas the mean life span for females was 867 ± 169 days versus 822 ± 161 days, IGF-1 deficient versus controls. Maximum life span for the males was 1120 ± 127 days versus 1002 ± 64 days, IGF-1 deficient versus control, whereas the maximum life span for females was 1143 ± 50 days versus 1075 ± 42 days, IGF-1 deficient versus control. The differences in mean and maximum life span were not significant in this cohort.
The life-span cohort established at the University of Texas Health Sciences Center consisted of 227 mice, 63 male IGF-1 deficient and 54 male controls and 64 female IGF-1 deficient and 46 female controls. Mean life span was 714 ± 247 versus 698 ± 227 for male IGF-1 deficient versus male controls and 692 ± 292 versus 732 ± 256 for female IGF-1 deficient versus female controls. The differences in mean life span between wild-type and IGF-1-deficient mice were not statistically significant. Maximum life span was 1204 ± 63 versus 1075 ± 62 for male IGF-1 deficient versus male controls and 1209 ± 130 versus 1079 ± 95 for female IGF-1 deficient versus female controls. In both cases, the maximum life-span difference between control animals and IGF-1-deficient animals is significant (p < .001).
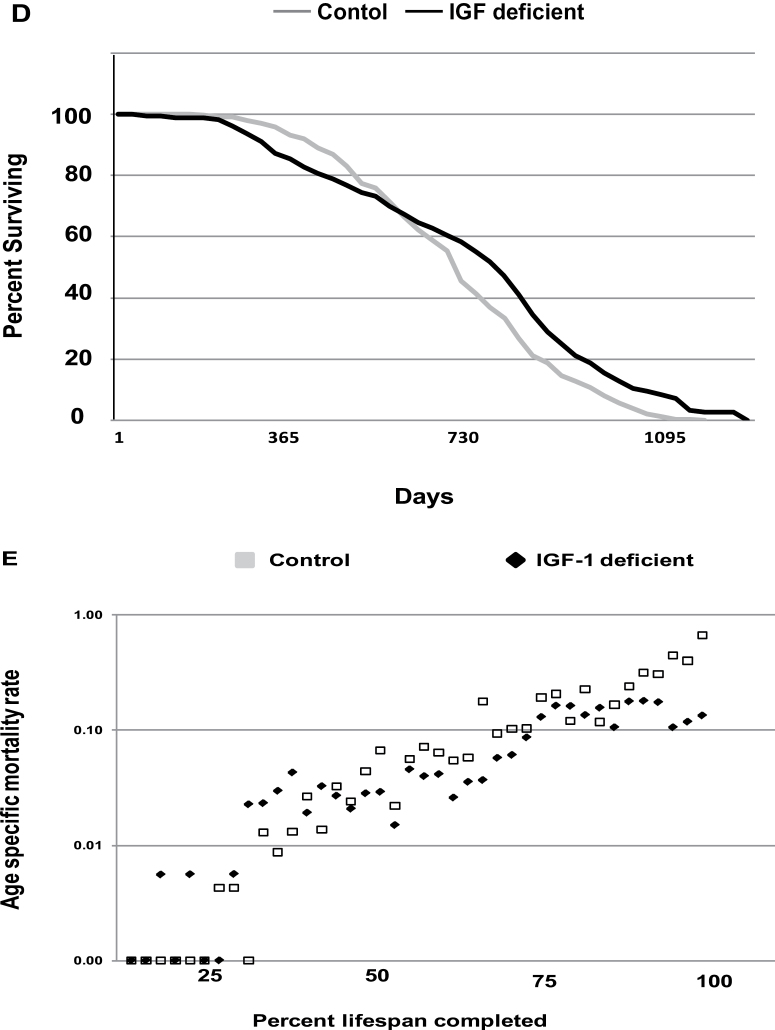
When the life-span data from all cohorts were combined, the mean life-span difference was 779 ± 289 versus 756 ± 218 for female IGF-1 deficient versus control and 738 ± 296 versus 704 ± 213 for male IGF-1 deficient versus male control (Table 2). Differences in maximum life span for the combined cohort were 1233 ± 92 versus 1118 ± 58 for female IGF-1 deficient versus control and 1172 ± 61 versus 1067 ± 54 for male IGF-1 deficient versus male control (Table 2). The differences in maximum life span were significant at p < .005, whereas the differences in mean life span were not significant. The survival curves for the life-span cohorts at each site are presented in Figure 1. The survival curve for all animals included in all three life span studies is also presented, as well as the relative mortality rate for all animals.
Figure 1.
Survival characteristics of IGF-1-deficient mice evaluated at three independent sites. Survival curves for life-span cohorts are presented from the three independent life-span analyses carried out as described in Materials and Methods. Panel A contains the survival curves for the life-span study carried out at the Lankenau Institute for Medical Research. Panel B contains the survival curve for the life-span study carried out at the Pennsylvania State University. Panel C contains the survival curve for the life-span study carried out at the University of Texas Health Sciences Center and San Antonio. Panel D contains a composite survival curve for all animals included in all three life-span studies. In Panel E, the age-specific mortality rates based upon the life table for Panel D were determined using 30-day increments throughout the life span.
Discussion
We present data on a life-span analysis of 418 mice of which 181 are homozygous for a mutant allele of the Igf1 gene leading to reduced IGF-1 production in all tissues tested to date. The mice producing reduced IGF-1 exhibit a significant increase in maximum life span but no significant change in mean life span relative to controls. The data suggest that a specific reduction in IGF-1 produces a significant increase in life span in mammals, but the increase is not commensurate with the level of reduction in IGF-1. The significance of the study lies in both the verification that IGF-1 directly influences life span in mice and the fact that the IGF-1 reduction did not produce an increase in mean life span. Both mean and maximal life span are important parameters for the assessment of life span; however, the implication of a change in either mean or maximum life span differs, and an examination of the age-specific mortality rates for control and IGF-1-deficient mice reveals a difference in the late-life mortality rate. The exponential increase in mortality rate that has been described in multiple species (25) is apparent in the control cohort (see Figure 1, Panel E). However, the IGF-1-deficient mice have an attenuation of this late-life increase in mortality rate. An effect on the late-life rise in mortality rate has been described in response to caloric restriction in rats but not in mice (44), and the change in late-life mortality we observe in the IGF-1-deficient mice suggests that lowering IGF-1 levels affect fundamental processes related to aging. A caveat to this conclusion is that the mean life span is not affected in our animals although there is a clear impact on the phenotype of the mice and on maximum life span. In addition, we must note that the IGF-1-deficient animals display an increase in early-life mortality that can be observed in the life-span curves for the Texas cohort and in the combined cohort. The reason for this early-life mortality is not clear, but it is of interest to note that the Drexel cohort was established through breeding of heterozygotes, whereas the Penn State and Texas cohorts were established through mating of homozygous IGF-1-deficient mice. Thus, the intrauterine environment differs between the cohorts. Given the documented influence of the intrauterine environment on adult phenotype (45), this may be one cause of some of the variation between cohorts in our analysis.
Regardless of the differences between the cohorts, in each case, the result is in contrast with multiple studies using mice with defects in the growth hormone axis such as the hypopituitary Ames and Snell dwarfs and the growth hormone receptor null mice (6–9). Ames dwarf mice are among the longest lived mouse lines that have been identified to date, displaying an increase in mean life span of 66% over controls (1206±32 vs 723±54), whereas the difference in mean life span in the Snell pituitary dwarf was reported to be 42% (1,178±235 days vs 832±158 days) (6,8). Growth hormone–deficient mice exhibit an increase of 37% in females (1031±41 vs 749±41), whereas the difference in mean life span between IGF-1-deficient and control mice was 3.5% (758±292 vs 730±215 days). Thus, the results presented in this study do not support the simple prediction that IGF-1 reduction alone is sufficient to extend life span in mammals and suggest that in there are additional factors that affect the fundamental mechanisms related to mammalian life span. Interestingly, there are also examples of growth hormone antagonism that fail to extend life span. For example, mice producing an antagonist of growth hormone do not display a life-span extension (9), and a recent analysis of these animals indicates an increasing adiposity with age, a phenotype not found in our IGF-1-deficient mice (46). An examination of tissue-specific IGF-1 levels in these growth hormone antagonist mice may provide information regarding the IGF status that may underlie aspects of the phenotype that are important to longevity.
One important element of the growth hormone/IGF-1 axis that has arisen during vertebrate evolution is growth hormone itself. Growth hormone appears to be an ancestral gene to the growth hormone family (growth hormone, prolactin, and chorionic somatomammotropin), which arose during vertebrate evolution (47). Interestingly, the growth hormone gene is divergent among species and appears to be the target of episodic evolution (48–51). Rapid evolution of the growth hormone gene has been characterized in primates (51), fish (52), and ruminants (53), and it has been proposed that functional switching to accommodate changes in secondary function may underlie these periods of rapid evolution for this gene locus (53). In contrast, IGF-1 is highly conserved across multiple species (54). Given the responsiveness of the growth hormone gene loci to evolutionary pressures, it is an attractive candidate for the acquisition of any additional functions related to life span during mammalian evolution.
In this context, a potentially important aspect of the phenotype associated with a reduction in IGF-1 level is the subsequent elevation of growth hormone due to loss of feedback inhibition at the level of the pituitary (55). Elevation of growth hormone has been found to reduce life span, and it produces a variety of pathologies including glom- erular and tubular pathologies in the kidney, liver fibrosis, and increased incidence of hepatocellular carcinoma (56). Previous studies indicate that the IGF-1-deficient mice do produce elevated levels of growth hormone (20), and we have verified the increase in growth hormone (4.4 ± 0.3 ng/mL vs 3.2 ± 0.9 ng/mL in females; 6.5 ± 4 ng/mL vs 2 ± 0.1 ng/mL) in IGF-1-deficient mice relative to controls; however, the levels of growth hormone in these animals is orders of magnitude lower than those observed in the short-lived growth hormone transgenics (56), and the dramatic pathologies identified in the growth hormone transgenic animals are not present in the IGF-1-deficient mice, for example, there is minimal impact on the size of the liver or other internal organs in the IGF-1-deficient mice (Table 1), whereas the growth hormone transgenic mice display enlarged internal organs (56). Interestingly, antagonizing growth hormone signaling through increased production of FGF21, a member of the fibroblast growth factor family (57), increases life span in mice (58).
The effect of altering IGF-1 levels on life span in a particular genetic background may also be dependent upon the age and tissue-specific effects of these changes. For example, IGF-1 has multiple roles in the CNS, inducing differentiation and survival of neurons, as well as potentiating learning and memory (59), and it has been proposed that the age-specific roles of IGF-1 may underlie the apparent contradiction between the protective role that IGF-1 plays in the CNS and reports, suggesting that mice with reduced IGF-1 levels maintain cognitive function during aging (60). In addition to its protective role in the brain, IGF-1 appears to provide benefit to the cardiovascular system. IGF-1 has been shown to reduce inflammatory processes related to atherosclerosis, and cardiac specific expression of IGF-1 provides protection against age-related changes in cardiac function and increases life span (61,62). It seems clear that IGF-1 may ameliorate some age-related decline in some tissues. These studies combined with reports that short-term growth hormone replacement early in life abrogates the life span of hypopituitary dwarfs (63), whereas adult onset of growth hormone deficiency extends life span (64), which has led to the suggestion that a reduction of growth hormone/IGF-1 signaling at critical stages of development may be sufficient to produce life-long benefits (65). Given these studies and our results, we suggest that there is, at the minimum, a requirement for reduced growth hormone levels in order to achieve significant life-span extension in rodents. Given the widespread perception that growth hormone injections may benefit human health and life span, this work underscores the need for additional investigation to reconciling the impact of IGF-1 and growth hormone on late-life events and the aging process.
Funding
This work was supported by National Institutes of Health training grants (T32 AG021890-05, AG223343) from the National Institute on Aging and by funds from the Drexel University Aging Initiative.
Acknowledgments
The support of the Lankenau Institute for Medical Research in the completion of the life-span study is gratefully acknowledged. We also thank Mark Tatar for the examination of dam effects and mortality rates on the Lankenau cohort.
References
- 1. Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322 [DOI] [PubMed] [Google Scholar]
- 2. Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110 [DOI] [PubMed] [Google Scholar]
- 3. Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946 [DOI] [PubMed] [Google Scholar]
- 4. Dorman JB, Albinder B, Shroyer T, Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141:1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957 [DOI] [PubMed] [Google Scholar]
- 6. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. [DOI] [PubMed] [Google Scholar]
- 7. Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123:121–130 [DOI] [PubMed] [Google Scholar]
- 8. Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613 [DOI] [PubMed] [Google Scholar]
- 10. Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–232 [DOI] [PubMed] [Google Scholar]
- 11. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A. 1997;94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59–72 [PubMed] [Google Scholar]
- 13. Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82 [PubMed] [Google Scholar]
- 14. Powell-Braxton L, Hollingshead P, Giltinan D, Pitts-Meek S, Stewart T. Inactivation of the IGF-I gene in mice results in perinatal lethality. Ann N Y Acad Sci. 1993;692:300–301 [DOI] [PubMed] [Google Scholar]
- 15. Powell-Braxton L, Hollingshead P, Warburton C, et al. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7(12B):2609–2617 [DOI] [PubMed] [Google Scholar]
- 16. Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82 [PubMed] [Google Scholar]
- 17. Liu JL, LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140:5178–5184 [DOI] [PubMed] [Google Scholar]
- 18. Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187 [DOI] [PubMed] [Google Scholar]
- 19. Bokov AF, Garg N, Ikeno Y, et al. Does reduced IGF-1R signaling in Igf1r+/- mice alter aging? PLoS One. 2011;6:e26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lembo G, Rockman HA, Hunter JJ, et al. Elevated blood pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. J Clin Invest. 1996;98:2648–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammers DW, Matheny RW, Jr, Sell C, et al. Impairment of IGF-I expression and anabolic signaling following ischemia/reperfusion in skeletal muscle of old mice. Exp Gerontol. 2011;46:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254 [DOI] [PubMed] [Google Scholar]
- 24. Birnboim HC, Wilkinson D, Sandhu JK, McLean JR, Ross W. Mutatect: a mouse tumour model for detecting radiation-induced mutations in vivo. Mutat Res. 1999;430:275–280 [DOI] [PubMed] [Google Scholar]
- 25. Arking R. The Biology of Aging: Observations and Principles. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 26. D’Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984;81:935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hammers DW, Matheny RW, Jr, Sell C, et al. Impairment of IGF-I expression and anabolic signaling following ischemia/reperfusion in skeletal muscle of old mice. Exp Gerontol. 2011;46:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crowe E, Sell C, Thomas JD, Johannes GJ, Torres C. Activation of proteasome by insulin-like growth factor-I may enhance clearance of oxidized proteins in the brain. Mech Ageing Dev. 2009;130:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162 [DOI] [PubMed] [Google Scholar]
- 30. Kadakia R, Arraztoa JA, Bondy C, Zhou J. Granulosa cell proliferation is impaired in the Igf1 null ovary. Growth Horm IGF Res. 2001;11:220–224 [DOI] [PubMed] [Google Scholar]
- 31. Zhou J, Chin E, Bondy C. Cellular pattern of insulin-like growth factor-I (IGF-I) and IGF-I receptor gene expression in the developing and mature ovarian follicle. Endocrinology. 1991;129:3281–3288 [DOI] [PubMed] [Google Scholar]
- 32. Zhou J, Bondy C. Anatomy of the human ovarian insulin-like growth factor system. Biol Reprod. 1993;48:467–482 [DOI] [PubMed] [Google Scholar]
- 33. Grigorescu F, Baccara MT, Rouard M, Renard E. Insulin and IGF-1 signaling in oocyte maturation. Horm Res. 1994;42:55–61 [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004;180:247–255 [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Zhou J, Bondy CA. Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 1999;13:1985–1990 [DOI] [PubMed] [Google Scholar]
- 36. Bikle D, Majumdar S, Laib A, et al. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–2329 [DOI] [PubMed] [Google Scholar]
- 37. Sell C, Dumenil G, Deveaud C, et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Resnicoff M, Coppola D, Sell C, Rubin R, Ferrone S, Baserga R. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 1994;54:4848–4850 [PubMed] [Google Scholar]
- 39. Takahara K, Tearle H, Ghaffari M, Gleave ME, Pollak M, Cox ME. Human prostate cancer xenografts in lit/lit mice exhibit reduced growth and androgen-independent progression. Prostate. 2011;71:525–537 [DOI] [PubMed] [Google Scholar]
- 40. Deitel K, Dantzer D, Ferguson P, et al. Reduced growth of human sarcoma xenografts in hosts homozygous for the lit mutation. J Surg Oncol. 2002;81:75–79 [DOI] [PubMed] [Google Scholar]
- 41. Yang XF, Beamer WG, Huynh H, Pollak M. Reduced growth of human breast cancer xenografts in hosts homozygous for the lit mutation. Cancer Res. 1996;56:1509–1511 [PubMed] [Google Scholar]
- 42. Wu Y, Cui K, Miyoshi K, et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–4388 [PubMed] [Google Scholar]
- 43. Dunn SE, Kari FW, French J, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672 [PubMed] [Google Scholar]
- 44. Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–1048 [DOI] [PubMed] [Google Scholar]
- 45. Sebert S, Sharkey D, Budge H, Symonds ME. The early programming of metabolic health: is epigenetic setting the missing link? Am J Clin Nutr. 2011;94(6 Suppl):1953S–1958S [DOI] [PubMed] [Google Scholar]
- 46. Berryman DE, Lubbers ER, Magon V, List EO, Kopchick JJ. A dwarf mouse model with decreased GH/IGF-1 activity that does not experience life- span extension: potential impact of increased adiposity, leptin, and insulin with advancing age. J Gerontol A Biol Sci Med Sci. 2013. [Epub ahead of print]. PMID: 23695394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kawauchi H, Sower SA. The dawn and evolution of hormones in the adenohypophysis. Gen Comp Endocrinol. 2006;148:3–14 [DOI] [PubMed] [Google Scholar]
- 48. Forsyth IA, Wallis M. Growth hormone and prolactin–molecular and functional evolution. J Mammary Gland Biol Neoplasia. 2002;7:291–312 [DOI] [PubMed] [Google Scholar]
- 49. Maniou Z, Wallis OC, Wallis M. Episodic molecular evolution of pituitary growth hormone in Cetartiodactyla. J Mol Evol. 2004;58:743–753 [DOI] [PubMed] [Google Scholar]
- 50. Wallis OC, Wallis M. Molecular evolution of growth hormone (GH) in Cetartiodactyla: cloning and characterization of the gene encoding GH from a primitive ruminant, the chevrotain (Tragulus javanicus). Gen Comp Endocrinol. 2001;123:62–72 [DOI] [PubMed] [Google Scholar]
- 51. Wallis OC, Wallis M. Evolution of growth hormone in primates: the GH gene clusters of the New World monkeys marmoset (Callithrix jacchus) and white-fronted capuchin (Cebus albifrons). J Mol Evol. 2006;63:591–601 [DOI] [PubMed] [Google Scholar]
- 52. Fukamachi S, Meyer A. Evolution of receptors for growth hormone and somatolactin in fish and land vertebrates: lessons from the lungfish and sturgeon orthologues. J Mol Evol. 2007;65:359–372 [DOI] [PubMed] [Google Scholar]
- 53. Wallis M. Function switching as a basis for bursts of rapid change during the evolution of pituitary growth hormone. J Mol Evol. 1997;44:348–350 [DOI] [PubMed] [Google Scholar]
- 54. Sparkman AM, Schwartz TS, Madden JA, et al. Rates of molecular evolution vary in vertebrates for insulin-like growth factor-1 (IGF-1), a pleiotropic locus that regulates life history traits. Gen Comp Endocrinol. 2012;178:164–173 [DOI] [PubMed] [Google Scholar]
- 55. Romero CJ, Pine-Twaddell E, Sima DI, et al. Insulin-like growth factor 1 mediates negative feedback to somatotroph GH expression via POU1F1/CREB binding protein interactions. Mol Cell Biol. 2012;32:4258–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68:71–87 [DOI] [PubMed] [Google Scholar]
- 57. Wu S, Levenson A, Kharitonenkov A, De Luca F. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J Biol Chem. 2012;287:26060–26067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang Y, Xie Y, Berglund ED, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239 [DOI] [PubMed] [Google Scholar]
- 60. Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6:799–806 [DOI] [PubMed] [Google Scholar]
- 62. Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932 [DOI] [PubMed] [Google Scholar]
- 65. Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]