Abstract
Background.
Growing evidence suggests that self-reported physical activity accounts for variability in cognitive function among older adults, and aerobic intervention may improve cognitive function in this population. However, much less is known about the longitudinal association between direct measures of cardiorespiratory fitness and cognitive function across the life span. The present study examined the prospective association between symptom-limited maximal oxygen consumption (VO2max) and longitudinal performance on a comprehensive neuropsychological battery.
Methods.
Up to 1,400 participants aged 19–94 years underwent initial VO2max assessment and completed subsequent tests of memory, attention, perceptuomotor speed, language, and executive function, in addition to cognitive screening measures, on up to six occasions (mean, M = 2; standard deviation, SD = 1) for up to 18 years (M = 7, SD = 3). Mixed-effects regression models were adjusted for demographic, biomedical, and behavioral confounders.
Results.
Analyses revealed significant longitudinal associations between baseline VO2max and trajectory of performance on multiple measures of verbal and visual memory, as well as on a cognitive screening test (all ps < .05). Individuals with lower VO2max demonstrated accelerated trajectories of cognitive decline over time.
Conclusions.
Baseline cardiorespiratory fitness is related to longitudinal neuropsychological performance, and memory appears to be a particularly vulnerable domain. Evidence that aerobic fitness is associated with accelerated cognitive decline emphasizes the possible importance of behavioral interventions to optimize cognitive aging over time.
Key Words: Cardiorespiratory fitness, Aerobic fitness, Maximal oxygen consumption, Cognitive function, Neuropsychology.
It is well known that the incidence and prevalence of cognitive decline and dementia are projected to increase substantially over the course of the 21st century due to a confluence of demographic trends. For instance, one in eight Americans aged 65 years and older currently has Alzheimer’s disease, and this rate is expected to double by 2050 (1). Efforts to identify risk and protective factors for cognitive decline have consequently intensified in recent years. A multitude of cardiovascular risk factors have received attention in this regard, and among these, physical fitness has been suggested as a useful intervention target to optimize cognitive aging (2). According to the most recent National Health Interview Survey in 2010, 62% of U.S. adults were classified as overweight or obese, and 79% did not meet federally recommended thresholds of physical activity (3).
Self-reported leisure time physical activity has been linked with cognitive function among older adults across several studies. In the National Health and Nutrition Examination Survey (NHANES-III), physical activity was correlated positively with concurrent performance on a brief mental status examination (4). Lower levels of self-reported physical activity were also associated with accelerated cognitive decline on global mental status measures during 2- to 11-year follow-ups (5–7), in addition to prospective risk of dementia (8,9). Nonetheless, such studies are limited by reliance on self-reported physical activity and the often-poor correspondence between leisure time physical activity and physiological measures of cardiorespiratory fitness.
Research incorporating physiological assessment of cardiorespiratory fitness is relatively sparse but also suggests that physical fitness is protective against decrements in cognitive function. For instance, a composite measure of estimated fitness, based on grip strength, 6-meter walk time, and forced expiratory volume from the lungs, was associated with global cognitive function in a group of 460 persons of age 79 years (10). Several smaller-scale cross-sectional studies (with n ≤ 100) have also linked higher maximal oxygen consumption (VO2max) on the treadmill test with better concurrent cognitive performance (11–14). Longitudinally, multiple studies have demonstrated associations between aerobic exercise interventions and modestly improved cognitive performance among older adults, particularly for executive functions (15,16).
To date, however, limited research has examined VO2 max, the widely accepted “gold standard” measure of cardiorespiratory fitness, as a predictor of longitudinal trajectories of cognitive function. Barnes and colleagues (17) indeed found an association of worse baseline VO2max with greater 6-year decline on the Modified Mini-Mental State Examination (Modified MMSE), a cognitive screening measure. Baseline VO2max was also associated with worse follow-up performance on measures of attention and executive function and on one measure of verbal memory. However, the absence of detailed neuropsychological assessment at baseline limited conclusions regarding cognitive change over time. Here, we examined the prospective association between baseline cardiorespiratory fitness, as measured by VO2max (symptom limited), and longitudinal performance on an extensive neuropsychological battery among up to 1,400 adults enrolled in the Baltimore Longitudinal Study of Aging (BLSA).
Methods
Participants
Participants were derived from the BLSA, a prospective study of community-dwelling volunteers initiated by the National Institute on Aging in 1958. Approximately every 2 years, participants visit the National Institute on Aging in Baltimore for medical, psychological, and cognitive testing. Data collection for the present study began in 1985, when aerobic fitness was introduced to the BLSA protocol, and continued through 2006. A total of 1,499 participants were available for the present study. Individuals with clinically significant cardiovascular disease or orthopedic or neuromuscular limitations at the time of aerobic fitness testing were not eligible for study inclusion. Further, persons with incident heart failure, stroke, transient ischemic attack, dementia, or other neurological disease (ie, Parkinson’s disease, Huntington’s disease, epilepsy, and multiple sclerosis) were censored from 2 years before diagnosis. Dementia diagnosis was determined according to previously published protocol (18). Following these exclusions, 1,400 participants were available for data analyses. Because the BLSA uses continuous enrollment procedures, participants have differential start times and ages, numbers of visits, and follow-up times in the project (see Table 1). During the course of the study, 115 (8.2%) participants died and 46 (3.3%) participants formally withdrew. Participants had an average of two visits (standard deviation, SD = 1; range = 1–6), and the average time between visits was 3 years (SD = 1; range = 0.5–7). Participants were followed for up to 18 years (mean = 7; SD = 3). Institutional Review Board approval was obtained from the Johns Hopkins Bayview Medical Center before 2002 and from the MedStar Research Institute afterward. All of the participants provided written informed consent, and all of the procedures followed were in accordance with institutional guidelines. The Institutional Review Board of the University of Maryland, Baltimore County, approved these data analyses.
Table 1.
Sample Size by Number of Visits
| Number of Visits | n (% of sample) |
|---|---|
| ≥1 | 1,400 (100.0) |
| ≥2 | 615 (43.9) |
| ≥3 | 204 (14.6) |
| ≥4 | 60 (4.3) |
| ≥5 | 11 (0.8) |
| 6 | 1 (0.1) |
Cardiorespiratory Fitness Assessment
Participants underwent a single, symptom-limited graded maximal treadmill exercise test following a modified Balke protocol with measurement of VO2 (19). Men walked at a constant 3.5 miles/hour (~5.6 km/hour) and women at a constant 3.0 miles/hour (~4.8 km/hour) on a motor-driven treadmill. Treadmill grade was increased by 3% every 2 minutes until self-determined exhaustion. Expired gas volumes were measured using either Tissot tanks or a Parkinson–Cowan gas meter (Waitsfield, VA). Expired O2 and CO2 concentrations were measured using either dedicated O2 and CO2 analyzers or a medical mass spectrometer (Perkin-Elmer MGA-1110, Boston, MA). Oxygen consumption was measured continuously and calculated every 30 seconds throughout the exercise. The highest value was termed peak VO2, or VO2max, expressed as milliliters O2 per kilogram per minute.
Neuropsychological Assessment
At each BLSA visit, standard neuropsychological tests (20) were administered by highly trained psychometricians. The numbers that follow each test indicate respective sample sizes because of test-specific missing data (presumed to be random with the exception of a more comprehensive battery administered to participants aged 60 and older). The Blessed Information–Memory–Concentration (I–M–C) test (n = 1,400) and the MMSE (n = 637) are cognitive screening measures that assess global cognitive status. The Digits Forward (n = 963) and Digits Backward (n = 965) portions of the Digit Span subtest of the Wechsler Adult Intelligence Scale–Revised assessed attention and concentration. The California Verbal Learning Test (CVLT; n = 890) measured verbal learning and memory, including immediate free recall, learning slope (average number of new words gained per trial), and short- and long-delay free recall. The Benton Visual Retention Test (BVRT; n = 1,099) evaluated immediate visual memory. The Trail Making Test Part A (n = 613) and Part B (n = 589) assessed attention, perceptuomotor speed, visuomotor scanning, and mental flexibility, an executive function. Letter Fluency (n = 616) and Category Fluency (n = 616) examined phonemic and semantic association fluency, respectively, in addition to executive function. The Boston Naming Test (n = 490) assessed confrontation naming, a language ability. The Card Rotations Test (n = 895) measured mental spatial rotation, a visuospatial function. For the Blessed I–M–C Test, BVRT, and Trail Making Test, worse performance is indicated by higher scores. For all other neuropsychological measures, better performance is indicated by higher scores. Longitudinal distributions of test-specific sample sizes were found to be highly similar across cognitive tests and comparable with the distribution presented in Table 1.
Covariates
Covariate selection was predicated on the theoretical potential for confounding of analyses, given known associations of certain risk factors and conditions with cardiorespiratory fitness and neuropsychological function. Age and education were assessed in years. Binary covariates included sex (male = 1; female = 0), race (white = 1; nonwhite = 0), and use of antihypertensive medications (yes = 1; no = 0). Resting brachial systolic and diastolic blood pressure values were obtained three times bilaterally with participants in the seated position following a 5-minute quiet resting period. Systolic and diastolic blood pressures were defined by Korotkoff phases I and V, respectively. Hypertension was defined as average systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medications. Body mass index was calculated as the ratio of weight (in kilograms) to height (in meters) squared, both measured with a clinical calibrated scale. Cardiovascular disease was defined time dependently as presence or absence (coded 0 or 1, respectively) of any of the following conditions: coronary artery disease, cardiac surgery, peripheral arterial disease, and diabetes mellitus. Inflammatory disease was defined time dependently as presence or absence (coded 0 or 1, respectively) of any of the following conditions: osteoarthritis, rheumatoid arthritis, rheumatism, hepatitis, or systemic lupus erythematosus. The Center for Epidemiological Studies-Depression scale assessed self-reported depressive symptomatology.
Statistical Analyses
All statistical analyses were performed using SAS version 9.2 (Cary, NC). Mixed-effects regression analyses using unstructured correlation matrices were conducted to examine longitudinal relations of continuous VO2max with cognitive function. This statistical approach handles inconsistent measurement intervals both within and across BLSA participants, remains unaffected by randomly missing data, and accounts for the correlation among repeated measurements on the same participants (21). Mixed-effects regression adequately handles variable numbers of data points per participant because one of its strengths is to use all available data, regardless of length of individual trajectories. VO2max measurement was obtained only at a baseline visit, whereas cognitive function and covariates were assessed at that visit and on up to five subsequent occasions. Results therefore demonstrate whether VO2max at baseline predicts trajectories of age-related change in cognitive performance thereafter.
Each cognitive measure was entered as a single-outcome variable in separate sequential mixed-effects regression models. Age, years of education, body mass index, and depressive symptoms were treated as continuous covariates, and sex, race, antihypertensive medications, hypertension, cardiovascular disease, and inflammatory disease were treated as dichotomous covariates. Except for sex and race, all covariates were treated as time-varying entities in all analyses. Age was modeled as a random effect to index time, as is recommended for studies with continuous enrollment procedures (22). The distribution of participants over time represents a sample of performance opportunities across ages and assumes that the effects of secular trends and methodological alterations are minimal and nonsignificant.
Baseline VO2max (representing overall differences in VO2max regardless of longitudinal changes) and a two-way interaction of VO2max with age (representing longitudinal change in cognitive outcomes associated with VO2max over time) served as fixed terms of interest. Graphs were generated using predicted scores associated with mean VO2max ± 1 SD. Quadratic age (to index nonlinear change over time) was examined but removed from the models because it was uninformative. Age-stratified models (age groups 19–39, 40–65, and 65 years and older) were computed to further examine nonlinearity.
An alpha level of p = .05 was used as a basis for interpretation because each neuropsychological outcome measure examines a distinct aspect of cognitive functioning. Significance of results following conservative Bonferroni correction (p = .003; .05/15 outcome variables) was also considered for the purposes of comparison.
Results
Table 2 shows sample characteristics at first assessment for the full sample and for participants with at least two study visits. The subsample with longitudinal data was highly similar to the full sample. Participants ranged in age from 19 to 94 years (mean = 54 years), with 50.5% being men and 73.6% white. The average participant was well educated, with the equivalent of a Bachelor’s degree (ie, > 16 years of education). Distributions of VO2max and cognitive test scores (presented in Supplementary Table 1) were comparable to known population distributions.
Table 2.
Characteristics of Study Sample at First Assessment
| Variable | Full Sample (n = 1,400) | Longitudinal Sample (n = 615) | ||
|---|---|---|---|---|
| Mean (SD or %) | Range | Mean (SD or %) | Range | |
| Age, y | 54.3 (16.2)* | 19–94 | 56.1 (14.6) | 20–89 |
| Sex, % men | 50.5 | — | 51.5 | — |
| Race, % white | 73.6 | — | 84.6 | — |
| Education, y | 16.7 (2.5) | 4–24 | 16.8 (2.5) | 4–24 |
| Body mass index, kg/m2 | 26.0 (4.3) | 17.0–46.4 | 25.8 (4.0) | 17.0–44.2 |
| Hypertension, % | 40.9 | — | 39.0 | — |
| Antihypertensives, % | 17.2 | — | 15.6 | — |
| Cardiovascular disease, % | 14.2 | — | 14.3 | — |
| Inflammatory disease, % | 42.7 | — | 46.8 | — |
| CES-D, total score | 6.5 (6.8) | 0–50 | 6.1 (6.4) | 0–46 |
| VO2max, mL O2/kg/min | 28.6 (7.9) | 8.2–61.9 | 28.4 (6.9) | 8.2–61.9 |
Notes: SD = standard deviation; CES-D = Center for Epidemiological Studies-Depression scale; VO2max = symptom-limited maximal oxygen consumption.
* The baseline age distribution was as follows: n = 257 aged 19–39 years, n = 755 aged 40–65 years, and n = 388 aged >60 years.
Mixed-effects regression analyses (Table 3) demonstrated significant interactions of VO2max and age (indicating change over time) for BVRT (b = −0.0040, p < .0001), Blessed I–M–C Test (b = −0.0006, p = .014), CVLT immediate free recall (b = 0.0083, p = .001), CVLT learning slope (b = 0.0005, p = .009), CVLT short-delay free recall (b = 0.0016, p = .023), and CVLT long-delay free recall (b = 0.0020, p = .006). If a conservative Bonferroni correction were applied to these results, the interactions of VO2max and age remain significant for BVRT and CVLT immediate free recall. All significant main effects of VO2max were qualified by higher-order age interactions. Results were nonsignificant for the Boston Naming Test, Card Rotation Test, Digit Span, Verbal Fluency, MMSE, and the Trail Making Test (all ps > .05). For complete results, please see Supplementary Table 2.
Table 3.
Results from Mixed-Effects Regression Models Predicting Neuropsychological Test Performance from Baseline VO2max and Covariates
| Neuropsychological Test | VO2max | VO2max × Age |
|---|---|---|
| b (SE) | b (SE) | |
| Benton Visual Retention Test | 0.1567 (0.0324)* | −0.0040 (0.0005)* |
| Blessed Information–Memory–Concentration Test | 0.0271 (0.0143) | −0.0006 (0.0002)* |
| Boston Naming Test | 0.0487 (0.2439) | −0.0006 (0.0034) |
| Card Rotation Test | 0.3685 (0.5946) | 0.0049 (0.0089) |
| CVLT, immediate free recall | −0.3193 (0.1568)* | 0.0083 (0.0025)* |
| CVLT, learning slope | −0.0209 (0.0107) | 0.0005 (0.0002)* |
| CVLT, short-delay free recall | −0.0603 (0.0435) | 0.0016 (0.0007)* |
| CVLT, long-delay free recall | −0.0751 (0.0437) | 0.0020 (0.0007)* |
| Digits Forward | −0.0082 (0.0338) | 0.0001 (0.0005) |
| Digits Backward | −0.0396 (0.0367) | 0.0009 (0.0006) |
| Category Fluency | −0.1575 (0.1461) | 0.0027 (0.0020) |
| Letter Fluency | −0.1174 (0.1555) | 0.0016 (0.0021) |
| Mini-Mental State Examination | 0.0218 (0.0669) | −0.0004 (0.0009) |
| Trail Making Test, Part A | 0.5996 (0.7553) | −0.0092 (0.0106) |
| Trail Making Test, Part B | 3.7315 (2.0557) | −0.0476 (0.0287) |
Notes: VO2max = symptom-limited maximal oxygen consumption; CVLT = California Verbal Learning Test; SE = standard error.
* p < .05; covariates included age, sex, race, education, body mass index, hypertension, antihypertensives, cardiovascular disease, inflammatory disease, and depressive symptoms.
Model goodness-of-fit information is presented in Supplementary Tables 3a, 3b, and 3c. Age-stratified results for the two groups aged 40–65 years and 65 years and older are presented in Supplementary Tables 4a and 4b; young adult models (ages 19–39 years) did not statistically converge due to insufficient sample size. Age-stratified results for middle-aged and older adults did not suggest any strong pattern of nonlinearity. Nine Age × Covariate interaction terms were added in supplementary analyses to adjust for covariates more conservatively. Results are presented in Supplementary Table 5 and are essentially unchanged from primary results, although p values for two Age × VO2max terms became nonsignificant (p = .08 and p = .11, respectively), presumably because of reduced power due to model expansion by nine terms.
Of note, because raw test scores were used in analyses, regression coefficients are not directly comparable across test measures. Several outcome measures, including Blessed I–M–C, Boston Naming Test, MMSE, and Trail Making Test Part B, did not meet regression distributional requirements. Following logarithmic transformation (log10) of these outcomes and associated resolution of distributional violations, we observed no meaningful changes in results.
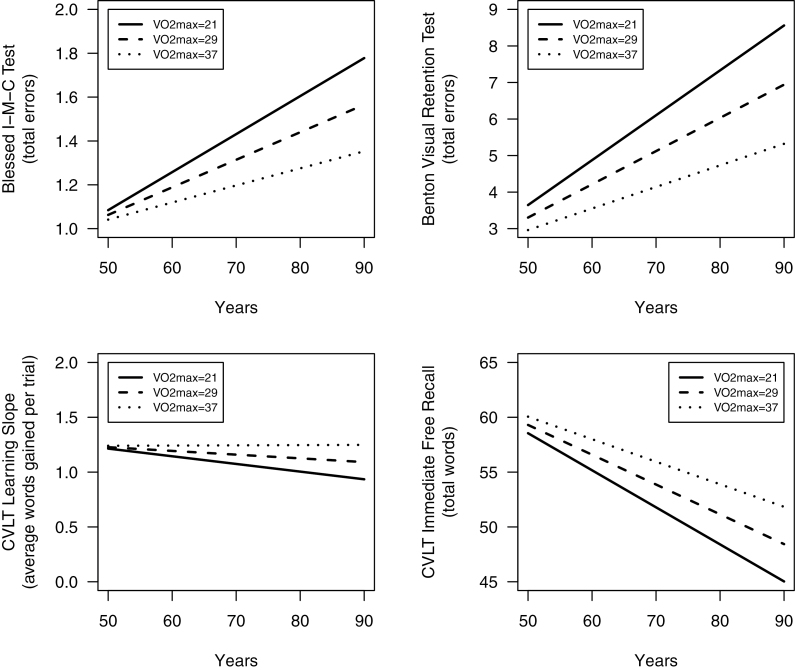
Figure 1 depicts significant VO2max × Age interactions for the Blessed I–M–C Test, BVRT, CVLT immediate free recall, and CVLT learning slope. Each graph depicts age-related change in cognitive performance as a function of VO2max using all information in the analyses, regardless of the number of repeated assessments. Figures begin at age 50 because the bulk of data reflect individuals aged 50 years and older, and most studies do not detect substantial age-related cognitive change prior to middle age.
Figure 1.
Longitudinal change in performance on the Blessed Information–Memory–Concentration (I–M–C) Test, Benton Visual Retention Test (BVRT), and California Verbal Learning Test (CVLT; immediate free recall and learning slope) as a function of baseline symptom-limited maximal oxygen consumption (VO2max; mL O2/kg/min). For the Blessed I–M–C Test and BVRT, worse performance is indicated by increasing scores over time, whereas for the CVLT measures, worse performance is indicated by decreasing scores over time. Covariates included age, sex, race, education, body mass index, hypertension, antihypertensives, cardiovascular disease, inflammatory disease, and depressive symptoms. Graphs were generated using predicted scores associated with mean VO2max ±1 SD.
Discussion
Few studies have examined physiological measures of cardiorespiratory fitness in relation to trajectories of cognitive function across the life span, and none have employed an extensive neuropsychological battery. Here, we found that individuals with reduced VO2max at baseline showed significantly greater prospective decline in performance on multiple measures of visual and verbal memory, including total errors on the BVRT, CVLT immediate free recall, CVLT learning slope, CVLT short-delay free recall, and CVLT long-delay free recall, as well as in a cognitive screening measure weighted heavily for memory, the Blessed I–M–C Test. In short, poorer baseline cardiorespiratory fitness was associated with accelerated memory decline, and greater cardiorespiratory fitness was associated with less prospective memory decline across the life span. As illustrated in Figure 1, effect size magnitudes were small, particularly at younger ages. Clinical significance of these findings on an individual level is therefore unclear, although overall public health significance may be meaningful.
Our findings are consistent with several existing literatures that have examined the link between physical fitness and cognition. For instance, multiple studies have reported significant associations between lower amounts of self-reported leisure time physical activity and (a) decrements in concurrent cognitive performance (4) and (b) longitudinal cognitive decline (5,6). Our study extends these findings in two important ways. First, we examined physiological assessment of cardiorespiratory fitness, rather than self-reported activity levels. Second, most studies relied on global mental status measures (eg, MMSE, Short Portable Mental Status Questionnaire), whereas we incorporated a comprehensive neuropsychological assessment battery. In the Nurses’ Health Study (7), self-reported physical activity was associated with 2-year performance declines on a global composite of four cognitive tests. Although this composite is certainly preferable to reliance on a single cognitive screening measure, all tests were administered over the telephone, which represents a less-ideal testing situation than face-to-face assessment (ie, with greater risks to validity).
The present findings complement the current literature linking lower VO2max with concurrent decrements in cognitive function (11–14). Further, our results extend those of Barnes and colleagues (17), who found worse baseline VO2max to be related to 6-year declines in global cognition and worse follow-up performance on measures of attention, executive function, and memory (CVLT immediate recall) but not delayed recall or verbal fluency (letter and category). We similarly found a longitudinal association with CVLT immediate recall (and absence of association with fluency), but we additionally identified significant effects across other measures of memory (visual immediate recall, verbal delayed recall). We also found no evidence of significant associations within the domains of attention and executive function. Notably, Barnes and colleagues did not include a detailed baseline neuropsychological assessment, so our findings more directly reflect domain-specific cognitive change over time and adequately account for baseline differences.
There was a clear predominance of memory findings in the present study. This pattern stands in contrast with prior literature noting sensitivity of executive functions to aerobic fitness interventions among older adults (15), although a more recent study (23) and Cochrane review (24) found evidence for impact on other cognitive domains of function, including memory. Importantly, the neurobiological mechanisms underlying prediction of life-span cognitive decline may be partially or wholly disparate than the mechanisms driving postintervention improvement of cognitive function, meaning that patterns of affected domains may also vary.
The most critical conclusion to draw from our results involves the likely importance of early intervention to improve cardiorespiratory fitness. Mounting evidence suggests a role of midlife cardiovascular risk factors in the development of not only vascular dementia but also Alzheimer’s disease (25), and memory difficulties enhance risk for all-cause dementia (26). Early intervention to improve cardiorespiratory fitness may modify long-term trajectories of cognitive function and decline in this regard, possibly staving off or even preventing dementia onset. Such intervention may be relevant to cognition as early as preadolescence (27), certainly prior to older adulthood (28). Moreover, decrements and declines in cognitive function carry important implications for everyday functional abilities (29), quality of life (30), and mortality (31), even in the absence of a clinical cognitive disorder.
Multiple mechanisms may account for associations between cardiorespiratory fitness and cognitive function. First, many other biomedical and behavioral cardiovascular risk factors are correlated with cardiorespiratory fitness (eg, obesity, hypertension), and these risk factors are also associated with neuropsychological function (32). Second, this constellation of risk factors is related to levels of subclinical cardiovascular disease, which in turn is associated with cognitive function and decline among healthy adults (33). Possible mechanisms linking cardiovascular risk factors and subclinical diseases with cognitive function include chronic cerebral hypoperfusion (34) and silent cerebral micro- and macrovascular disease. Third, research has revealed compelling evidence for a direct impact of aerobic exercise on brain structure and function (35). In rats, aerobic exercise leads to improved cerebral perfusion and angiogenesis (36), and neuroplastic effects have also been noted (37). Specifically, aerobic exercise is associated with neurogenesis and increased neuronal preservation, and elevations in brain-derived neurotrophic factor and nerve growth factor are thought to play a role (38). Interestingly, these exercise effects appear to be particularly true for the hippocampus (39). In fact, rats demonstrate enhanced spatial learning and memory following only 1 week of prolonged aerobic exercise (40), and older adults have shown increases in hippocampal size and memory performance following a 1-year aerobic exercise intervention (41).
Strengths of this investigation include its longitudinal design, physiological assessment of cardiorespiratory fitness, frequency of neuropsychological testing, length of follow-up, use of an extensive neuropsychological battery, and inclusion of time-dependent covariates. The study was limited by its use of a single baseline assessment of VO2max, and VO2max assessment was symptom maximal. In addition, the study was based on a convenience sample of typically highly educated participants. The homogeneity and nonrepresentative nature of the sample may limit the generalizability of the study, although the sample’s homogeneity may also restrict the influences of confounding demographic variables. Future research examining longitudinal covariation of VO2max and cognitive function would be quite valuable. Furthermore, the present study’s pattern of memory findings awaits replication across other samples to make statements regarding its generalizability.
In sum, findings from the current study suggest a link between cardiorespiratory fitness and longitudinal trajectories of neuropsychological function across the life span. Lower baseline VO2max was associated with accelerated memory decline over time. Early behavioral intervention to improve cardiorespiratory fitness carries the potential to modify patterns of memory decline with aging, thereby carrying the potential to delay or prevent ultimate dementia.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (Z01-AG000195).
References
- 1. Thies W, Bleiler L. Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7:208–244 [DOI] [PubMed] [Google Scholar]
- 2. Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. J Appl Physiol. 2011;111:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2010. Washington, DC: U.S. Government Printing Office; 2012 [Google Scholar]
- 4. Obisesan TO, Umar N, Paluvoi N, Gillum RF. Association of leisure-time physical activity with cognition by apolipoprotein-E genotype in persons aged 60 years and over: the National Health and Nutrition Examination Survey (NHANES-III). Clin Interv Aging. 2012;7:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ku PW, Stevinson C, Chen LJ. Prospective associations between le isure-time physical activity and cognitive performance among older adults across an 11-year period. J Epidemiol. 2012;22:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–2321 [DOI] [PubMed] [Google Scholar]
- 7. Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461 [DOI] [PubMed] [Google Scholar]
- 8. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651 [DOI] [PubMed] [Google Scholar]
- 9. Rovio S, Kå, reholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711 [DOI] [PubMed] [Google Scholar]
- 10. Deary IJ, Whalley LJ, Batty GD, Starr JM. Physical fitness and lifetime cognitive change. Neurology. 2006;67:1195–1200 [DOI] [PubMed] [Google Scholar]
- 11. Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging. 2010;31:2047–2057 [DOI] [PubMed] [Google Scholar]
- 12. Etnier JL, Caselli RJ, Reiman EM, et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39:199–207 [DOI] [PubMed] [Google Scholar]
- 13. Netz Y, Dwolatzky T, Zinker Y, Argov E, Agmon R. Aerobic fitness and multidomain cognitive function in advanced age. Int Psychogeriatr. 2011;23:114–124 [DOI] [PubMed] [Google Scholar]
- 14. Newson RS, Kemps EB. Cardiorespiratory fitness as a predictor of successful cognitive ageing. J Clin Exp Neuropsychol. 2006;28:949–967 [DOI] [PubMed] [Google Scholar]
- 15. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130 [DOI] [PubMed] [Google Scholar]
- 16. Maki Y, Ura C, Yamaguchi T, et al. Effects of intervention using a community-based walking program for prevention of mental decline: a randomized controlled trial. J Am Geriatr Soc. 2012;60:505–510 [DOI] [PubMed] [Google Scholar]
- 17. Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465 [DOI] [PubMed] [Google Scholar]
- 18. Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077 [DOI] [PubMed] [Google Scholar]
- 19. Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988;65:1147–1151 [DOI] [PubMed] [Google Scholar]
- 20. Lezak MD, Howieson DB, Loring DW, eds. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004 [Google Scholar]
- 21. Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–355 [Google Scholar]
- 22. McArdle JJ, Bell RQ. An introduction to latent growth models for developmental data analysis. In: Little TD, Schnabel KU, Baumert J, eds. Modeling Longitudinal and Multilevel Data: Practical Issues, Applied Approaches, and Specific Examples. Mahwah, NJ: Erlbaum; 2000:69–107 [Google Scholar]
- 23. Klusmann V, Evers A, Schwarzer R, et al. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2010;65:680–688 [DOI] [PubMed] [Google Scholar]
- 24. Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008; April:CD005381. [DOI] [PubMed] [Google Scholar]
- 25. White L, Launer L. Relevance of cardiovascular risk factors and ischemic cerebrovascular disease to the pathogenesis of Alzheimer disease: a review of accrued findings from the Honolulu-Asia Aging Study. Alzheimer Dis Assoc Disord. 2006;20(suppl 2):S79–S83 [DOI] [PubMed] [Google Scholar]
- 26. Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Progression to dementia in clinical subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22:27–34 [DOI] [PubMed] [Google Scholar]
- 27. Wu CT, Pontifex MB, Raine LB, et al. Aerobic fitness and response variability in preadolescent children performing a cognitive control task. Neuropsychology. 2011;25:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301 [DOI] [PubMed] [Google Scholar]
- 29. Lee Y, Kim JH, Lee KJ, Han G, Kim JL. Association of cognitive status with functional limitation and disability in older adults. Aging Clin Exp Res. 2005;17:20–28 [DOI] [PubMed] [Google Scholar]
- 30. Waldstein SR, Wendell CR, Hosey MM. Applications of neurocognitive assessment in behavioral medicine. In: Steptoe A, ed. Handbook of Behavioral Medicine. New York, NY: Springer; 2010:125–136 [Google Scholar]
- 31. Sachs GA, Carter R, Holtz LR, et al. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann Intern Med. 2011;155:300–308 [DOI] [PubMed] [Google Scholar]
- 32. Waldstein SR, Wendell CR. Neurocognitive function and cardiovascular disease. J Alzheimers Dis. 2010;20:833–842 [DOI] [PubMed] [Google Scholar]
- 33. Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke. 2009;40:3180–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruitenberg A, den Heijer T, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794 [DOI] [PubMed] [Google Scholar]
- 35. Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H. The effects of aerobic activity on brain structure. Front Psychol. 2012;3:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046 [DOI] [PubMed] [Google Scholar]
- 37. Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring). 2006;14:345–356 [DOI] [PubMed] [Google Scholar]
- 38. Neeper SA, Gó, mez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56 [PubMed] [Google Scholar]
- 39. Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23:941–955 [DOI] [PubMed] [Google Scholar]
- 40. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590 [DOI] [PubMed] [Google Scholar]
- 41. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]