Abstract
Background.
The use of potentially inappropriate medications in older adults can lead to known adverse drug events, but long-term effects are less clear. We therefore conducted a prospective cohort study of older women to determine whether PIM use is associated with risk of functional impairment or low cognitive performance.
Methods.
We followed up 1,429 community-dwelling women (≥75 years) for a period of 5 years at four clinical sites in the United States. The primary predictor at baseline was PIM use based on 2003 Beers Criteria. We also assessed anticholinergic load using the Anticholinergic Cognitive Burden scale. Outcomes included scores on a battery of six cognitive tests at follow-up and having one or more incident impairments in instrumental activities of daily living. Regression models were adjusted for baseline age, race, education, smoking, physical activity, a modified Charlson Comorbidity Index, and cognitive score.
Results.
The mean ± SD age of women at baseline was 83.2 ± 3.3. In multivariate models, baseline PIM use and higher ACB scores were significantly associated with poorer performance in category fluency (PIM: p = .01; ACB: p = .02) and immediate (PIM: p = .04; ACB: p = .03) and delayed recall (PIM: p = .04). Both PIM use (odds ratio [OR]: 1.36 [1.05–1.75]) and higher ACB scores (OR: 1.11 [1.04–1.19]) were also strongly associated with incident functional impairment.
Conclusions.
The results provide suggestive evidence that PIM use and increased anticholinergic load may be associated with risk of functional impairment and low cognitive performance. More cautious selection of medications in older adults may reduce these potential risks.
Key Words: Dementia, Cognitive aging, Medication, Epidemiology, Physical function.
High rates of medication consumption among older populations has led to the development of criteria to identify potentially inappropriate medications (PIMs), whose adverse effects can outweigh their therapeutic benefit (1). Despite growing literature highlighting the risks of PIM use, prevalence remains high in a variety of population settings, with PIMs prescribed to approximately 1 in 4 community-dwelling older adults (2,3) and to up to 1 in 2 in residential aged care facilities (4). In addition, PIM use can lead to an increased risk of preventable adverse drug events (1) as well as increased healthcare costs (5). Of particular concern are the many PIMs that act on the central nervous system, which may lead to cognitive impairment and affect an older adult’s ability to perform everyday tasks. Therefore, when possible, it becomes essential to limit the prescription of PIMs in older populations in order to improve their health outcomes and quality of life.
Much research on the functional outcomes of PIM use is based on cross-sectional or case–control data providing limited insight into long-term effects. A few longitudinal studies have shown that PIM use can be associated with specific adverse functional outcomes, including increased risk of falls, mortality, and hospitalization (6,7). Fewer studies have investigated the role of PIMs among the oldest old or in those at risk of cognitive decline, with some evidence indicating that individual classes of PIMs, such as anticholinergics and benzodiazepines, may be associated with cognitive impairment (8–10). The long-term effects of anticholinergics are of particular interest as they both account for a large portion of PIM use (11,12) and can have strong acute central effects and sedative properties.
To expand on existing findings, we conducted a longitudinal study in a population of women older than 75 years of age to test the hypothesis that PIM use is associated with increased risk of functional impairment and low cognitive performance. We also aimed to determine whether anticholinergic load in particular is associated with a higher risk of functional impairment and low cognitive performance.
Methods
Study Population
We studied participants enrolled in the Study of Osteoporotic Fractures, a multicenter, prospective study of women older than 65 years at baseline (13). In brief, 9,704 primarily Caucasian women who were able to walk were recruited using mailing lists at four sites in the United States from 1986 to 1988 (Minneapolis, Minnesota; Portland, Oregon; Baltimore, Maryland; Monongahela Valley, Pennsylvania). From 1997 to 1998, an additional cohort of 662 African American women was recruited. To focus on the population of women older than 75 years of age, we used the visit occurring between 2002 and 2004 as the baseline time point for the present study. Women were followed up for a mean of 4.9 years until the follow-up visit occurring between 2006 and 2008. Of the 4,606 women enrolled at baseline, 1,039 had incomplete medication inventory, 1,227 died, and 911 had incomplete data on cognitive or functional status or were lost to follow-up, resulting in an analytic cohort of 1,429 women. Compared with the other women enrolled at baseline, women in the analytic cohort were more likely to be younger, African American, and physically active, and to have a higher body mass index, drink alcohol more frequently, and less likely to have ever smoked or have comorbidities. This study was approved by an institutional review board (University of California, San Francisco), and written informed consent was obtained from all participants.
Measurements
At baseline, age was calculated and anthropometric measures were recorded. Lifestyle factors and medical history were recorded via questionnaire. Depression and anxiety were measured using the 15-item Geriatric Depression Scale (14) and the Goldberg Anxiety Scale (15), respectively. Baseline cognitive function was measured using the Mini Mental State Examination (MMSE). Sleep quality was measured using the total score on the Pittsburgh Sleep Quality Index (PSQI), a validated 19-item self-administered questionnaire designed to measure sleep quality and disturbances (16). To conduct medication inventory, women were asked to bring in all prescription and over-the-counter medications taken in the 30 days prior to the baseline visit. Medications were classified using the Iowa Drug Information System coding (17). A medication was considered a PIM if it was classified as such for older adults with cognitive impairment according to the 2003 Beers criteria (1). These medication classes included barbiturates, anticholinergics, antispasmodics, muscle relaxants, and central nervous system stimulants. In addition, benzodiazepines and sedative-hypnotics were added to this list as earlier literature has indicated these classes of medications can contribute to cognitive impairment in older adults (2,8). Medications were categorized into PIM classes using standard references and existing literature (2,17,18). Some medications belonged to more than one category.
We also measured anticholinergic load using the total score on the Anticholinergic Cognitive Burden (ACB) scale (18). The ACB score assigns a value between 0 and 3 for a given medication. A medication is assigned a 0 if there is no anticholinergic activity, and a 1 if there is possible anticholinergic activity suggested by serum anticholinergic activity or in vitro affinity to muscarinic receptors. For medications with known clinically relevant anticholinergic effects, a 2 or 3 is given, based on the drug’s ability to cross the blood–brain barrier and its association with delirium. The ACB score for each participant was computed by summing these values for each reported medication. The scale was developed through a systematic review by an interdisciplinary panel and validated in subsequent studies (9,19).
Outcome Assessment
We assessed cognitive function at follow-up using a battery of seven cognitive tests, including Trails B, Modified Mini Mental State Examination (3MS), California Verbal Learning Test, Second Edition (CVLT-II) short form, Backward Digit Span, and category and verbal fluency. The Trails B test assesses visual attention and information processing speed (20). The 3MS is designed to measure several cognitive domains, including orientation, registration, attention, recall, and visuospatial ability (21). The CVLT-II measures several aspects of verbal memory (22). For the present study, we analyzed CVLT-II scores on immediate and 10-minute delayed recall. Backward Digit Span is a test of working memory and information processing, requiring participants to repeat a series of numbers in the reverse order they were given (23). Category and verbal fluency measure semantic memory (24). The test required participants to recite as many words as possible that belonged to the category “vegetable,” or began with the letter “F.”
Functional impairment was measured by having one or more new impairments in instrumental activities of daily living (IADL) since baseline. IADLs included walking 2–3 blocks on level ground, climbing 10 steps without resting, preparing meals, doing heavy housework, and shopping for groceries or clothes. For each IADL, a “yes” response on a questionnaire (eg, “Do you have any difficulty walking 2 or 3 blocks outside on level ground?”) was considered an impairment.
Statistical Analysis
To determine whether any differences existed for baseline characteristics between PIM users and nonusers, we first conducted bivariate analyses using one-way analysis of variance tests for normally distributed variables, Kruskal-Wallis tests for non-normal continuous variables, and chi-square tests for categorical variables. To test the association between PIM use or ACB score and cognitive outcomes, we used both unadjusted and adjusted linear regression models. Similarly, we used both unadjusted and adjusted logistic regression models to evaluate the association between PIM use or ACB score and one or more new IADL impairments. With the exception of 3MS scores, as transformation of non-normal variables did not significantly change results, only models using untransformed variables were reported for purposes of interpretability. To achieve a normal distribution, 3MS scores were transformed by taking the square root of the number of errors on the test. Covariates in the fully adjusted models included demographic characteristics (age, race, education), and those lifestyle factors or comorbidities found to be associated with PIM use (p < .10). Comorbidities were not included individually but collectively adjusted for using a modified Charlson Comorbidity Index (25) (data for some comorbidities in the index were not available). The final models included adjustments for age, race, years of education, smoking (ever/never), physical activity (“takes walks for exercise”), and a modified Charlson Comorbidity Index. For the cognitive outcomes, baseline Mini Mental State Examination score was also included. Because some of the most frequently reported PIMs were indicated for anxiety, sleep, or depression, which can be associated with cognitive and functional status, we conducted sensitivity analysis by further adjusting for these factors. All analyses were performed using SAS 9.2 software (SAS Institute, Inc., Cary, North Carolina).
Results
Population Characteristics
Table 1 displays baseline characteristics of the 1,429 women in our study. There were 395 PIM users (27.6%), and the mean ± SD ACB score was 1.6 ± 1.9. for the women. PIM users were more likely to exercise less (p = .03) and have increased comorbidities compared with women who did not use PIMs. The most common class of PIMs was anticholinergics (n = 300; 76.0%), followed by antispasmodics (n = 100; 25.3%), benzodiazepines (n = 98; 24.8%), sedative-hypnotics (n = 44; 11.1%), central nervous system stimulants (n = 17; 4.3%), muscle relaxants (n = 10; 2.5%), and barbiturates (n = 9; 2.3%). The most common individual PIMs accounting for more than 90% of all PIM use were diphenhydramine (n = 83; 21.0%), oxybutynin (n = 54; 13.7%), meclizine (n = 40; 10.1%), paroxetine (n = 30; 7.6%), lorazepam (n = 28; 7.1%), codeine (n = 28; 7.1%); amitriptyline (n = 25; 6.3%); tolterodine (n = 23; 5.8%); temazepam (n = 21; 5.3%), alprazolam (n = 20; 5.1%) and chlorpheniramine (n = 20; 5.1%).
Table 1.
Baseline Characteristics of Participants (n = 1,429)
| Characteristic | PIM users (n = 395) | Nonusers (n = 1,034) | p |
|---|---|---|---|
| Age (mean ± SD) | 83.0±3.1 | 82.8±3.1 | .24 |
| Caucasian (n, %) | 356 (90.1%) | 906 (87.9%) | .23 |
| Education (years, mean ± SD) | 12.9±2.5 | 12.8±2.5 | .85 |
| Smoking history (ever, n, %) | 149 (37.8%) | 336 (32.6%) | .06 |
| Alcohol (drinks/wk in past 30 days, mean ± SD) | 1.11±2.6 | 1.04±2.9 | .21 |
| Takes walks for exercise (n, %) | 144 (36.8%) | 440 (43.1%) | .03 |
| Body mass index (mean ± SD) | 27.2±4.5 | 27.3±4.3 | .88 |
| COPD (n, %) | 65 (16.5%) | 107 (10.4%) | .02 |
| Hypertension (n, %) | 246 (62.4%) | 604 (58.5%) | .17 |
| Myocardial infarction (n, %) | 53 (13.5%) | 96 (9.3%) | .02 |
| Osteoarthritis (n, %) | 190 (48.2%) | 352 (34.1%) | <.001 |
| Stroke (n, %) | 47 (11.9%) | 106 (10.3%) | .36 |
| Type II diabetes (n, %) | 40 (10.2%) | 111 (10.8%) | .74 |
| Mini Mental State Examination score (mean ± SD) | 28.3±1.5 | 28.4±1.7 | .62 |
| No. of IADL impairments (mean ± SD) | 1.4±1.5 | 0.9±1.3 | <.001 |
Notes: COPD = chronic obstructive pulmonary disease; IADL = instrumental activities of daily living.
Use of PIMs
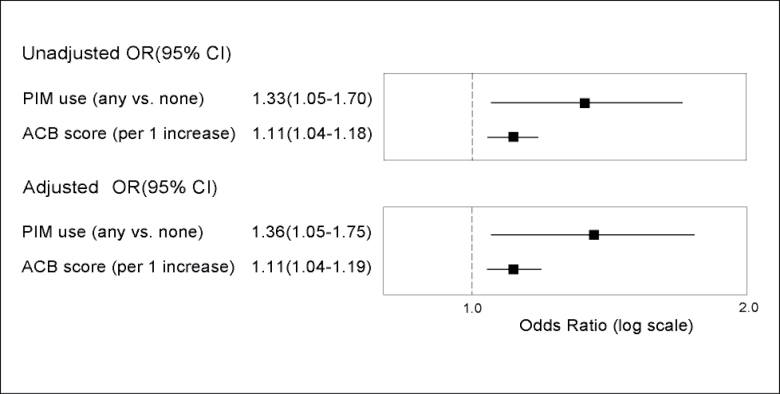
As shown in Figure 1, PIM use was significantly associated with one or more new IADL impairments in the unadjusted model (OR: 1.33 [1.05–1.70]) and after adjustment for age, race, education, smoking, physical activity, and a modified Charlson Comorbidity Index (OR: 1.36 [1.05–1.75]). Table 2 shows that women who used PIMs at baseline generally had poorer performance on cognitive tests at follow-up compared with nonusers. PIM use was significantly associated with a decreased mean number of words recalled in both CVLT-II tests. For immediate recall, the difference in mean ± SE number of words recalled for PIM users relative to nonusers was –0.81 ± 0.33 (p = .02). Adjusting for covariates slightly attenuated the effect, but it remained significant (mean ± SE: –0.72 ± 0.32 words; p = .02). The CVLT-II delayed recall test showed similar results (unadjusted mean ± SE: –0.38±0.16 words [p = .02]; adjusted mean ± SE: −0.33 ± 0.16 words [p = .04]). For the Trails B test, PIM users on average took longer to complete the test compared with nonusers (mean ± SE: 24.0 ± 10.2 seconds; p = .02). However, after adjustment, the effect size noticeably decreased and the association was no longer significant (mean ± SE: 8.5 ± 8.8 seconds; p = .33). A similar pattern was seen for the 3MS, where only in the unadjusted model did PIM users perform significantly worse (unadjusted mean ± SE: 0.17 ± 0.08; p = .03; adjusted mean ± SE: 0.11 ± 0.07 seconds; p = .13). In the category fluency test, the mean number of words recited was significantly fewer in PIM users compared with that in nonusers in both unadjusted (mean ± SE: −0.62 ± 0.21; p = .004) and adjusted models (mean ± SE: −0.53 ± 0.21; p = .01). Both the backward digit span and the verbal fluency test showed very small effect sizes and no significant differences between PIM users and nonusers. After further adjustment for anxiety, sleep, and depression, results were largely unchanged.
Figure 1.
Association of potentially inappropriate medications (PIM) use or increase in Anticholinergic Cognitive Burden (ACB) score with ≥1 new instrumental activities of daily living (IADL) impairments. Odds ratios from unadjusted and adjusted logistic regression models showing the association between PIM use or increase in ACB score and ≥1 new IADL impairments. Adjusted models include as covariates age, race, education, smoking, physical activity, and a modified Charlson Comorbidity Index.
Table 2.
Association Between Baseline Potentially Inappropriate Medication (PIM) Use and Cognitive Performance (Mean Cognitive Test Scores, by PIM Use)
| Unadjusted mean (SE) | Adjusted mean (SE)* | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | PIM users | Nonusers | Difference | p | PIM users | Nonusers | Difference | p |
| CVLT-II immediate recall (0–36) | 22.81 (0.28) | 23.62 (0.17) | −0.81 (0.33) | .02 | 22.97 (0.27) | 23.69 (0.17) | −0.72 (0.32) | .02 |
| CVLT-II delayed recall (0–9) | 4.84 (0.14) | 5.22 (0.09) | −0.38 (0.16) | .02 | 4.92 (0.14) | 5.25 (0.08) | −0.33 (0.16) | .04 |
| Category fluency | 10.07 (0.18) | 10.69 (0.11) | −0.63 (0.21) | .004 | 10.19 (0.19) | 10.72 (0.11) | −0.53 (0.22) | .01 |
| Verbal fluency | 10.54 (0.21) | 10.59 (0.13) | −0.05 (0.25) | .85 | 10.66 (0.21) | 10.61 (0.13) | 0.05 (0.25) | .84 |
| Trails B | 205.4 (8.7) | 181.4 (5.2) | 24.0 (10.2) | .02 | 188.2 (7.5) | 179.7 (4.5) | 8.5 (8.8) | .33 |
| 3MS (transformed)† | 3.44 (0.07) | 3.27 (0.04) | 0.17 (0.08) | .03 | 3.33 (0.06) | 3.22 (0.04) | 0.11 (0.07) | .13 |
| Backward digit span (0–14) | 5.51 (0.11) | 5.47 (0.06) | 0.04 (0.12) | .72 | 5.57 (0.10) | 5.51 (0.06) | 0.06 (0.12) | .65 |
Notes:*adjusted for age, race, education, smoking, physical activity, modified Charlson Comorbidity Index, and baseline Mini Mental State Examination score.
†3MS score was transformed by taking the square root of the number of errors.
Anticholinergic Load
A one-unit increase in ACB score was significantly associated with one or more new IADL impairments in both unadjusted (OR: 1.11 [1.04–1.18]) and adjusted (OR: 1.11 [1.04–1.19]) models (Figure 1). Similar to PIM use, increased baseline anticholinergic load was generally associated with worse cognitive performance at follow-up, although overall effects were smaller (Table 3). For the CVLT-II test of immediate recall, each increase in ACB score was significantly associated with a fewer mean number of words recalled (unadjusted mean ± SE: −0.16±0.08 [p = .04]; adjusted mean ± SE: −0.17±0.08 [p = .03]). However, for delayed recall, the association was not significant and the effect size was small. For category fluency, an increase in ACB score was significantly associated with fewer mean number of words recited in unadjusted (mean ± SE: −0.14±0.05; p = .006) and adjusted (mean ± SE: −0.12±0.05; p = .02) models. Results for verbal fluency were similar but did not reach significance (unadjusted mean ± SE: −0.12 ± 0.06 [p =.05]; adjusted mean ± SE: −0.11±0.06 [p = .07]). For Trails B and the 3MS, a higher ACB score was not significantly associated with worse performance on either test. Similar to PIM use, ACB score was not significantly associated with the backward digit span test, and effect sizes were very small. Results were similar after further adjustment for anxiety, sleep, and depression.
Table 3.
Association Between Baseline Anticholinergic Load and Cognitive Performance (Mean Difference of Cognitive Test Scores, Per One Unit Increase in ACB Score)
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| Outcome | Mean (SE) | p | Mean (SE) | p |
| CVLT-II immediate recall (0–36) | −0.16 (0.08) | .04 | −0.17 (0.08) | .03 |
| CVLT-II delayed recall (0–9) | −0.04 (0.04) | .29 | −0.03 (0.04) | .48 |
| Category fluency | −0.14 (0.05) | .006 | −0.12 (0.05) | .02 |
| Verbal fluency | −0.12 (0.06) | .05 | −0.11 (0.06) | .07 |
| Trails B | 3.91 (2.47) | .11 | 0.96 (0.22) | .66 |
| 3MS† | 0.02 (0.02) | .26 | 0.01 (0.02) | .68 |
| Backward digit span (0–14) | 0.02 (0.03) | .50 | 0.03 (0.03) | .41 |
Notes :*adjusted for age, race, education, smoking, physical activity, modified Charlson Comorbidity Index, and baseline Mini Mental State Examination score.
†3MS score was transformed by taking the square root of the number of errors.
Discussion
In a population of women 75 years of age and older at baseline, we found that PIM use was generally associated with worse scores on cognitive tests after 5 years of follow-up. Results for anticholinergic load were qualitatively similar and showed a smaller effect size. PIM use and increased anticholinergic load were also associated with a higher risk of worse functional impairment at follow-up. After adjusting for demographic variables, lifestyle factors and comorbidities, results largely remained unchanged. These findings are consistent with earlier prospective cohort studies showing that PIM use can not only lead to short-term adverse events (6,7) but also to long-term cognitive (26) and functional adverse outcomes, affecting an older adult’s ability to perform everyday tasks and function independently. Earlier studies have also shown that anticholinergic load in particular can worsen future cognitive function (26,27). In addition, while many other studies have shown a high prevalence of PIM use among the young old (28,29), the present study shows that PIM use remains high even in women into their eighth and ninth decades of life, a population at particularly high risk for cognitive and functional decline.
In our study, the most consistent and significant associations were seen between PIM use or increased anticholinergic load and lower scores in tests of memory function. In addition, performance in category fluency was particularly affected, while differences in verbal fluency were less pronounced, a pattern frequently seen in individuals with mild cognitive impairment (30) and Alzheimer’s disease (31). Moreover, baseline Mini Mental State Examination scores were not significantly different between PIM users and nonusers, suggesting that existing cognitive impairment may not have contributed to group differences at follow-up. Both PIM use and anticholinergic load were significantly associated with having new IADL impairments at follow-up in both unadjusted and adjusted models. It is possible that PIM use can be especially detrimental on IADL because of both peripheral effects, which can directly impair function (1), and central effects impairing the ability to perform even basic activities of daily living (32).
If PIM use can lead to detrimental effects on cognitive and functional outcomes, strategies to reduce PIM use will need to be implemented. PIM criteria are used extensively in research and increasingly for the regulation and quality assessment of health care. However, an efficient method is needed to apply these criteria in often demanding and busy clinical settings. Recent randomized controlled trials have shown that consultation with a physician familiar with the criteria or computerized warnings can significantly reduce inappropriate prescription in older adults (33,34). Other methods to limit the use of PIMs can include improved health education or modified medication warnings. Finally, although caution is needed when deciding whether an older adult should be administered a PIM, a careful balance always needs to be struck, considering the risk–benefit ratio as well as individual characteristics. In certain situations, treatment using PIMs may in fact be the best available option. Regardless, the relatively high rate of PIM use both in this study and other ambulatory populations of older adults (2) suggests that some of the most frequently reported PIMs may still be avoided. For example, diphenhydramine, likely used for insomnia or allergy relief, was the most frequently reported PIM in this cohort. However, alternative treatments may be more appropriate as diphenhydramine use in older adults is consistently considered high-risk (35) with limited data on effectiveness (36). Similarly, recent studies question the risk–benefit ratio of the second most frequently reported PIM, oxybutynin, and suggest other treatments such as bladder training to treat urinary incontinence (37,38). Lastly, several of the most frequently reported PIMs included benzodiazepines. Although once a first-line treatment for anxiety, the latest Beers’ criteria now lists all benzodiazepines all potentially inappropriate, and numerous studies consistently show a variety of cognitive and functional adverse effects as well as increased risk of mortality (39).
Various limitations arise when conducting a longitudinal study in an older population. With high levels of mortality and morbidity, many factors, particularly sleep disorders and psychiatric comorbidities, can be associated with both PIM use and cognitive and/or functional outcomes. It may be difficult to adequately adjust for all these factors, thus leading to residual confounding. Although we found significant associations between PIM use or ACB score and cognitive test scores, the effect size was small. However, both PIM use and ACB score showed considerable variability, which may have attenuated the findings. Additionally, our study consisted of only female participants, making these findings less generalizable to a male population. Gender differences include a higher prevalence of PIM use in older women than in older men, as well as qualitative differences in the classes of PIMs most often used (40,41). Because only medications taken within 30 days prior to the baseline visit were recorded, the actual duration of PIM use is not known. Moreover, medications taken outside the 30-day period can be omitted, leading to misclassification and/or underreporting of actual PIM use. Lastly, there may be selection bias from both loss to follow-up and the women without medication inventory at baseline. However, if participants who were not in the analytic cohort throughout the study period were more likely to be PIM users and have more cognitive and functional decline than those who remained, results would most likely be biased toward the null. Strengths of this study include the prospective design, which is not subject to issues such as the recall bias common in retrospective studies or the causal limitations inherent in cross-sectional and case–control studies. In addition, many baseline characteristics, including common confounders such as age or education, were not significantly different between groups, suggesting the results may not be strongly affected by confounding.
The results of this study provide support to the hypothesis that PIM use can lead to both increased difficulties in everyday functioning and poorer cognitive function compared with nonusers. Even in a population of women into their seventh and eighth decades of life, nearly 1 in 3 were taking at least one PIM. Increased awareness of inappropriate medications is needed among health professionals, caregivers, as well as older adults themselves. Future prospective studies are needed to confirm the long-term cognitive and functional effects of PIM use.
Funding
This work was supported by the National Institute of Aging at the National Institutes of Health (grants K24 AG 031155, R01 AG 026720) and the Alzheimer’s Association (grant IIRG-08-88872). The Study of Osteoporotic Fractures is supported by the National Institutes of Health. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Conflict of Interest
Dr. Steinman received an honorarium for serving on the American Geriatrics Society 2012 Beers Criteria Update Expert Panel. Dr. Ensrud reports receiving in-kind travel support from Merck Sharp & Dohme to attend annual Data Monitoring Committee meetings. Dr. Yaffe has served on data safety monitoring boards for Pfizer Inc, Medivation, Inc., Takeda Pharmaceutials Inc., and the NIH (NIMH and NIA trials); has served as a consultant for Novartis.
References
- 1. Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–2724. 10.1001/archinte. 163.22.2716 [DOI] [PubMed] [Google Scholar]
- 2. Mort JR, Aparasu RR. Prescribing potentially inappropriate psychotropic medications to the ambulatory elderly. Arch Intern Med. 2000;160:2825–2831 [DOI] [PubMed] [Google Scholar]
- 3. Barton C, Sklenicka J, Sayegh P, Yaffe K. Contraindicated medication use among patients in a memory disorders clinic. Am J Geriatr Pharmacother. 2008;6:147–152. 10.1016/j.amjopharm. 2008.08.002 [DOI] [PubMed] [Google Scholar]
- 4. Somers M, Rose E, Simmonds D, Whitelaw C, Calver J, Beer C. Quality use of medicines in residential aged care. Aust Fam Physician. 2010;39:413–416 [PubMed] [Google Scholar]
- 5. Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting. Med Care. 2005;43:1171–1176 [DOI] [PubMed] [Google Scholar]
- 6. Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50:1629–1637 [DOI] [PubMed] [Google Scholar]
- 7. Dedhiya SD, Hancock E, Craig BA, Doebbeling CC, Thomas J., 3rd Incident use and outcomes associated with potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother. 2010;8:562–570. 10.1016/S1543-5946(10)80005-4 [DOI] [PubMed] [Google Scholar]
- 8. Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18:37–48 [DOI] [PubMed] [Google Scholar]
- 9. Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Billioti de Gage S, Begaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai HY, Hwang SJ, Chen YC, Chen TJ, Lin MH, Chen LK. Prevalence of the prescribing of potentially inappropriate medications at ambulatory care visits by elderly patients covered by the Taiwanese National Health Insurance program. Clin Ther. 2009;31:1859–1870. 10.1016/j.clinthera.2009.08.023 [DOI] [PubMed] [Google Scholar]
- 12. Egger SS, Bachmann A, Hubmann N, Schlienger RG, Krahenbuhl S. Prevalence of potentially inappropriate medication use in elderly patients: comparison between general medical and geriatric wards. Drugs Aging. 2006;23:823–837 [DOI] [PubMed] [Google Scholar]
- 13. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773 [DOI] [PubMed] [Google Scholar]
- 14. Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontologist. 1986;5:165–173 [Google Scholar]
- 15. Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. BMJ. 1988;297:897–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 17. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411 [DOI] [PubMed] [Google Scholar]
- 18. Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4:311–320 [Google Scholar]
- 19. Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75:152–159. 10.1212/WNL.0b013e3181e7f2ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276 [Google Scholar]
- 21. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318 [PubMed] [Google Scholar]
- 22. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Second Edition (CVLT-II). San Antonio, TX: Psychological Corp; 2000 [Google Scholar]
- 23. The Psychological Corporation WAIS-III/WMS-III Technical Manual: Updated. San Antonio, TX; 2002 [Google Scholar]
- 24. Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York, NY: Oxford University Press; 1991 [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 26. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477–1483. 10.1111/j.1532-5415.2011.03491.x [DOI] [PubMed] [Google Scholar]
- 27. Carriere I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169:1317–1324. 10.1001/archinternmed.2009.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bongue B, Naudin F, Laroche ML, et al. Trends of the potentially inappropriate medication consumption over 10 years in older adults in the East of France. Pharmacoepidemiol Drug Saf. 2009;18:1125–1133. 10.1002/pds.1762 [DOI] [PubMed] [Google Scholar]
- 29. Weston AL, Weinstein AM, Barton C, Yaffe K. Potentially inappropriate medication use in older adults with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2010;65:318–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy KJ, Rich JB, Troyer AK. Verbal fluency patterns in amnestic mild cognitive impairment are characteristic of Alzheimer’s type dementia. J Int Neuropsychol Soc. 2006;12:570–574 [DOI] [PubMed] [Google Scholar]
- 31. Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42:1212–1222. 10.1016/j.neuropsychologia.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 32. Hall JR, Vo HT, Johnson LA, Barber RC, O’, Bryant SE. The Link between Cognitive Measures and ADLs and IADL Functioning in Mild Alzheimer’s: What Has Gender Got to Do with It? Int J Alzheimers Dis. 2011;2011:276734. 10.4061/2011/276734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Terrell KM, Perkins AJ, Dexter PR, Hui SL, Callahan CM, Miller DK. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc. 2009;57:1388–1394. 10.1111/j.1532-5415.2009.02352.x [DOI] [PubMed] [Google Scholar]
- 34. Gallagher PF, O’, Connor MN, O’, Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89:845–854. 10.1038/clpt.2011.44 [DOI] [PubMed] [Google Scholar]
- 35. Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 2. Management of sleep disorders in older people. CMAJ. 2007;176:1449–1454. 10.1503/cmaj.070335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montgomery P, Lilly J. Insomnia in the elderly. Clin Evid (Online). 2007;2007. [PMC free article] [PubMed] [Google Scholar]
- 37. Chancellor M, Boone T. Anticholinergics for overactive bladder therapy: central nervous system effects. CNS Neurosci Ther. 2012;18:167–174. 10.1111/j.1755-5949.2011.00248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moga DC, Carnahan RM, Lund BC, et al. Risks and Benefits of Bladder Antimuscarinics Among Elderly Residents of Veterans Affairs Community Living Centers. J Am Med Dir Assoc. 2013. 10.1016/j.jamda.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 39. Halme AS, Beland SG, Preville M, Tannenbaum C. Uncovering the source of new benzodiazepine prescriptions in community-dwelling older adults’. Int J Geriatr Psychiatry. 2012;28:248–255. 10.1002/gps.3818 [DOI] [PubMed] [Google Scholar]
- 40. Johnell K, Weitoft GR, Fastbom J. Sex differences in inappropriate drug use: a register-based study of over 600,000 older people. Ann Pharmacother. 2009;43:1233–1238. 10.1345/aph.1M147 [DOI] [PubMed] [Google Scholar]
- 41. Bierman AS, Pugh MJ, Dhalla I, et al. Sex differences in inappropriate prescribing among elderly veterans. Am J Geriatr Pharmacother. 2007;5:147–161. 10.1016/j.amjopharm.2007.06.005 [DOI] [PubMed] [Google Scholar]